Gasification of Solid Recovered Fuels with Variable Fractions of Polymeric Materials †
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Gasification Experiments
2.3. Calculation of Gasification Performance Indicators
2.4. Analysis Procedures for Feedstocks and Gasification Products
- Proximate analysis (with moisture, volatile matter and ash contents determined by ASTM E949-88, ASTM E897-88 and ASTM E830-87, respectively; fixed carbon was calculated by difference). The final results for each parameter were determined through the average of two experimental replicates;
- Ultimate analysis (the contents of N, C, H and S were quantified in a ThermoFisher Scientific Flash 2000 CHNS-O analyser; the concentrations of O were calculated by difference);
- Higher heating value—HHV (using a calorimetric bomb IKA C200);
- Chlorine concentration (using an X-ray fluorescent analyser Thermo Scientific Niton XL 3T GOLDD+). Two readings of chlorine concentrations were performed to obtain the final results through the average of experimental values;
- Mineral composition of ashes (using the same procedure as for chlorine concentration). Again, this composition was obtained through the average of two readings, using the analyser;
- Apparent density (by following EN 15103). Final results were determined through the average of two replicates.
3. Results and Discussion
3.1. Characterisation of Raw Residues and Solid Recovered Fuels
| Property | Mixture | |||
|---|---|---|---|---|
| SRF1 | SRF2 | SRF3 | ||
| Proximate analysis (wt %) * | Moisture | 11.9 ± 0.3 | 9.4 ± 1.1 | 6.0 ± 0.3 |
| Volatile matter | 92.4 ± 0.7 | 93.9 ± 0.4 | 85.5 ± 0.8 | |
| Fixed carbon | 1.6 ± 0.8 | 0.0 ± 0.9 | 9.5 ± 1.2 | |
| Ash | 6.0 ± 0.1 | 6.1 ± 0.5 | 5.0 ± 0.4 | |
| Ultimate analysis (wt % daf) | C | 51.7 ± 0.0 | 52.2 ± 0.0 | 52.7 ± 0.0 |
| H | 6.3 ± 0.0 | 6.5 ± 0.0 | 6.6 ± 0.0 | |
| N | 1.5 ± 0.0 | 1.4 ± 0.0 | 1.3 ± 0.0 | |
| S | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| O ** | 40.4 ± 0.0 | 39.8 ± 0.0 | 39.3 ± 0.0 | |
| Ash composition (wt % db) | CaO | 34.7 ± 1.09 | 41.5 ± 0.13 | 45.0 ± 0.88 |
| SiO2 | 25.0 ± 0.51 | 24.7 ± 0.66 | 23.1 ± 0.00 | |
| Al2O3 | 13.9 ± 0.32 | 18.7 ± 0.96 | 21.3 ± 3.00 | |
| Fe2O3 | 6.6 ± 0.63 | 4.2 ± 0.17 | 4.9 ± 0.00 | |
| K2O | 2.5 ± 0.11 | 1.2 ± 0.00 | 1.1 ± 0.02 | |
| H/C mass ratio | 0.12 | 0.12 | 0.13 | |
| O/C mass ratio | 0.78 | 0.76 | 0.75 | |
| Higher heating value (HHV, MJ/kg db) | 18.5 ± 0.0 | 21.0 ± 0.0 | 24.7 ± 0.0 | |
| Chlorine (wt % db) | 0.2 ± 0.0 | 0.9 ± 0.1 | 1.6 ± 0.1 | |
| Apparent density (kg/m3 wb) | 152 ± 5 | 117 ± 2 | 93 ± 8 | |
3.2. Assessment of Gasification Performance
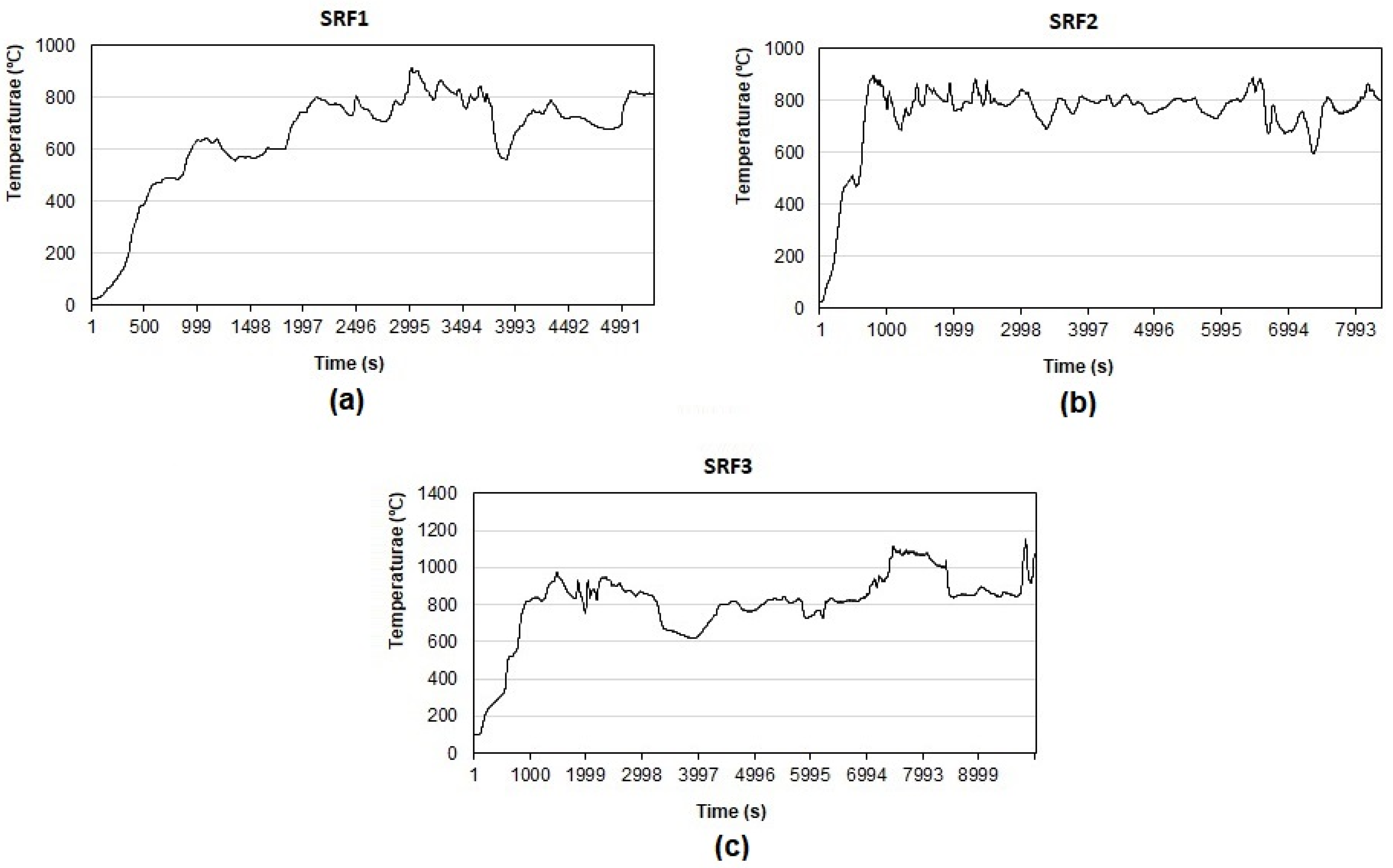
3.3. Analysis of Gasification Products
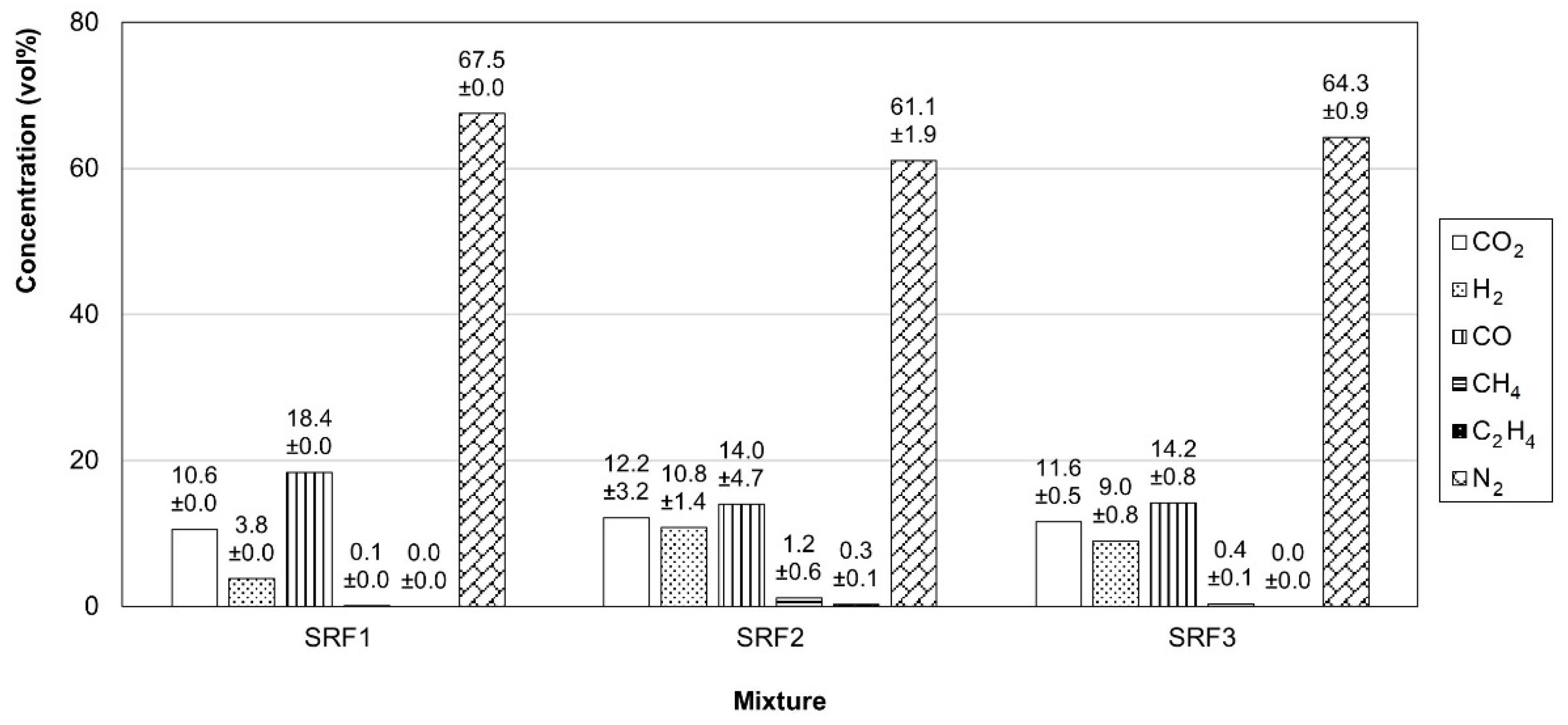
3.3.1. Gas Properties
3.3.2. Char Properties
3.4. Summary of the Influence of SRF Composition in Gasification Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eurostat. Your Key to European Statistics. Available online: https://ec.europa.eu/eurostat (accessed on 1 March 2019).
- Ferreira, S.; Monteiro, E.; Calado, L.; Silva, V.; Brito, P.; Vilarinho, C. Experimental and modeling analysis of brewers’ spent grains gasification in a downdraft reactor. Energies 2019, 12, 4413. [Google Scholar] [CrossRef]
- Pordata. Base de Dados Portugal Contemporâneo. Available online: https://www.pordata.pt (accessed on 1 March 2019).
- Bianchini, G.; Marrocchino, E.; Tassinari, R.; Vaccaro, C. Recycling of construction and demolition waste materials: A chemical-mineralogical appraisal. Waste Manag. 2005, 25, 149–159. [Google Scholar] [CrossRef]
- Nasrullah, M.; Vainikka, P.; Hannula, J.; Hurme, M.; Oinas, P. Elemental balance of SRF production process: Solid recovered fuel produced from municipal solid waste. Waste Manag. Res. 2016, 34, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Portal do Instituto Nacional de Estatística-Portugal. Available online: https://www.ine.pt (accessed on 1 March 2019).
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Luz, F.C.; Rocha, M.H.; Lora, E.E.S.; Venturini, O.J.; Andrade, R.V.; Leme, M.M.V.; Del Olmo, O.A. Techno-economic analysis of municipal solid waste gasification for electricity generation in Brazil. Energy Convers. Manag. 2015, 103, 321–337. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Resources, properties and utilization of tar. Resour. Conserv. Recycl. 2010, 54, 905–915. [Google Scholar] [CrossRef]
- García, G.; Arauzo, J.; Gonzalo, A.; Sánchez, J.L.; Ábrego, J. Influence of feedstock composition in fluidised bed co-gasification of mixtures of lignite, bituminous coal and sewage sludge. Chem. Eng. J. 2013, 222, 345–352. [Google Scholar] [CrossRef]
- Taupe, N.C.; Lynch, D.; Wnetrzak, R.; Kwapinska, M.; Kwapinski, W.; Leahy, J.J. Updraft gasification of poultry litter at farm-scale-A case study. Waste Manag. 2016, 50, 324–333. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion-A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Zaccariello, L.; Mastellone, M.L. Fluidized-bed gasification of plastic waste, wood and their blends with coal. Energies 2015, 8, 8052–8068. [Google Scholar] [CrossRef]
- Borgianni, C.; De Filippis, P.; Pochetti, F.; Paolucci, M. Gasification process of wastes containing PVC. Fuel 2002, 81, 1827–1833. [Google Scholar] [CrossRef]
- Khodaei, H.; Olson, C.; Patino, D.; Rico, J.; Jin, Q.; Boateng, A. Multi-objective utilization of wood waste recycled from construction and demolition (C&D): Products and characterization. Waste Manag. 2022, 149, 228–238. [Google Scholar] [CrossRef]
- Yufeng, D.; Tongyao, J.; Meng, Y.; Han, S.; Jiang, J. Pyrolysis characteristics of excavated waste and generation mechanism of gas products. J. Clean. Prod. 2022, 370, 133489. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Lee, I.; Jeon, J.; Ryu, C.; Park, S.; Jung, S.; Park, Y. Influence of reaction conditions on bio-oil production from pyrolysis of construction waste wood. Renew. Energy 2014, 65, 41–48. [Google Scholar] [CrossRef]
- Nuss, P.; Gardner, K.H.; Jambeck, J.R. Comparative life cycle assessment (LCA) of construction and demolition (C&D) derived biomass and U.S. northeast forest residuals gasification for electricity production. Environ. Sci. Technol. 2013, 47, 3463–3471. [Google Scholar] [CrossRef]
- Hwang, I.H.; Kobayashi, J.; Kawamoto, K. Characterization of products obtained from pyrolysis and steam gasification of wood waste, RDF and RPF. Waste Manag. 2014, 34, 402–410. [Google Scholar] [CrossRef]
- Willeboer, W. The amer demolition wood gasification project. Biomass Bioenergy 1998, 15, 245–249. [Google Scholar] [CrossRef]
- Littlejohns, J.; Butler, J.; Luque, L.; Austin, k. Experimental investigation of bioenergy production from small-scale gasification of landfill-diverted wood wastes. Waste Biomass Valoriz. 2020, 11, 6885–6901. [Google Scholar] [CrossRef]
- Lotfi, S.; Littlejohns, J.; Austin, K.; Luque, L. Modeling as a tool for the optimal design of a downdraft gasifier operating on waste feedstock. Waste Biomass Valoriz. 2021, 12, 6569–6589. [Google Scholar] [CrossRef]
- Peres, S.; Loureiro, E.; Santos, H.; Vanderley e Silva, F.; Gusmao, A. The production of gaseous biofuels using biomass waste from construction sites in Recife, Brazil. Processes 2020, 8, 457. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y. Experimental study on non-woody biomass gasification in a downdraft gasifier. Int. J. Hydrogen Energy 2012, 37, 4935–4944. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction-Practical Design and Theory, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Akkache, S.; Hernández, A.B.; Teixeira, G.; Gelix, F.; Roche, N.; Ferrasse, J.H. Co-gasification of wastewater sludge and different feedstock: Feasibility study. Biomass Bioenergy 2016, 89, 201–209. [Google Scholar] [CrossRef]
- Patel, V.R.; Patel, D.; Varia, N.S.; Patel, R.N. Co-gasification of lignite and waste wood in a pilot-scale (10 kWe) downdraft gasifier. Energy 2017, 119, 834–844. [Google Scholar] [CrossRef]
- You, S.; Tong, H.; Armin-Hoiland, J.; Tong, Y.W.; Wang, C.H. Techno-economic and greenhouse gas savings assessment of decentralized biomass gasification for electrifying the rural areas of Indonesia. Appl. Energy 2017, 208, 495–510. [Google Scholar] [CrossRef]
- Silva, R.B.; Fragoso, R.; Sanches, C.; Costa, M.; Martins-Dias, S. Which chlorine ions are currently being quantified as total chlorine on solid alternative fuels? Fuel Process. Technol. 2014, 128, 61–67. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2002, 24, 125–140. [Google Scholar] [CrossRef]
- Özyuguran, A.; Yaman, S. Prediction of calorific value of biomass from proximate analysis. Energy Procedia 2017, 107, 130–136. [Google Scholar] [CrossRef]
- Berrueco, C.; Recari, J.; Abelló, S.; Farriol, X.; Montané, D. Experimental investigation of solid recovered fuel (SRF) gasification: Effect of temperature and equivalence ratio on process performance and release of minor contaminants. Energy Fuel 2015, 29, 7419–7427. [Google Scholar] [CrossRef]
- Recari, J.; Berrueco, C.; Abelló, S.; Montané, D.; Farriol, X. Gasification of two solid recovered fuels (SRFs) in a lab-scale fluidized bed reactor: Influence of experimental conditions on process performance and release of HCl, H2S, HCN and NH3. Fuel Process. Technol. 2016, 142, 107–114. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Yang, W.; Alam, M.; Seo, Y. A comparative study of the gasification of solid refuse fuel in downdraft fixed bed and bubbling fluidized bed reactors. Waste Biomass Valoriz. 2020, 11, 2345–2356. [Google Scholar] [CrossRef]
- Khosasaeng, T.; Suntivarakorn, R. Effect of Equivalence Ratio on an Efficiency of Single Throat Downdraft Gasifier Using RDF from Municipal solid waste. Energy Procedia 2017, 138, 784–788. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; André, R.N.; Miranda, M.; Gulyurtlu, I.; Cabrita, I. Co-gasification study of biomass mixed with plastic wastes. Fuel 2002, 81, 291–297. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Nipattummakul, N.; Gupta, A.K. Characteristics of syngas from co-gasification of polyethylene and woodchips. Appl. Energy 2011, 88, 165–174. [Google Scholar] [CrossRef]
- Kungkajit, C.; Prateepchaikul, G.; Kaosol, T. Influence of Plastic Waste for Refuse-Derived Fuel on Downdraft Gasification. Energy Procedia 2015, 79, 528–535. [Google Scholar] [CrossRef]
- Etutu, T.G.; Laohalidanond, K.; Kerdsuwan, S. Gasification of municipal solid waste in a downdraft gasifier: Analysis of tar formation. Songklanakarin J. Sci. Technol. 2016, 38, 221–228. [Google Scholar]
- Laurence, L.C.; Ashenafi, D. Syngas Treatment Unit for Small Scale Gasification-Application to IC Engine Gas Application to IC Engine Gas Quality Requirement. J. Appl. Fluid Mech. 2012, 5, 95–103. [Google Scholar]
- Galvagno, S.; Casciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam gasification of tyre waste, poplar, and refuse-derived fuel: A comparative analysis. Waste Manag. 2009, 29, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen production from biomass gasification using biochar as a catalyst/support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y. Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification. Renew. Sustain. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Pozzi, P.; Vezzali, V. Char valorization in construction materials. In Wastes: Solutions, Treatments and Opportunities; Vilarinho, C., Castro, F., Gonçalves, M., Fernando, A., Eds.; Taylor & Francis Group: London, UK, 2020; Volume III, pp. 230–235. [Google Scholar]

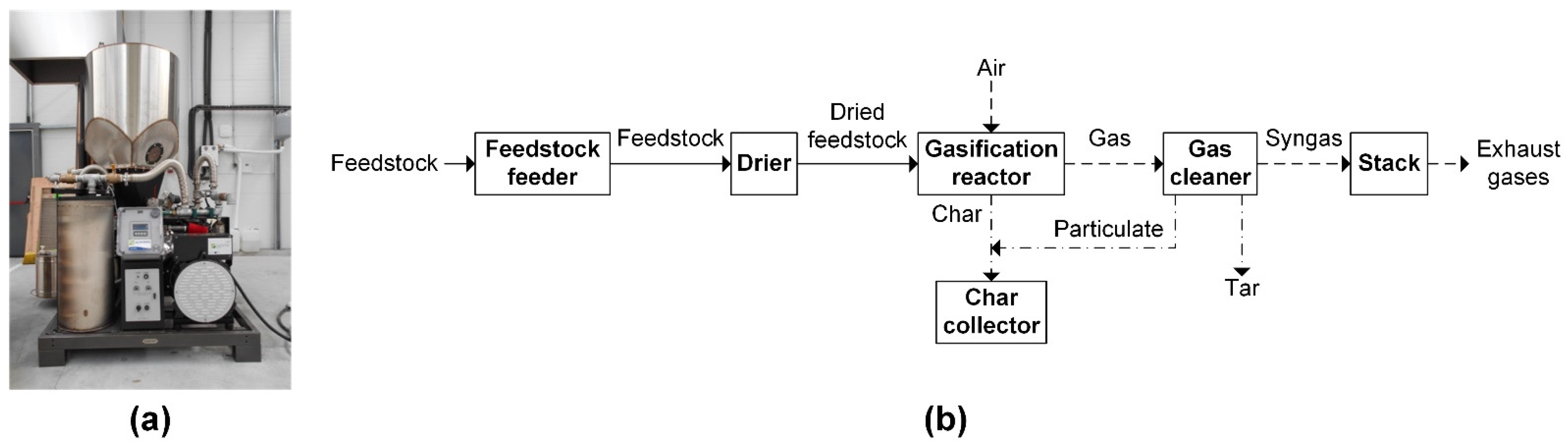
| Code | Description | ELW * Code | Category | Annual Mass Fraction (wt % wb) |
|---|---|---|---|---|
| R1 | Wood from construction and demolition wastes (C&DW) | 17 02 01 | Lignocellulosic | 65.9 (R1 + R2) |
| R2 | Wood from municipal solid wastes (MSW) | 20 01 38 | Lignocellulosic | |
| R3 | Paper/card (MSW) | 20 01 01 | Lignocellulosic | 9.2 |
| R4 | Plastic packages | 15 01 02 | Polymeric | 0.7 |
| R5 | Composite packages | 15 01 05 | Polymeric | 4.9 |
| R6 | Mixture of packages | 15 01 06 | Polymeric | 4.2 |
| R7 | Plastics (C&DW) | 17 02 03 | Polymeric | 9.9 |
| R8 | Plastics (MSW) | 20 01 39 | Polymeric | 2.8 |
| R9 | Polymeric insulations (C&DW) | 17 06 04 | Polymeric | 2.4 |
| Mixture | General Composition | Fraction of Residues (wt % wb *) |
|---|---|---|
| SRF1 | 100 wt % lignocellulosic | R1 + R2 **: 87.7 R3: 12.3 |
| SRF2 | 90 wt % lignocellulosic + 10 wt % polymeric | R1 + R2: 78.8 R3: 11.1 R4: 0.3 R5: 2.0 R6: 1.7 R7: 4.0 R8: 1.1 R9: 1.0 |
| SRF3 | 80 wt % lignocellulosic + 20 wt % polymeric | R1 + R2: 70.1 R3: 9.8 R4: 0.6 R5: 3.9 R6: 3.4 R7: 8.0 R8: 2.3 R9: 1.9 |
| Property | Residue | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 + R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | ||
| Proximate analysis (wt %) * | Moisture | 12.5 ± 0.1 | 6.2 ± 0.2 | 0.6 ± 0.0 | 6.1 ± 0.0 | 4.3 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.2 | 1.0 ± 0.1 |
| Volatile matter | 85.7 ± 0.1 | 82.0 ± 1.5 | 98.0 ± 0.1 | 94.0 ± 0.6 | 85.7 ± 0.4 | 81.8 ± 0.5 | 98.1 ± 0.1 | 93.6 ± 0.2 | |
| Fixed carbon | 6.3 ± 0.7 | 0.0 ± 1.5 | 0.0 ± 0.2 | 0.0 ± 0.8 | 5.2 ± 0.5 | 7.3 ± 0.9 | 0.4 ± 0.2 | 4.5 ± 0.3 | |
| Ash | 8.0 ± 0.6 | 18.0 ± 0.0 | 2.0 ± 0.1 | 6.0 ± 0.2 | 9.1 ± 0.1 | 10.9 ± 0.4 | 1.5 ± 0.1 | 1.9 ± 0.1 | |
| Ultimate analysis (wt % daf **) | C | 52.3 ± 0.0 | 47.2 ± 0.0 | 85.6 ± 0.0 | 42.8 ± 0.0 | 46.0 ± 0.0 | 57.1 ± 0.0 | 73.6 ± 0.0 | 72.3 ± 0.0 |
| H | 6.3 ± 0.0 | 6.4 ± 0.0 | 14.3 ± 0.0 | 5.8 ± 0.0 | 6.5 ± 0.0 | 7.4 ± 0.0 | 13.1 ± 0.0 | 7.5 ± 0.0 | |
| N | 1.7 ± 0.0 | 0.4 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.0 | 0.8 ± 0.0 | 3.4 ± 0.0 | |
| S | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| O *** | 39.6 ± 0.0 | 45.9 ± 0.0 | 0.1 ± 0.0 | 51.1 ± 0.0 | 47.5 ± 0.0 | 35.1 ± 0.0 | 12.5 ± 0.0 | 16.8 ± 0.0 | |
| Ash composition (wt % db) | CaO | 17.26 ± 1.30 | 42.19 ± 0.06 | 23.69 ± 1.39 | 54.66 ± 0.22 | 36.72 ± 0.00 | 22.66 ± 0.00 | 36.22 ± 2.07 | 17.61 ± 0.14 |
| SiO2 | 14.58 ± 1.69 | 16.32 ± 0.02 | 17.97 ± 0.49 | 18.03 ± 0.36 | 16.21 ± 0.06 | 6.72 ± 0.24 | 18.48 ± 0.30 | 57.67 ± 0.80 | |
| Al2O3 | 4.78 ± 0.45 | 8.24 ± 0.26 | 4.65 ± 1.38 | 8.37 ± 1.02 | 8.22 ± 0.25 | 0.00 ± 0.00 | 3.59 ± 0.60 | 2.49 ± 0.13 | |
| Fe2O3 | 5.43 ± 1.46 | 1.37 ± 0.01 | 4.59 ± 0.23 | 2.69 ± 0.07 | 1.47 ± 0.06 | 1.39 ± 0.03 | 4.68 ± 0.27 | 6.11 ± 0.33 | |
| K2O | 1.60 ± 0.27 | 0.11 ± 0.01 | 1.35 ± 0.07 | 0.64 ± 0.00 | 0.33 ± 0.00 | 0.08 ± 0.00 | 0.58 ± 0.04 | 0.52 ± 0.01 | |
| H/C mass ratio (daf) | 0.12 | 0.13 | 0.17 | 0.13 | 0.14 | 0.13 | 0.18 | 0.10 | |
| O/C mass ratio (daf) | 0.76 | 0.97 | 0.00 | 1.19 | 1.03 | 0.61 | 0.17 | 0.23 | |
| HHV (MJ/kg db) | 18.5 ± 0.0 | 14.9 ± 0.1 | 44.9 ± 0.1 | 17.0 ± 0.1 | 26.5 ± 2.3 | 25.0 ± 0.5 | 38.2 ± 3.3 | 30.3 ± 0.1 | |
| Chlorine (wt % db) | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 7.3 ± 0.0 | 0.1 ± 0.0 | 44.1 ± 0.3 | |
| Apparent density (kg/m3 wb) | 262 ± 0 | 37 ± 1 | 26 ± 0 | 25 ± 2 | 43 ± 3 | 543 ± 11 | 69 ± 17 | 19 ± 2 | |
| Indicator | Mixture | |||
|---|---|---|---|---|
| SRF1 | SRF2 | SRF3 | ||
| Average temperature (°C) * | 780 | 783 | 805 | |
| Test duration (h) | 1.5 | 2.3 | 2.8 | |
| SRF feed rate (kg/h) | 6.6 | 4.1 | 3.1 | |
| Equivalence ratio (ER) | 0.39 | 0.33 | 0.45 | |
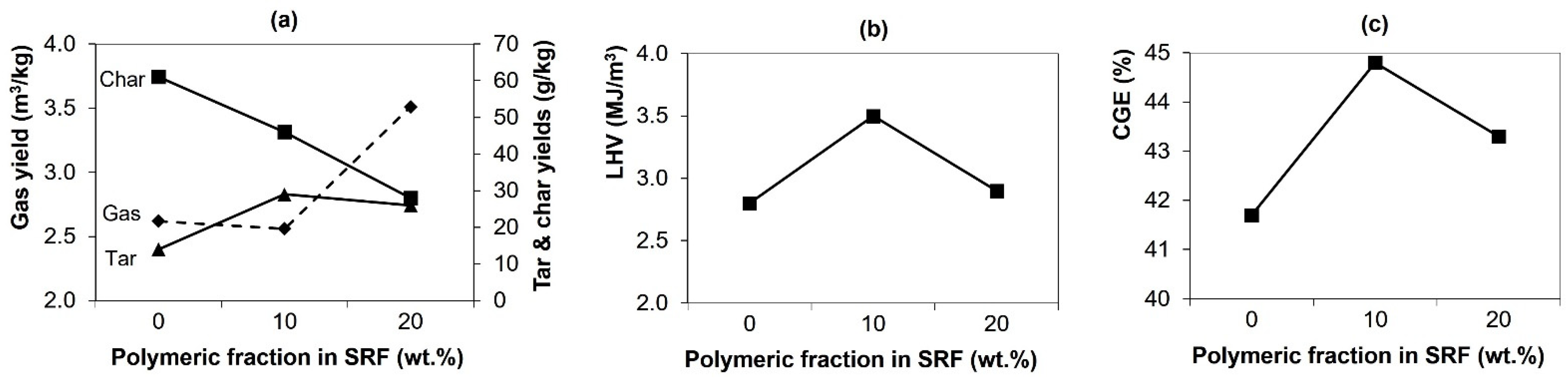
| Gas yield (m3/kg waste) | 2.62 | 2.56 | 3.51 | |
| Char yield (g/kg waste) | 61 | 46 | 28 | |
| Tar yield | g/kg waste | 14 | 29 | 26 |
| g/m3 gas | 5.3 | 11.3 | 7.4 | |
| Cold-gas efficiency (CGE, %) | 41.7 | 44.8 | 43.3 | |
| Carbon conversion efficiency (CCE, %) | 67.1 | 64.2 | 84.4 | |
| Mixture | Lower Heating Value (LHV, MJ/Nm3) | Density (kg/Nm3) | Tar Amount (g/m3) | HCl Concentration (g/Nm3) |
|---|---|---|---|---|
| SRF1 | 2.8 ± 0.0 | 1.28 ± 0.00 | 5.3 | 1.1 ± 0.1 |
| SRF2 | 3.5 ± 0.5 | 1.20 ± 0.03 | 11.3 | 1.9 ± 0.0 |
| SRF3 | 2.9 ± 0.2 | 1.22 ± 0.01 | 7.4 | 1.7 ± 0.3 |
| Property | Mixture | |||
|---|---|---|---|---|
| SRF1 | SRF2 | SRF3 | ||
| HHV (MJ/kg db) | 0.0 ± 0.0 1 | 5.8 ± 0.1 | 4.6 ± 0.0 | |
| Ash (wt % db) | 90.7 ± 0.1 | 79.9 ± 0.4 | 81.7 ± 0.0 | |
| Chlorine (wt % db) | 3.4 ± 0.1 | 2.9 ± 0.0 | 4.2 ± 0.2 | |
| Mineral oxides in ash (wt % db) | CaO | 40.3 ± 0.1 | 32.4 ± 0.3 | 29.5 ± 1.5 |
| SiO2 | 12.3 ± 0.2 | 15.8 ± 0.6 | 15.9 ± 0.4 | |
| Al2O3 | 3.9 ± 0.2 | 4.5 ± 0.4 | 4.9 ± 0.2 | |
| Fe2O3 | 5.1 ± 0.4 | 7.5 ± 1.9 | 10.4 ± 2.3 | |
| K2O | 2.1 ± 0.1 | 3.4 ± 0.1 | 2.7 ± 0.0 | |
| Heavy metals in ash (ppmw db) | Cd | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Cr | 799 ± 56 | 804 ± 68 | 1564 ± 584 | |
| Cu | 892 ± 41 | 3757 ± 769 | 2988 ± 391 | |
| Hg | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Ni | 599 ± 31 | 421 ± 25 | 713 ± 53 | |
| Pb | 494 ± 18 | 514 ± 82 | 410 ± 33 | |
| Zn | 888 ± 14 | 1382 ± 3 | 1568 ± 36 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, O.; Calado, L.; Panizio, R.M.; Nobre, C.; Monteiro, E.; Brito, P.; Gonçalves, M. Gasification of Solid Recovered Fuels with Variable Fractions of Polymeric Materials. Energies 2022, 15, 8139. https://doi.org/10.3390/en15218139
Alves O, Calado L, Panizio RM, Nobre C, Monteiro E, Brito P, Gonçalves M. Gasification of Solid Recovered Fuels with Variable Fractions of Polymeric Materials. Energies. 2022; 15(21):8139. https://doi.org/10.3390/en15218139
Chicago/Turabian StyleAlves, Octávio, Luís Calado, Roberta M. Panizio, Catarina Nobre, Eliseu Monteiro, Paulo Brito, and Margarida Gonçalves. 2022. "Gasification of Solid Recovered Fuels with Variable Fractions of Polymeric Materials" Energies 15, no. 21: 8139. https://doi.org/10.3390/en15218139
APA StyleAlves, O., Calado, L., Panizio, R. M., Nobre, C., Monteiro, E., Brito, P., & Gonçalves, M. (2022). Gasification of Solid Recovered Fuels with Variable Fractions of Polymeric Materials. Energies, 15(21), 8139. https://doi.org/10.3390/en15218139











