Experimental Thermodynamic Characterization of the Chalcopyrite-Based Compounds in the Ag–In–Te System for a Potential Thermoelectric Application
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- the GCs with PE of the mentioned phase regions are characterized by different EMF values at the same temperature, Figure 2 (right);
- as the phase region approaches the position of silver composition, the EMF values of cells decrease [41]; and
- subsequent determination of thermodynamic data of the In2Te5 compound.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.-T.; Sun, Q.; Zhong, Y.; Deng, Q.; Gan, L.; Lv, F.-L.; Shi, X.-L.; Chen, Z.-G.; Ang, R. High-performance in n-type PbTe-based thermoelectric materials achieved by synergistically dynamic doping and energy filtering. Nano Energy 2022, 91, 106706. [Google Scholar] [CrossRef]
- Zazakowny, K.; Kosonowski, A.; Lis, A.; Cherniushok, O.; Parashchuk, T.; Tobola, J.; Wojciechowski, K.T. Phase Analysis and Thermoelectric Properties of Cu-Rich Tetrahedrite Prepared by Solvothermal Synthesis. Materials 2022, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Lin, S.; Zhou, B.; Gao, B.; Pei, Y. Promising Thermoelectric Ag5−δTe3 with Intrinsic Low Lattice Thermal Conductivity. ACS Energy Lett. 2017, 2, 2470–2477. [Google Scholar] [CrossRef]
- Cherniushok, O.; Cardoso-Gil, R.; Parashchuk, T.; Grin, Y.; Wojciechowski, K.T. Phase Equilibria and Thermoelectric Properties in the Pb–Ga–Te System in the Vicinity of the PbGa6Te10 Phase. Inorg. Chem. 2021, 60, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Irshad, K.; Islam, N.; Zahir, H.; Pasha, A.A.; AbdelGawad, A.F. Thermal performance investigation of Therminol55/MWCNT+CuO nanofluid flow in a heat exchanger from an exergy and entropy approach. Case Stud. Therm. Eng. 2022, 34, 102010. [Google Scholar] [CrossRef]
- Cherniushok, O.; Parashchuk, T.; Tobola, J.; Luu, S.D.N.; Pogodin, A.; Kokhan, O.; Studenyak, I.; Barchiy, I.; Piasecki, M.; Wojciechowski, K.T. Entropy-Induced Multivalley Band Structures Improve Thermoelectric Performance in p-Cu7P(SxSe1–x)6 Argyrodites. ACS Appl. Mater. Interfaces 2021, 13, 39606–39620. [Google Scholar] [CrossRef]
- Tesfaye, F.; Iloeje, C.O. Thermodynamic Considerations for Improved Renewable Energy Production. JOM 2021, 73, 1484–1486. [Google Scholar] [CrossRef]
- Ravi, V.; Firdosy, S.; Caillat, T.; Brandon, E.; Van Der Walde, K.; Maricic, L.; Sayir, A. Thermal Expansion Studies of Selected High-Temperature Thermoelectric Materials. J. Electron. Mater. 2009, 38, 1433–1442. [Google Scholar] [CrossRef]
- Verma, A. Bond-stretching force constant of AIBIIIC2V I and AIIBIVC2V chalcopyrite semiconductors. Solid State Commun. 2009, 149, 1236–1239. [Google Scholar] [CrossRef]
- Skomedal, G.; Holmgren, L.; Middleton, H.; Eremin, I.; Isachenko, G.; Jaegle, M.; Tarantik, K.; Vlachos, N.; Manoli, M.; Kyratsi, T.; et al. Design, assembly and characterization of silicide-based thermoelectric modules. Energy Convers. Manag. 2016, 110, 13–21. [Google Scholar] [CrossRef]
- Mouacher, R.; Seddik, T.; Rezini, B.; Haq, B.U.; Batouche, M.; Uğur, G.; Uğur, S.; Belfedal, A. First-principles calculations of electronic and optical properties of AgGa1-xTlxS2 alloys: Analyses and design for solar cell applications. J. Solid State Chem. 2022, 309, 122996. [Google Scholar] [CrossRef]
- Ando Junior, O.H.; Maran, A.L.O.; Henao, N.C. A review of the development and applications of thermoelectric microgenerators for energy harvesting. Renew. Sustain. Energy Rev. 2018, 91, 376–393. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Jafarian, M.; Vashaee, D.; Ghomashchi, R. Thermal Management Systems and Waste Heat Recycling by Thermoelectric Generators—An Overview. Energies 2021, 14, 5646. [Google Scholar] [CrossRef]
- Ochieng, A.O.; Megahed, T.F.; Ookawara, S.; Hassan, H. Comprehensive review in waste heat recovery in different thermal energy-consuming processes using thermoelectric generators for electrical power generation. Process Saf. Environ. Prot. 2022, 162, 134–154. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, K.; Chen, H.; Ren, Q.; Yue, Z.; Wei, T.-R.; Qiu, P.; Chen, L.; Shi, X. Entropy engineering induced exceptional thermoelectric and mechanical performances in Cu2-Ag Te1-2S Se. Acta Mater. 2021, 224, 117512. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, K.; Kosuga, A. Relationship between electrical properties and electronic structure of the thermoelectric Ag–In–Te system with chalcopyrite structure and its related defect-containing structure. Scr. Mater. 2019, 162, 272–276. [Google Scholar] [CrossRef]
- Bahari, Z.; Rivet, J.; Dugué, J. Diagramme de phases du système Ag2Te-In2Te3. Comptes Rendus L’académie Des. Sci. Ser. IIC Chem. 1998, 1, 411–415. [Google Scholar] [CrossRef]
- Bahari, Z.; Rivet, J.; Legendre, B.; Dugué, J. Study of the Ag–In–Te ternary system: I. Description of the triangle Ag2Te–In2Te3–Te. J. Alloys Compd. 1999, 282, 164–174. [Google Scholar] [CrossRef]
- Bahari, Z.; Rivet, J.; Legendre, B.; Dugué, J. Study of the Ag–In–Te ternary system: II. Description of the quadrilateral Ag–Ag2Te–In2Te3–In. J. Alloys Compd. 1999, 289, 99–115. [Google Scholar] [CrossRef]
- Charoenphakdee, A.; Kurosaki, K.; Muta, H.; Uno, M.; Yamanaka, S. Thermal Conductivity of the Ternary Compounds: AgMTe2 and AgM5Te8 (M = Ga or In). Mater. Trans. 2009, 50, 1603–1606. [Google Scholar] [CrossRef] [Green Version]
- Rangasami, C.; Malar, P.; Osipowicz, T.; Jain, M.K.; Kasiviswanathan, S. Structure of melt-quenched AgIn3Te5. Powder Diffr. 2011, 26, 248–255. [Google Scholar] [CrossRef]
- Rangasami, C. Vibrational modes of AgIn3Te5 and effect of laser irradiation. Vib. Spectrosc. 2018, 94, 66–73. [Google Scholar] [CrossRef]
- Moroz, M.; Tesfaye, F.; Demchenko, P.; Prokhorenko, M.; Kogut, Y.; Pereviznyk, O.; Prokhorenko, S.; Reshetnyak, O. Solid-state electrochemical synthesis and thermodynamic properties of selected compounds in the Ag–Fe–Pb–Se system. Solid State Sci. 2020, 107, 106344. [Google Scholar] [CrossRef]
- Moroz, M.; Tesfaye, F.; Demchenko, P.; Prokhorenko, M.; Prokhorenko, S.; Reshetnyak, O. Non-activation synthesis and thermodynamic properties of ternary compounds of the Ag–Te–Br system. Thermochim. Acta 2021, 698, 178862. [Google Scholar] [CrossRef]
- Moroz, M.; Tesfaye, F.; Demchenko, P.; Prokhorenko, M.; Yarema, N.; Lindberg, D.; Reshetnyak, O.; Hupa, L. The Equilibrium Phase Formation and Thermodynamic Properties of Functional Tellurides in the Ag–Fe–Ge–Te System. Energies 2021, 14, 1314. [Google Scholar] [CrossRef]
- Moroz, M.; Tesfaye, F.; Demchenko, P.; Prokhorenko, M.; Prokhorenko, S.; Lindberg, D.; Reshetnyak, O.; Hupa, L. Synthesis and Thermodynamic Investigation of Energy Materials in the Ag-Te-Cl System by the Solid-State Galvanic Cells. JOM 2021, 73, 1487–1494. [Google Scholar] [CrossRef]
- Diffractometer Stoe WinXPOW, version 3.03; Stoe & Cie Gmb: Darmstadt, Germany, 2010.
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent Developments of the Program FULLPROF, in Commission on Powder Diffraction (IUCr). Newsletter 2001, 26, 12–19. [Google Scholar]
- Rajan, H.; Uchida, H.; Bryan, D.L.; Swaminathan, R.; Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Miner. 2003, 88, 247–250. [Google Scholar]
- Villars, P.; Cenzual, K. (Eds.) Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds, Release 2014/15; ASM International: Materials Park, OH, USA, 2014. [Google Scholar]
- Moroz, M.V.; Prokhorenko, M. Determination of thermodynamic properties of Ag3SBr superionic phase using EMF technique. Russ. J. Electrochem. 2015, 51, 886–889. [Google Scholar] [CrossRef]
- Prokhorenko, M.; Moroz, M.V.; Demchenko, P.Y. Measuring the thermodynamic properties of saturated solid solutions in the Ag2Te-Bi-Bi2Te3 system by the electromotive force method. Russ. J. Phys. Chem. A 2015, 89, 1330–1334. [Google Scholar] [CrossRef]
- Moroz, M.V.; Demchenko, P.Y.; Prokhorenko, M.V.; Reshetnyak, O.V. Thermodynamic Properties of Saturated Solid Solutions of the Phases Ag2PbGeS4, Ag0.5Pb1.75GeS4 and Ag6.72Pb0.16Ge0.84S5.20 of the Ag-Pb-Ge-S System Determined by EMF Method. J. Phase Equilib. Diffus. 2017, 38, 426–433. [Google Scholar] [CrossRef]
- Tesfaye, F.; Taskinen, P. Electrochemical study of the thermodynamic properties of matildite (β-AgBiS2) in different temperature and compositional ranges. J. Solid State Electrochem. 2014, 18, 1683–1694. [Google Scholar] [CrossRef]
- Moroz, M.; Tesfaye, F.; Demchenko, P.; Prokhorenko, M.; Lindberg, D.; Reshetnyak, O.; Hupa, L. Phase Equilibria and Thermodynamics of Selected Compounds in the Ag–Fe–Sn–S System. J. Electron. Mater. 2018, 47, 5433–5442. [Google Scholar] [CrossRef]
- Osadchii, E.G.; Rappo, O.A. Determination of standard thermodynamic properties of sulfides in the Ag-Au-S system by means of a solid-state galvanic cell. Am. Miner. 2004, 89, 1405–1410. [Google Scholar] [CrossRef]
- Hasanova, G.S.; Aghazade, A.I.; Imamaliyeva, S.Z.; Yusibov, Y.A.; Babanly, M.B. Refinement of the Phase Diagram of the Bi-Te System and the Thermodynamic Properties of Lower Bismuth Tellurides. JOM 2021, 73, 1511–1521. [Google Scholar] [CrossRef]
- Hasanova, G.S.; Aghazade, A.I.; Babanly, D.M.; Imamaliyeva, S.Z.; Yusibov, Y.A.; Babanly, M.B. Experimental study of the phase relations and thermodynamic properties of Bi-Se system. J. Therm. Anal. 2021, 147, 6403–6414. [Google Scholar] [CrossRef]
- Babanly, M.; Yusibov, Y.; Babanly, N. The EMF Method with Solid-State Electrolyte in the Thermodynamic Investigation of Ternary Copper and Silver Chalcogenides. In Electromotive Force and Measurement in Several Systems; Kara, S., Ed.; InTech: Houston, TX, USA, 2011; pp. 57–78. ISBN 978-953-307-728-4. [Google Scholar]
- Vassiliev, V.; Lysenko, V.; Bros, J. Thermodynamic study of the Ag-In-Sn system by the EMF method. J. Alloys Compd. 2019, 790, 370–376. [Google Scholar] [CrossRef]
- Babanly, N.; Orujlu, E.; Imamaliyeva, S.; Yusibov, Y. Thermodynamic investigation of silver-thallium tellurides by EMF method with solid electrolyte Ag4RbI5. J. Chem. Thermodyn. 2018, 128, 78–86. [Google Scholar] [CrossRef]
- Imamaliyeva, S.Z.; Musayeva, S.S.; Babanly, D.M.; Jafarov, Y.I.; Taghiyev, D.B.; Babanly, M.B. Determination of the thermodynamic functions of bismuth chalcoiodides by EMF method with morpholinium formate as electrolyte. Thermochim. Acta 2019, 679, 178319. [Google Scholar] [CrossRef]
- Gravetter, F.J.; Wallnau, L.B. Statistics for the Behavioral Sciences, 10th ed.; Cengage Learning: Boston, MA, USA, 2017; ISBN 978-1-305-50491-2. [Google Scholar]
- Voronin, M.V.; Osadchii, E.G. Thermodynamic Properties of Silver and Bismuth Sulfosalt Minerals, Pavonite (AgBi3S5) and Matildite (AgBiS2) and Implications for Ore Deposits. Econ. Geol. 2013, 108, 1203–1210. [Google Scholar] [CrossRef]
- Voronin, M.V.; Osadchii, E.G.; Brichkina, E.A. Thermochemical properties of silver tellurides including empressite (AgTe) and phase diagrams for Ag–Te and Ag–Te–O. Phys. Chem. Miner. 2017, 44, 639–653. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substance; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Mills, K.C. Thermodynamic Data for Inorganic Sulphides, Selenides, Tellurides; Butterworths: London, UK, 1974. [Google Scholar]
- Zlomanov, V.P.; Sheiman, M.S.; Legendre, B. Phase diagram and thermodynamic properties of phases in the In-Te system. J. Phase Equilibria Diffus. 2001, 22, 339–344. [Google Scholar] [CrossRef]
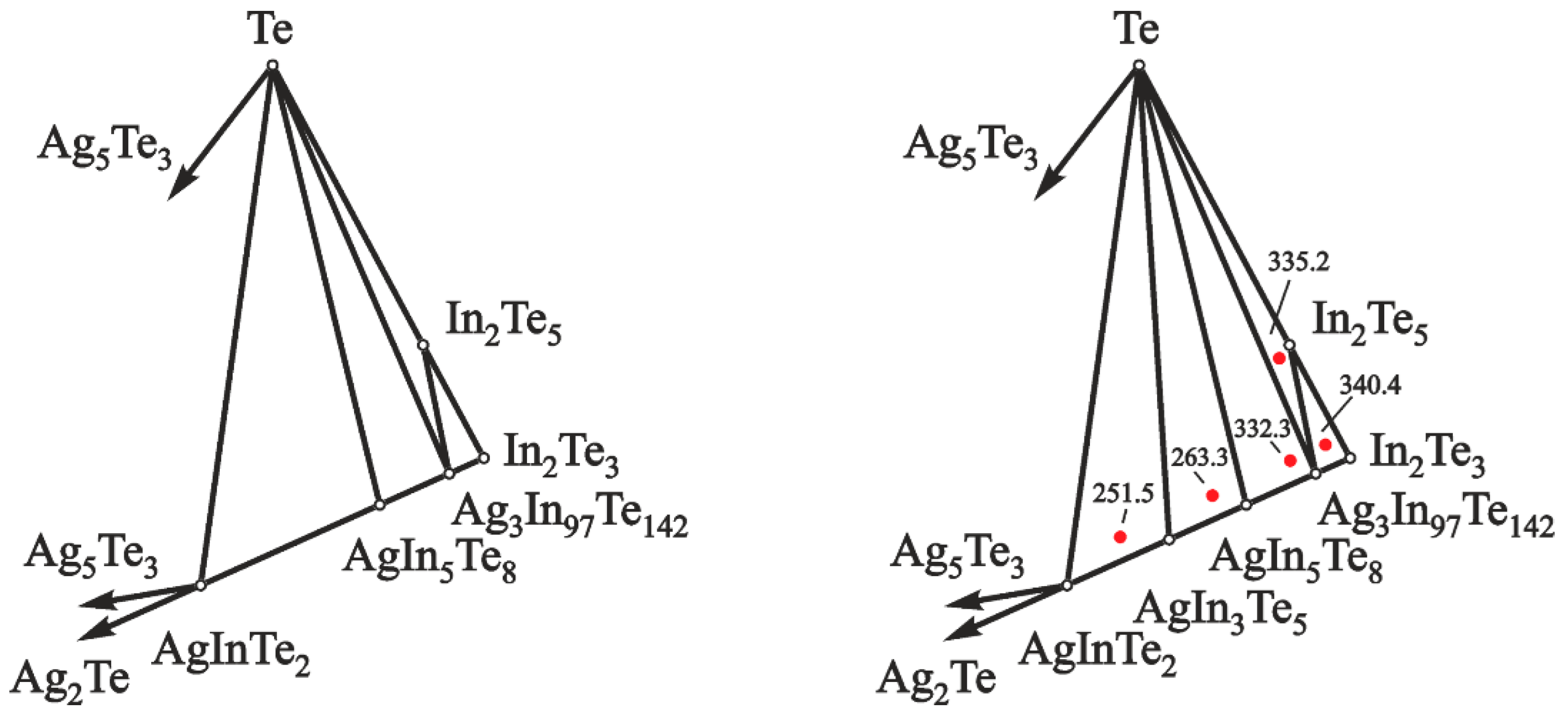

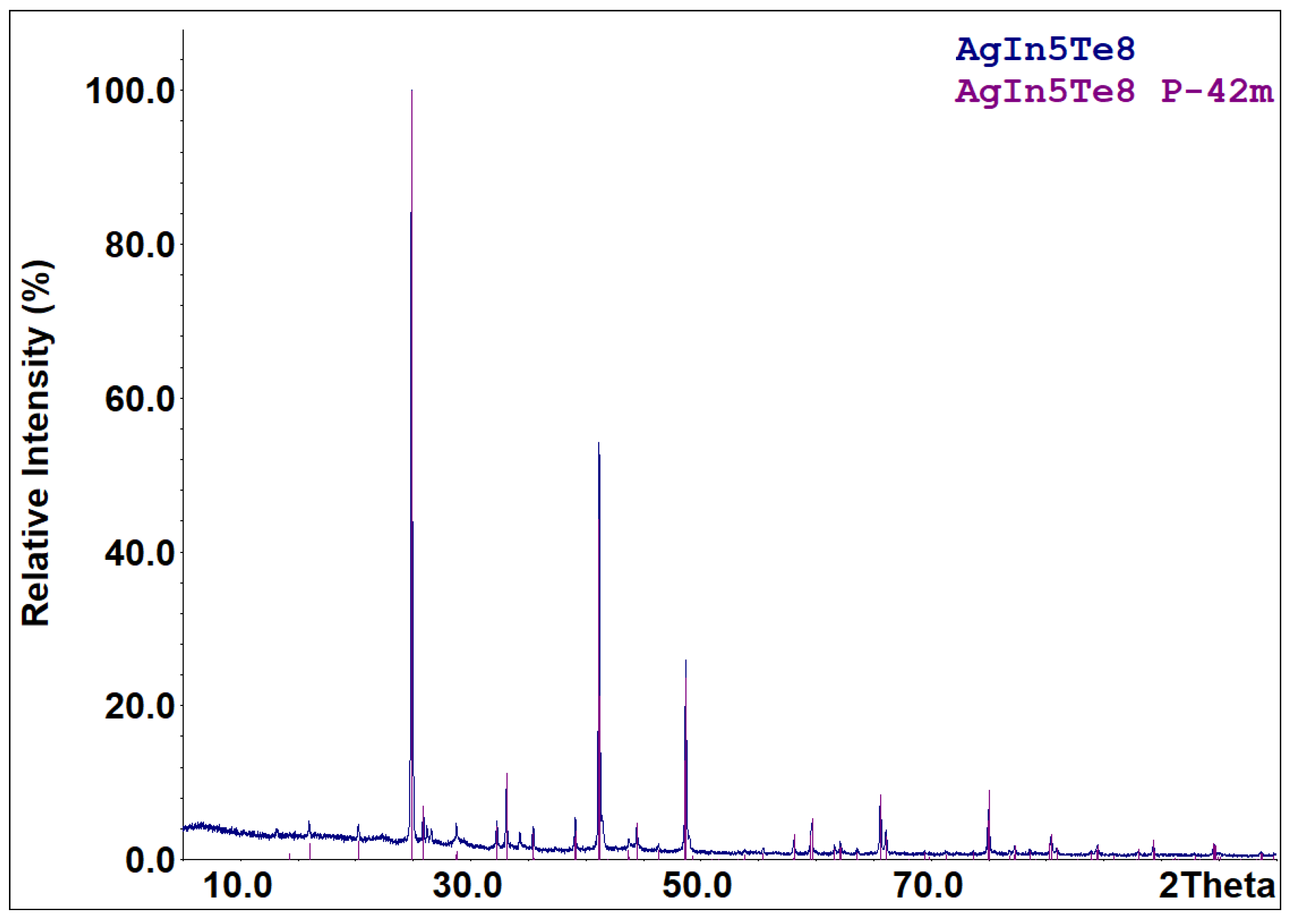
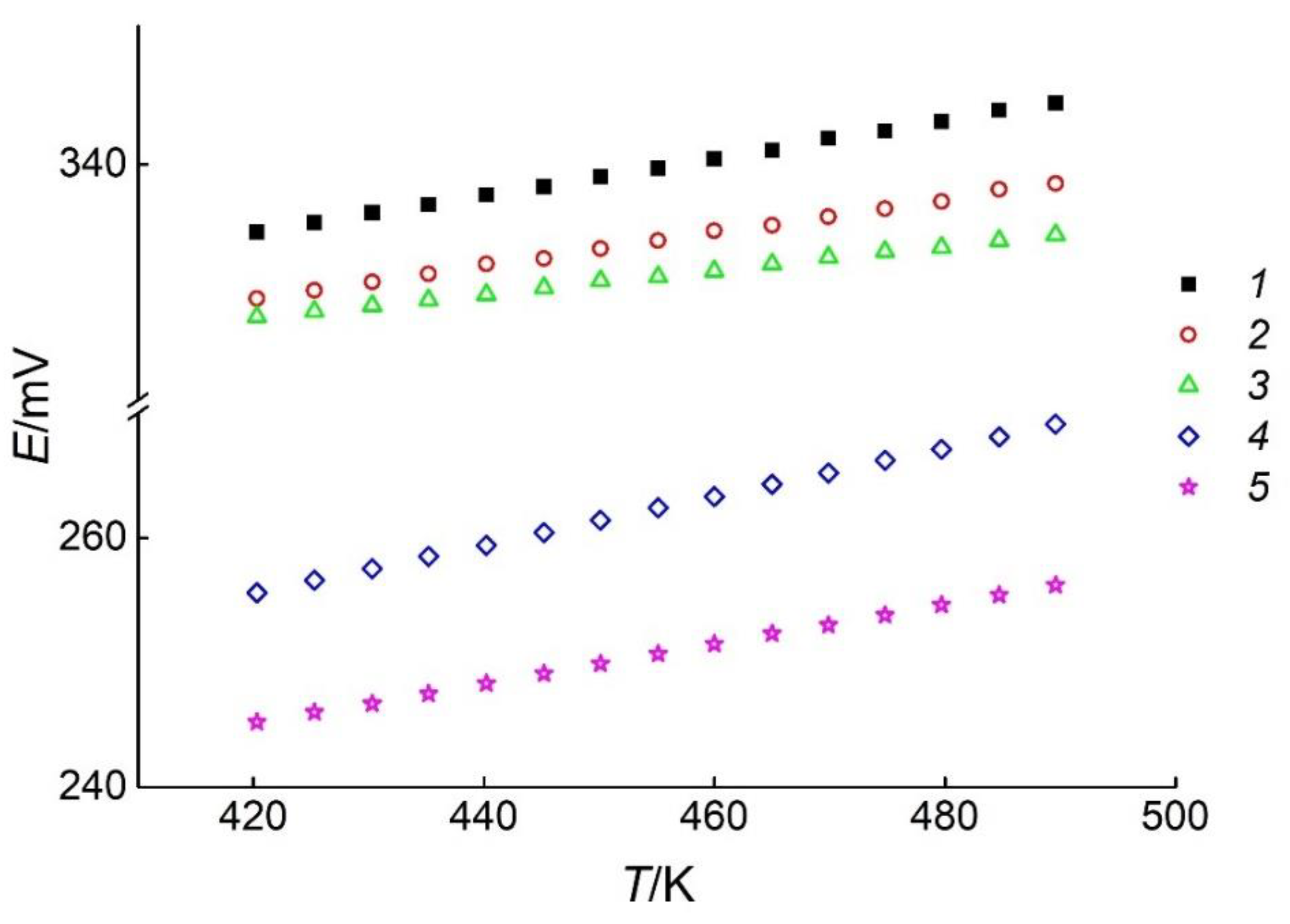
| T/K | Phase Region | ||||
|---|---|---|---|---|---|
| (I) | (II) | (III) | (IV) | (V) | |
| E/mV | E/mV | E/mV | E/mV | E/mV | |
| 420.3 | 335.1 | 330.3 | 329.0 | 255.6 | 245.2 |
| 425.3 | 335.8 | 330.9 | 329.4 | 256.6 | 246.0 |
| 430.3 | 336.5 | 331.5 | 329.8 | 257.5 | 246.7 |
| 435.2 | 337.1 | 332.1 | 330.2 | 258.5 | 247.5 |
| 440.2 | 337.8 | 332.8 | 330.6 | 259.4 | 248.3 |
| 445.2 | 338.4 | 333.2 | 331.1 | 260.4 | 249.1 |
| 450.1 | 339.1 | 333.9 | 331.6 | 261.4 | 249.9 |
| 455.1 | 339.7 | 334.5 | 331.9 | 262.4 | 250.7 |
| 460.0 | 340.4 | 335.2 | 332.3 | 263.3 | 251.5 |
| 465.2 | 341.0 | 335.6 | 332.8 | 264.3 | 252.3 |
| 469.9 | 341.9 | 336.2 | 333.3 | 265.2 | 253.0 |
| 474.8 | 342.4 | 336.8 | 333.7 | 266.2 | 253.8 |
| 479.7 | 343.1 | 337.3 | 334.0 | 267.1 | 254.6 |
| 484.7 | 343.9 | 338.2 | 334.5 | 268.1 | 255.4 |
| 489.6 | 344.4 | 338.6 | 334.9 | 269.1 | 256.2 |
| Phase region | |
| (I) | |
| (II) | |
| (III) | |
| (IV) | |
| (V) |
| Reaction | |||
|---|---|---|---|
| kJ·mol –1 | J·(mol·K) −1 | ||
| (R1) | 368.9 ± 0.3 | 322.4 ± 1.0 | 156.0 ± 2.1 |
| (R2) | 365.5 ± 0.4 | 324.1 ± 1.2 | 138.7 ± 2.5 |
| (R3) | 2519.5 ± 1.7 | 2316.8 ± 4.9 | 680.1 ± 10.7 |
| (R4) | 44.73 ± 0.03 | 33.55 ± 0.08 | 37.52 ± 0.17 |
| (R5) | 43.56 ± 0.02 | 34.42 ± 0.07 | 30.67 ± 0.16 |
| Phases | Reference | |||
|---|---|---|---|---|
| kJ⋅mol −1 | J⋅(mol⋅K) −1 | |||
| Ag | 0 | 0 | 42.677 | [48] |
| In | 0 | 0 | 57.823 | [48] |
| Te | 0 | 0 | 49.497 | [48] |
| In2Te3 | 182.733 | 191.627 | 234.304 | [48] |
| In2Te5 | – | 191.6 ± 2.1 | – | [49] |
| In2Te5 | – | 188.0 ± 1.3 | – | [50] |
| In2Te5 | 182.7 ± 1.9 | 191.6 ± 3.4 | 333.4 ± 4.7 | Present study |
| Ag3In97Te147 | 8954 ± 189 | 9374 ± 327 | 11,605 ± 425 | Present study |
| AgIn5Te8 | 487.6 ± 11.3 | 507.1 ± 19.3 | 662.2 ± 20.5 | Present study |
| AgIn3Te5 | 301.5 ± 6.5 | 311.0 ± 10.2 | 431.8 ± 18.2 | Present study |
| AgInTe2 | 115.0 ± 3.1 | 115.1 ± 4.3 | 199.1 ± 6.6 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroz, M.; Tesfaye, F.; Demchenko, P.; Mastronardo, E.; Mysina, O.; Prokhorenko, M.; Prokhorenko, S.; Lindberg, D.; Reshetnyak, O.; Hupa, L. Experimental Thermodynamic Characterization of the Chalcopyrite-Based Compounds in the Ag–In–Te System for a Potential Thermoelectric Application. Energies 2022, 15, 8180. https://doi.org/10.3390/en15218180
Moroz M, Tesfaye F, Demchenko P, Mastronardo E, Mysina O, Prokhorenko M, Prokhorenko S, Lindberg D, Reshetnyak O, Hupa L. Experimental Thermodynamic Characterization of the Chalcopyrite-Based Compounds in the Ag–In–Te System for a Potential Thermoelectric Application. Energies. 2022; 15(21):8180. https://doi.org/10.3390/en15218180
Chicago/Turabian StyleMoroz, Mykola, Fiseha Tesfaye, Pavlo Demchenko, Emanuela Mastronardo, Oksana Mysina, Myroslava Prokhorenko, Serhiy Prokhorenko, Daniel Lindberg, Oleksandr Reshetnyak, and Leena Hupa. 2022. "Experimental Thermodynamic Characterization of the Chalcopyrite-Based Compounds in the Ag–In–Te System for a Potential Thermoelectric Application" Energies 15, no. 21: 8180. https://doi.org/10.3390/en15218180
APA StyleMoroz, M., Tesfaye, F., Demchenko, P., Mastronardo, E., Mysina, O., Prokhorenko, M., Prokhorenko, S., Lindberg, D., Reshetnyak, O., & Hupa, L. (2022). Experimental Thermodynamic Characterization of the Chalcopyrite-Based Compounds in the Ag–In–Te System for a Potential Thermoelectric Application. Energies, 15(21), 8180. https://doi.org/10.3390/en15218180









