Co-Torrefaction Progress of Biomass Residue/Waste Obtained for High-Value Bio-Solid Products
Abstract
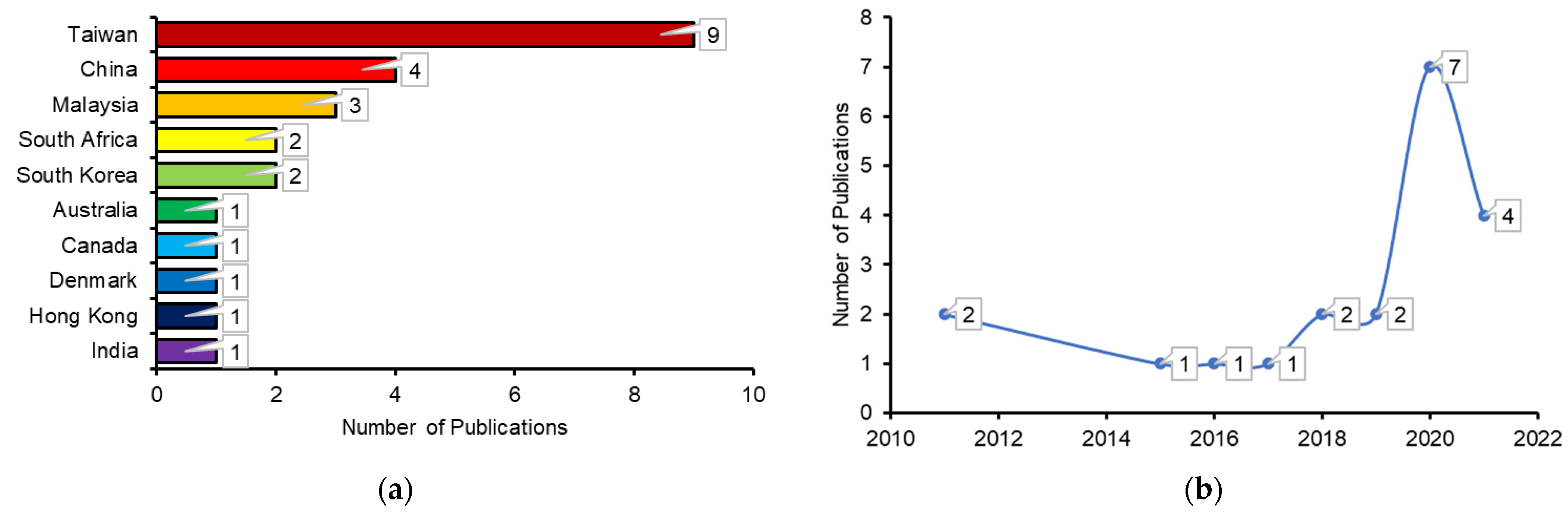
:1. Introduction
2. Biomass Residue and Their Analysis
2.1. Ultimate Analysis
2.2. Proximate Analysis
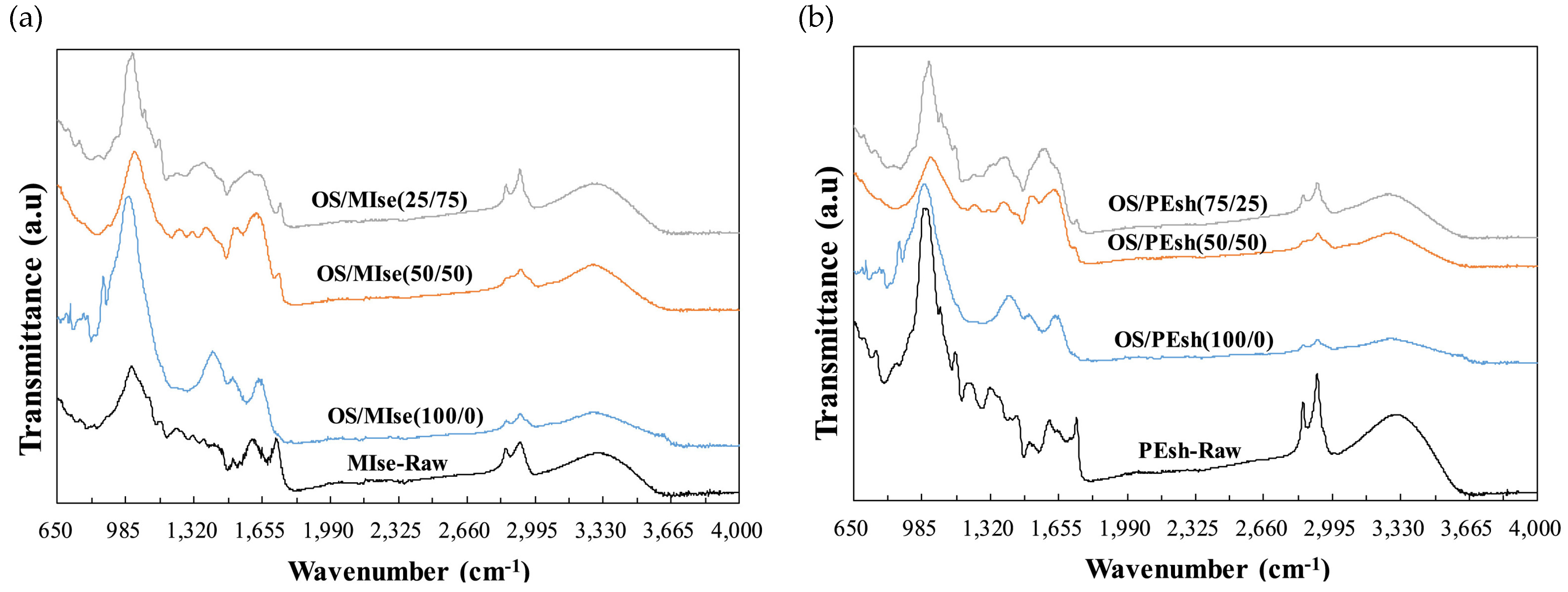
2.3. FTIR Analysis
3. Co-Torrefaction Mechanism and Operation Parameters
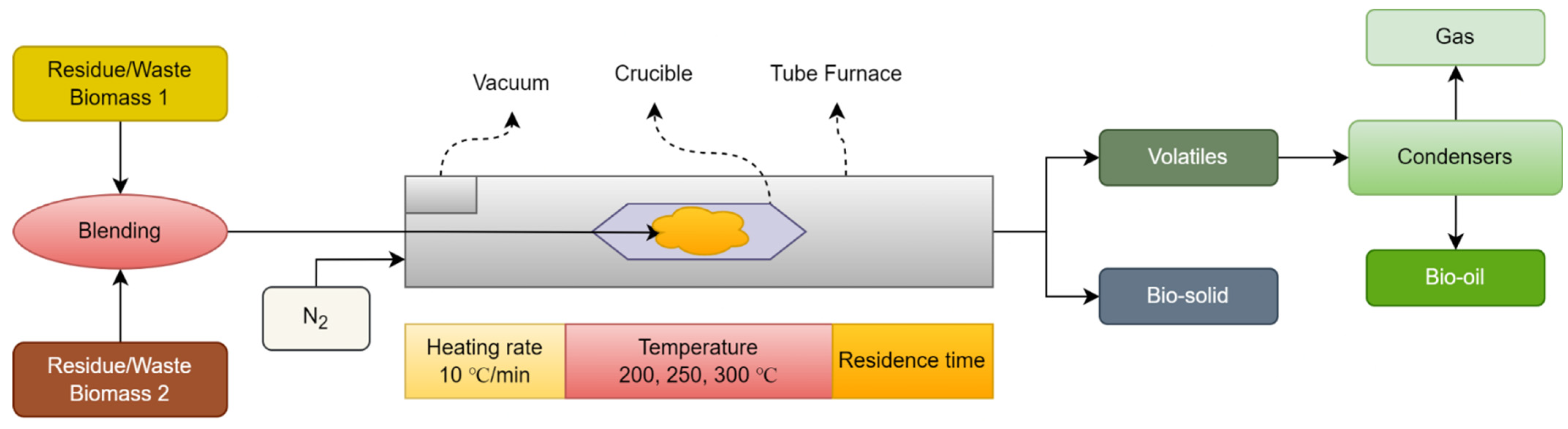
3.1. Co-Torrefaction Process
3.2. Synergistic Effect
3.3. Operating Parameters
3.3.1. Studying the Role of Temperature and Residence Time on Mass and Energy Yields
3.3.2. Studying the Role of Temperature and Residence Time on HHV
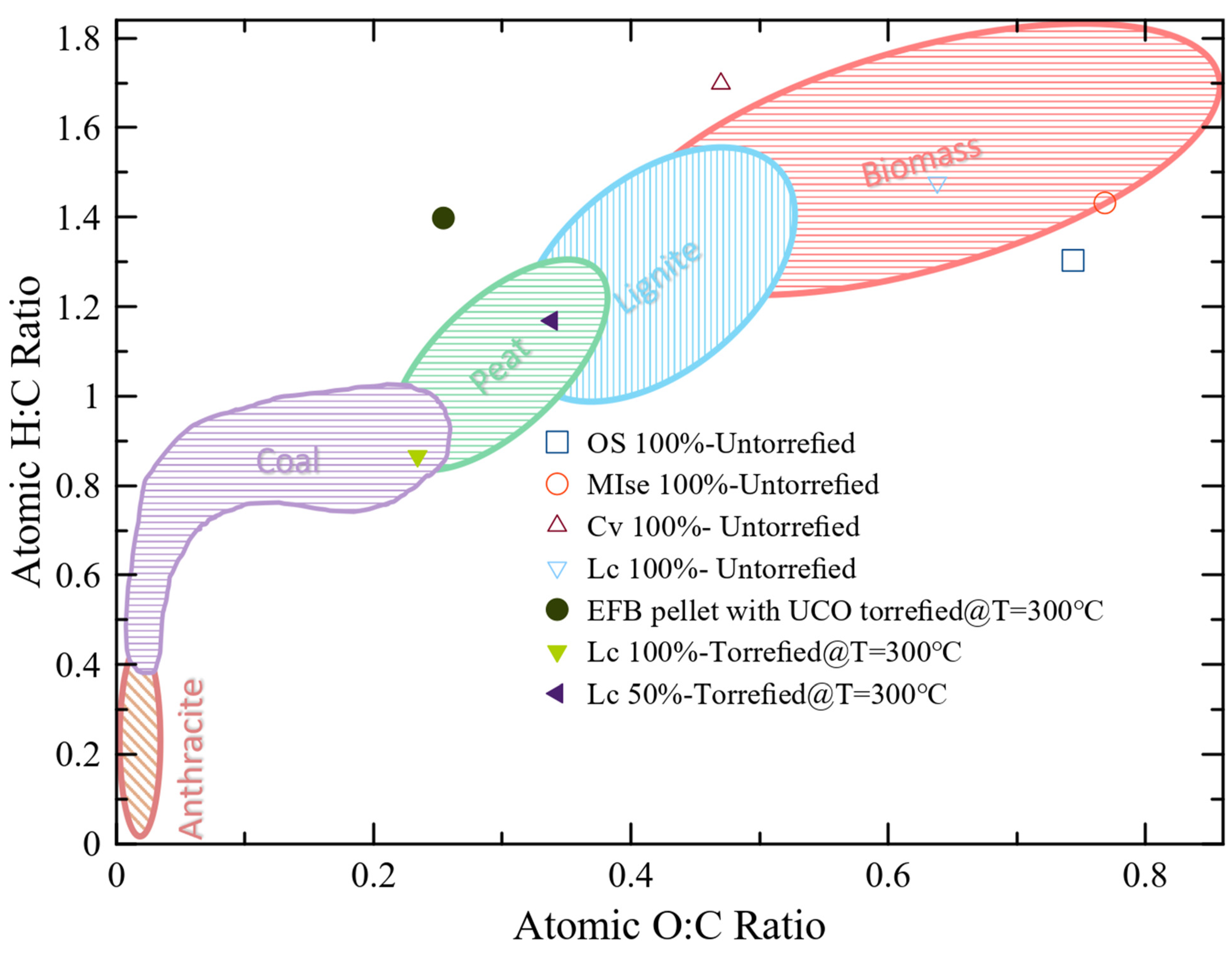
3.3.3. Van Krevelen Diagram
4. Reactor for Co-Torrefaction Technology
4.1. Conventional/Fixed-Bed Reactor
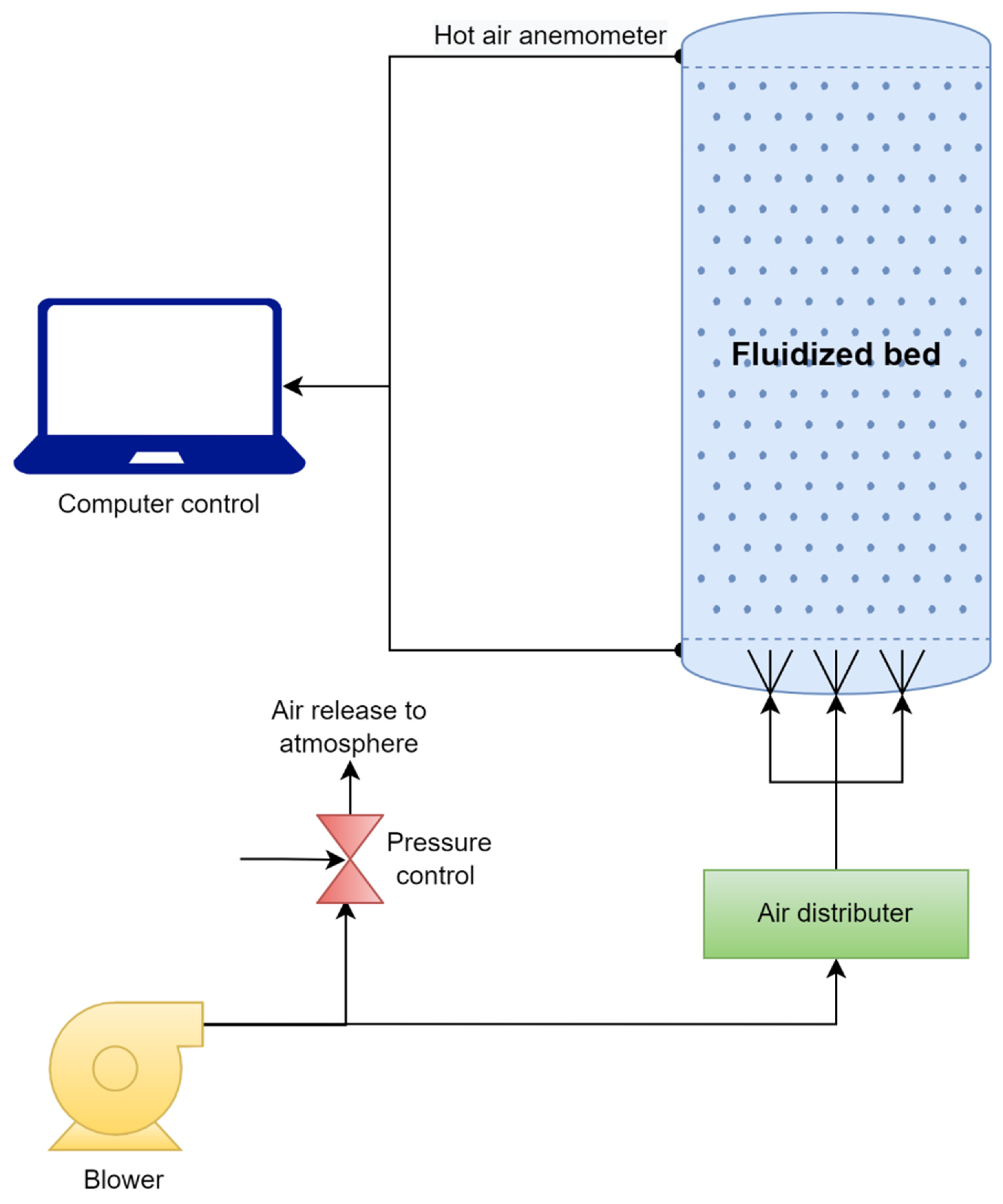
4.2. Fluidizing-Bed Reactor
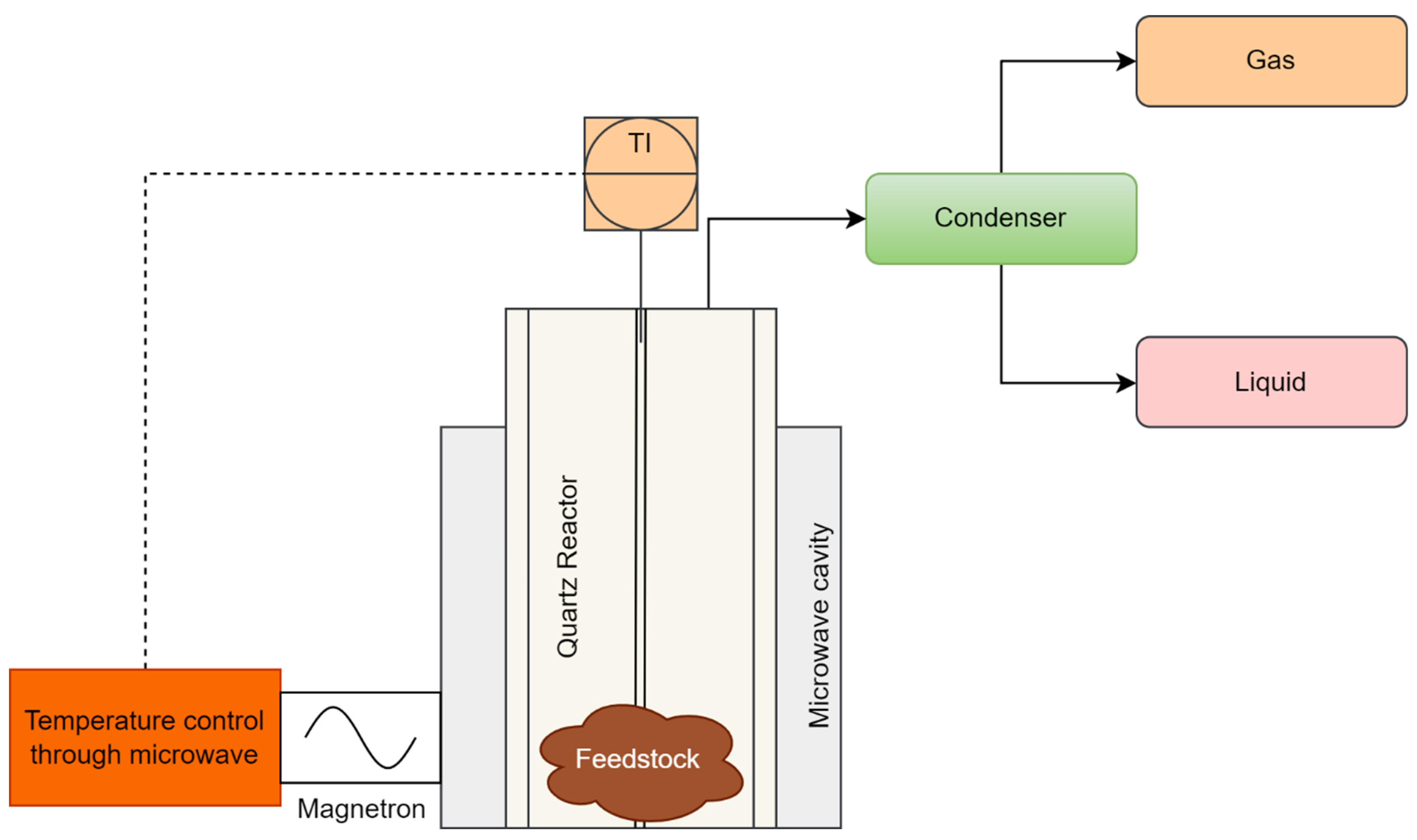
4.3. Microwave Reactor
4.4. Co-Torrefaction in a Batch Reactor
5. Application of the Co-Torrefaction Process
5.1. Biochar Enhancement
5.2. CO2 Adsorption through Bio-Solid via the Co-Torrefaction Method
5.3. Renewable Fuel for Gasification
6. Circular Economy
7. Research Gaps and Recommendations
- Co-torrefaction techniques depend on the activation energies to degrade cellulose, hemicelluloses, and lignin.
- Co-torrefaction may be examined at the microscopic level by identifying unique functional groups and determining the energy required to cleave bonding bonds.
- Utilizing Fourier transform infrared (FTIR) and Raman spectroscopy to study the spontaneous co-torrefaction process.
- Using the Hunter colorimeter, determine the level of co-torrefaction severity predicated on color changes.
- Thermogravimetric analysis was utilized to investigate the kinetics of weight reduction.
- Investigating how various temperatures affect the structure of biomass.
- Integration of co-torrefaction and densification as part of an integrated operation.
- Method to calculate the energy required for the production of condensable and non-condensable products through co-torrefaction.
- The off-gassing and spontaneous combustion behaviors of co-torrefied biomass stored at various storage temperatures are suggested.
- The recommendation process of the co-torrefaction process.
- It is essential to comprehend the environmental aspects of alternative fuel techniques if one is interested in generating environmentally friendly fuels.
- It is possible to significantly reduce emissions by increasing the properties of biomass fuels. Consequently, biomass must be processed before being used in energy applications to optimize its fuel properties. Considering the environmental impact of pre-treatment procedures is essential because they use large amounts of energy and other resources.
- Life cycle analysis (LCA) is the most widely used method for determining whether a bioenergy system is environmentally feasible.
- Thus, a key component in controlling the release of prospective greenhouse gas emissions throughout the co-torrefaction process is the thermal energy source employed in drying through the co-torrefaction process. Therefore, such emissions can be reduced by adopting renewable fuels as a heat source.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Si, B.; Lu, J.; Watson, J.; Zhang, Y.; Liu, Z. Elemental migration and characterization of products during hydrothermal liquefaction of cornstalk. Bioresour. Technol. 2017, 243, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.E.; Abdalla, S.A.; Taqvi, S.A.; Naqvi, S.R.; Chen, W.-H. Investigation of Biomass Integrated Air Gasification Regenerative Gas Turbine Power Plants. Energies 2022, 15, 741. [Google Scholar] [CrossRef]
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Mahari, W.A.W.; Xia, C.; Yek, P.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum pyrolysis incorporating microwave heating and base mixture modification: An integrated approach to transform biowaste into eco-friendly bioenergy products. Renew. Sustain. Energy Rev. 2020, 127, 109871. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Taqvi, S.A.A.; Mehran, M.T.; Khoja, A.H.; Naqvi, M.; Bokhari, A.; Saidina Amin, N.A. Chapter 2-Catalytic pyrolysis of biomass using shape-selective zeolites for bio-oil enhancement. In Bioenergy Resources and Technologies; Azad, A.K., Khan, M.M.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 39–60. [Google Scholar]
- Zafar, M.W.; Shahbaz, M.; Hou, F.; Sinha, A. From nonrenewable to renewable energy and its impact on economic growth: The role of research & development expenditures in Asia-Pacific Economic Cooperation countries. J. Clean. Prod. 2019, 212, 1166–1178. [Google Scholar]
- Qayyum, M.; Khoja, A.H.; Naqvi, S.R.; Ejaz, H.; Nawar, A.; Ansari, A.A. Development of Cost-Effective Fertilizer-Based Media for the Microalgae Cultivation Aimed at Effective Biomass Production. NUST J. Eng. Sci. 2020, 13, 45–51. [Google Scholar]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Chen, W.-H.; Farooq, W.; Shahbaz, M.; Naqvi, S.R.; Ali, I.; Al-Ansari, T.; Saidina Amin, N.A. Current status of biohydrogen production from lignocellulosic biomass, technical challenges and commercial potential through pyrolysis process. Energy 2021, 226, 120433. [Google Scholar] [CrossRef]
- khan, M.; Raza Naqvi, S.; Ullah, Z.; Ali Ammar Taqvi, S.; Nouman Aslam Khan, M.; Farooq, W.; Taqi Mehran, M.; Juchelková, D.; Štěpanec, L. Applications of machine learning in thermochemical conversion of biomass-A review. Fuel 2023, 332, 126055. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chong, C.C.; Lee, S.P.; Lim, J.W.; Wu, T.Y.; Cheng, C.K. Syngas from palm oil mill effluent (POME) steam reforming over lanthanum cobaltite: Effects of net-basicity. Renew. Energy 2020, 148, 349–362. [Google Scholar] [CrossRef]
- Farooq, W.; Ali, I.; Raza Naqvi, S.; Sajid, M.; Abbas Khan, H.; Adamu, S. Evolved Gas Analysis and Kinetics of Catalytic and Non-Catalytic Pyrolysis of Microalgae Chlorella sp. Biomass With Ni/θ-Al2O3 Catalyst via Thermogravimetric Analysis. Front. Energy Res. 2021, 9, 775037. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Global Renew. Energy resources and use in 2050. In Managing Global Warming; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–235. [Google Scholar]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Chong, C.T.; Lam, W.H.; Anuar, T.N.S.T.; Ma, N.L.; Ibrahim, M.D.; Lam, S.S. Microwave co-pyrolysis of waste polyolefins and waste cooking oil: Influence of N2 atmosphere versus vacuum environment. Energy Convers. Manag. 2018, 171, 1292–1301. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Jusoh, A.; Chong, C.T.; Lee, C.L.; Chase, H.A. Pyrolysis using microwave absorbents as reaction bed: An improved approach to transform used frying oil into biofuel product with desirable properties. J. Clean. Prod. 2017, 147, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Uemura, Y.; Sellappah, V.; Trinh, T.H.; Hassan, S.; Tanoue, K.-I. Torrefaction of empty fruit bunches under biomass combustion gas atmosphere. Bioresour. Technol. 2017, 243, 107–117. [Google Scholar] [CrossRef]
- Atabani, A.E.; Pugazhendhi, A.; Almomani, F.; Rene, E.R.; Naqvi, S.R. Recent advances in the thermochemical transformation of biomass to bio-oil, biochar and syngas and its upgrading methods. Process Saf. Environ. Prot. 2022, 168, 624–625. [Google Scholar] [CrossRef]
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Štěpanec, L.; Juchelková, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef]
- Zheng, N.-Y.; Lee, M.; Lin, Y.-L. Co-processing textile sludge and lignocellulose biowaste for biofuel production through microwave-assisted wet torrefaction. J. Clean. Prod. 2020, 268, 122200. [Google Scholar] [CrossRef]
- Inayat, M.; Shahbaz, M.; Naqvi, S.R.; Sulaiman, S.A. Chapter 3-Advance strategies for tar elimination from biomass gasification techniques. In Bioenergy Resources and Technologies; Azad, A.K., Khan, M.M.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 61–88. [Google Scholar]
- Sajdak, M. Impact of plastic blends on the product yield from co-pyrolysis of lignin-rich materials. J. Anal. Appl. Pyrolysis 2017, 124, 415–425. [Google Scholar] [CrossRef]
- Wu, W.; Qiu, K. Vacuum co-pyrolysis of Chinese fir sawdust and waste printed circuit boards. Part I: Influence of mass ratio of reactants. J. Anal. Appl. Pyrolysis 2014, 105, 252–261. [Google Scholar] [CrossRef]
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of torrefaction on physiochemical characteristics and grindability of stem wood, stump and bark. Appl. Energy 2018, 227, 137–148. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Mahari, W.A.W.; Kong, S.H.; Foong, S.Y.; Peng, W.; Ting, H.; Liew, R.K.; Xia, C.; Sonne, C.; Tabatabaei, M.; et al. Pilot-scale co-processing of lignocellulosic biomass, algae, shellfish waste via thermochemical approach: Recent progress and future directions. Bioresour. Technol. 2022, 347, 126687. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Lam, S.S.; Tsang, Y.F.; Yek, P.N.Y.; Liew, R.K.; Osman, M.S.; Peng, W.; Lee, W.H.; Park, Y.-K. Co-processing of oil palm waste and waste oil via microwave co-torrefaction: A waste reduction approach for producing solid fuel product with improved properties. Process Saf. Environ. Prot. 2019, 128, 30–35. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, I.; Naqvi, S.R.; Li, K.; Mehran, M.T.; Khoja, A.H.; Alarabi, A.A.; Atabani, A.E. Investigation of slow pyrolysis mechanism and kinetic modeling of Scenedesmus quadricauda biomass. J. Anal. Appl. Pyrolysis 2021, 158, 105149. [Google Scholar] [CrossRef]
- Madhu, P.; Vidhya, L.; Vinodha, S.; Wilson, S.; Sekar, S.; Patil, P.P.; Kaliappan, S.; Prabhakar, S. Co-pyrolysis of Hardwood Combined with Industrial Pressed Oil Cake and Agricultural Residues for Enhanced Bio-Oil Production. J. Chem. 2022, 2022, 9884766. [Google Scholar] [CrossRef]
- Viegas, C.; Nobre, C.; Correia, R.; Gouveia, L.; Gonçalves, M. Optimization of Biochar Production by Co-Torrefaction of Microalgae and Lignocellulosic Biomass Using Response Surface Methodology. Energies 2021, 14, 7330. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Sharma, H.B.; Dubey, B.K. Binderless fuel pellets from hydrothermal carbonization of municipal yard waste: Effect of severity factor on the hydrochar pellets properties. J. Clean. Prod. 2020, 277, 124295. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Kethobile, E.; Ketlogetswe, C.; Gandure, J. Torrefaction of non-oil Jatropha curcas L.(Jatropha) biomass for solid fuel. Heliyon 2020, 6, e05657. [Google Scholar] [CrossRef]
- Zheng, N.-Y.; Lee, M.; Lin, Y.-L.; Samannan, B. Microwave-assisted wet co-torrefaction of food sludge and lignocellulose biowaste for biochar production and nutrient recovery. Process Saf. Environ. Prot. 2020, 144, 273–283. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Chen, X.; Peng, W.; Liew, R.K.; Cheng, C.K.; Sonne, C.; Sii, H.S.; Lam, S.S. Microwave co-torrefaction of waste oil and biomass pellets for simultaneous recovery of waste and co-firing fuel. Renew. Sustain. Energy Rev. 2021, 152, 111699. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. Energy Procedia 2019, 158, 4435–4440. [Google Scholar] [CrossRef]
- Rizkiana, J.; Zahra, A.; Wulandari, W.; Saputra, W.; Andrayukti, R.; Sianipar, A.; Sasongko, D. Effects of Coal and Biomass Types towards the Quality of Hybrid Coal Produced via Co-Torrefaction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012028. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y. Biowaste-to-biochar through microwave-assisted wet co-torrefaction of blending mango seed and passion shell with optoelectronic sludge. Energy 2021, 225, 120213. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Q.; Yan, Y.; Peng, F.; Sun, R.; Ren, J. Hemicellulose from plant biomass in medical and pharmaceutical application: A critical review. Curr. Med. Chem. 2019, 26, 2430–2455. [Google Scholar] [CrossRef] [PubMed]
- Rago, Y.P.; Collard, F.-X.; Görgens, J.F.; Surroop, D.; Mohee, R. Torrefaction of biomass and plastic from municipal solid waste streams and their blends: Evaluation of interactive effects. Fuel 2020, 277, 118089. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Chen, F.; Zhang, J.; Zhou, J. Insight into a new phenolic-leaching pretreatment on bamboo pyrolysis: Release characteristics of pyrolytic volatiles, upgradation of three phase products, migration of elements, and energy yield. Renew. Sustain. Energy Rev. 2021, 136, 110444. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y.; Hsu, C.-H. Torrefaction of fruit peel waste to produce environmentally friendly biofuel. J. Clean. Prod. 2021, 284, 124676. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Hu, L.; Liao, Q.; Fang, S.; Gao, R. Dynamic Simulation Based on a Simplified Model of 1/3 Coking Coal Molecule and Its Formation Characteristics of Hydration Films. ACS Omega 2021, 6, 33339–33353. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and opportunities in biomass ash management and its utilization in novel applications. Renew. Sustain. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- López-González, D.; Fernandez-Lopez, M.; Valverde, J.; Sanchez-Silva, L. Thermogravimetric-mass spectrometric analysis on combustion of lignocellulosic biomass. Bioresour. Technol. 2013, 143, 562–574. [Google Scholar] [CrossRef]
- Sarwar, A.; Ali, M.; Khoja, A.H.; Nawar, A.; Waqas, A.; Liaquat, R.; Naqvi, S.R.; Asjid, M. Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J. CO2 Util. 2021, 46, 101476. [Google Scholar] [CrossRef]
- Darmstadt, H.; Garcia-Perez, M.; Chaala, A.; Cao, N.-Z.; Roy, C. Co-pyrolysis under vacuum of sugar cane bagasse and petroleum residue: Properties of the char and activated char products. Carbon 2001, 39, 815–825. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Gierlinger, N.; Goswami, L.; Schmidt, M.; Burgert, I.; Coutand, C.; Rogge, T.; Schwanninger, M. In situ FT-IR microscopic study on enzymatic treatment of poplar wood cross-sections. Biomacromolecules 2008, 9, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sawatari, C. A Fourier transform infra-red spectroscopic analysis of the character of hydrogen bonds in amorphous cellulose. Polymer 1996, 37, 393–399. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Hydrogen bonding in lignin: A Fourier transform infrared model compound study. Biomacromolecules 2005, 6, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Pérez, L.E.; Grandón, H.; Flores, M.; Segura, C.; Kelley, S.S. Steam torrefaction of Eucalyptus globulus for producing black pellets: A pilot-scale experience. Bioresour. Technol. 2017, 238, 194–204. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Hidayat, W.; Rubiyanti, T.; Sulistio, Y.; Iryani, D.A.; Haryanto, A.; Amrul, A.; Yoo, J.; Kim, S.; Lee, S.; Hasanudin, U. Effects of Torrefaction Using COMB Dryer/Pyrolizer on the Properties of Rubberwood (Hevea brasiliensis) and Jabon (Anthocephalus cadamba) Pellets. 2021. Available online: http://repository.lppm.unila.ac.id/id/eprint/32920 (accessed on 5 October 2022).
- Cheong, K.Y.; Kong, S.H.; Liew, R.K.; Wong, C.C.; Wong, C.S.; Ngu, H.J.; Yek, P.N.Y. Integration of microwave co-torrefaction with helical lift for pellet fuel production. Green Process. Synth. 2022, 11, 404–410. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C.; Liu, S.-H.; Wu, W. Thermal characterization of oil palm fiber and eucalyptus in torrefaction. Energy 2014, 71, 40–48. [Google Scholar] [CrossRef]
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Co-torrefaction of sewage sludge and leucaena by using microwave heating. Energy 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Microwave torrefaction of sewage sludge and leucaena. J. Taiwan Inst. Chem. Eng. 2017, 70, 236–243. [Google Scholar] [CrossRef]
- Tian, H.; Jiao, H.; Cai, J.; Wang, J.; Yang, Y.; Bridgwater, A.V. Co-pyrolysis of Miscanthus Sacchariflorus and coals: A systematic study on the synergies in thermal decomposition, kinetics and vapour phase products. Fuel 2020, 262, 116603. [Google Scholar] [CrossRef]
- Assad Munawar, M.; Hussain Khoja, A.; Hassan, M.; Liaquat, R.; Raza Naqvi, S.; Taqi Mehran, M.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Raza, J.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Shakir, S.; Liaquat, R.; Tahir, M.; Ali, G. Methane decomposition for hydrogen production over biomass fly ash-based CeO2 nanowires promoted cobalt catalyst. J. Environ. Chem. Eng. 2021, 9, 105816. [Google Scholar] [CrossRef]
- Tang, B.; Feng, X.; Huang, S.; Bin, L.; Fu, F.; Yang, K. Variation in rheological characteristics and microcosmic composition of the sewage sludge after microwave irradiation. J. Clean. Prod. 2017, 148, 537–544. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Mehdi, R.; Raza, N.; Naqvi, S.R.; Khoja, A.H.; Mehran, M.T.; Farooq, M.; Tran, K.-Q. A comparative assessment of solid fuel pellets production from torrefied agro-residues and their blends. J. Anal. Appl. Pyrolysis 2021, 156, 105125. [Google Scholar] [CrossRef]
- Cao, Y.; He, M.; Dutta, S.; Luo, G.; Zhang, S.; Tsang, D.C. Hydrothermal carbonization and liquefaction for sustainable production of hydrochar and aromatics. Renew. Sustain. Energy Rev. 2021, 152, 111722. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, K.; Zhang, Z.; Li, C.; Rahman, M.M.; Cai, J. Distributed activation energy model for lignocellulosic biomass torrefaction kinetics with combined heating program. Energy 2022, 239, 122228. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent coffee ground characterization, pelletization test and emissions assessment in the combustion process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef] [PubMed]
- Tumuluru, J.S.; Ghiasi, B.; Soelberg, N.R.; Sokhansanj, S. Biomass Torrefaction Process, Product Properties, Reactor Types, and Moving Bed Reactor Design Concepts. Front. Energy Res. 2021, 462, 728140. [Google Scholar] [CrossRef]
- Shagali, A.A.; Hu, S.; Li, H.; Chi, H.; Qing, H.; Xu, J.; Jiang, L.; Wang, Y.; Su, S.; Xiang, J. Thermal behavior, synergistic effect and thermodynamic parameter evaluations of biomass/plastics co–pyrolysis in a concentrating photothermal TGA. Fuel 2023, 331, 125724. [Google Scholar] [CrossRef]
- Brachi, P.; Chirone, R.; Miccio, M.; Ruoppolo, G. Fluidized bed torrefaction of biomass pellets: A comparison between oxidative and inert atmosphere. Powder Technol. 2019, 357, 97–107. [Google Scholar] [CrossRef]
- Pitsukha, E.; Teplitskii, Y.S.; Buchilko, É. Characteristic Features of Fluidization of Bidisperse Beds in Suffosion Conditions. J. Eng. Phys. Thermophys. 2017, 90, 1379–1385. [Google Scholar] [CrossRef]
- Teplitskii, Y.; Kovenskii, V. Velocity of full fluidization of a bed of polydisperse granular materials. J. Eng. Phys. Thermophys. 2009, 82, 291–295. [Google Scholar] [CrossRef]
- Saleem, F.; Abbas, A.; Rehman, A.; Khoja, A.H.; Naqvi, S.R.; Arshad, M.Y.; Zhang, K.; Harvey, A. Decomposition of benzene as a biomass gasification tar in CH4 carrier gas using non-thermal plasma: Parametric and kinetic study. J. Energy Inst. 2022, 102, 190–195. [Google Scholar] [CrossRef]
- Is’yomin, R.; Kuz’min, S.; Mikhalyov, A.; Milovanov, O.Y.; Klimov, D.; Nebyvaev, A.; Khaskhachikh, V. Fluidization of a Multicomponent Bed in a Reactor for Co-Torrefaction of Waste Coal and Biomass. J. Eng. Phys. Thermophys. 2020, 93, 750–756. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Cheng, Y.W.; Liew, R.K.; Wan Mahari, W.A.; Ong, H.C.; Chen, W.-H.; Peng, W.; Park, Y.-K.; Sonne, C.; Kong, S.H.; et al. Progress in the torrefaction technology for upgrading oil palm wastes to energy-dense biochar: A review. Renew. Sustain. Energy Rev. 2021, 151, 111645. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, W.-H.; Lim, S.; Ong, H.C.; Ubando, A.T. Synergistic interaction and biochar improvement over co-torrefaction of intermediate waste epoxy resins and fir. Environ. Technol. Innov. 2020, 21, 101218. [Google Scholar] [CrossRef]
- Chen, X.; Hou, J.; Gu, Q.; Wang, Q.; Gao, J.; Sun, J.; Fang, Q. A non-bisphenol-A epoxy resin with high Tg derived from the bio-based protocatechuic Acid:Synthesis and properties. Polymer 2020, 195, 122443. [Google Scholar] [CrossRef]
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Marczak, M.; Ratajczyk-Szufa, J. Torrefaction process of millet and cane using batch reactor. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2020; pp. 371–379. [Google Scholar]
- Fan, Y.; Tippayawong, N.; Wei, G.; Huang, Z.; Zhao, K.; Jiang, L.; Zheng, A.; Zhao, Z.; Li, H. Minimizing tar formation whilst enhancing syngas production by integrating biomass torrefaction pretreatment with chemical looping gasification. Appl. Energy 2020, 260, 114315. [Google Scholar] [CrossRef]
- Huang, C.-L.; Bao, L.-J.; Luo, P.; Wang, Z.-Y.; Li, S.-M.; Zeng, E.Y. Potential health risk for residents around a typical e-waste recycling zone via inhalation of size-fractionated particle-bound heavy metals. J. Hazard. Mater. 2016, 317, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Chih, Y.-K.; Chen, W.-H.; Tran, K.-Q. Hydrogen production from methanol partial oxidation through the catalyst prepared using torrefaction liquid products. Fuel 2020, 279, 118419. [Google Scholar] [CrossRef]
- Hashmi, S.; Taqvi, S.A.A.; Abideen, Z.; Ahmed, J.P.; Talha, M.; Bhatti, M.A.; Shahid, H.; Naqvi, S.R.; Almomani, F. Simulation of steam gasification of halophyte biomass for syngas production using Aspen Plus®. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lu, K.-M.; Lee, W.-J.; Liu, S.-H.; Lin, T.-C. Non-oxidative and oxidative torrefaction characterization and SEM observations of fibrous and ligneous biomass. Appl. Energy 2014, 114, 104–113. [Google Scholar] [CrossRef]
- Shalini, S.; Joseph, K.; Yan, B.; Karthikeyan, O.; Palanivelu, K.; Ramachandran, A. Solid Waste Management Practices in India and China: Sustainability Issues and Opportunities. In Waste Management Policies and Practices in BRICS Nations; CRC Press: Boca Raton, FL, USA, 2021; pp. 73–114. [Google Scholar]
- Ihsanullah, I.; Khan, M.T.; Zubair, M.; Bilal, M.; Sajid, M. Removal of pharmaceuticals from water using sewage sludge-derived biochar: A review. Chemosphere 2022, 289, 133196. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Effects of lignocellulosic composition and microwave power level on the gaseous product of microwave pyrolysis. Energy 2015, 89, 974–981. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.R.; Prins, W.; van Swaaij, W.P.; van de Beld, B.; Elliott, D.C.; Neuenschwander, G.G.; Kruse, A. Biomass gasification in near-and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandre, J.-M. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wang, G.-j.; Kuang, J.-h.; Zhang, Y.-l.; Luo, Y.-H. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Felix, C.B.; Ubando, A.T.; Chen, W.-H.; Goodarzi, V.; Ashokkumar, V. COVID-19 and industrial waste mitigation via thermochemical technologies towards a circular economy: A state-of-the-art review. J. Hazard. Mater. 2022, 423, 127215. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Qayimova, Z. Prospects for the development of Islamic banking services market in commercial banks of Uzbekistan. Центр Научных Публикаций (buxdu. uz) 2021, 7. [Google Scholar]
- Singh, R.P.; Tyagi, V.V.; Allen, T.; Ibrahim, M.H.; Kothari, R. An overview for exploring the possibilities of energy generation from municipal solid waste (MSW) in Indian scenario. Renew. Sustain. Energy Rev. 2011, 15, 4797–4808. [Google Scholar] [CrossRef]
- Corrado, S.; Sala, S. Bio-economy contribution to circular economy. In Designing Sustainable Technologies, Products and Policies; Springer: Cham, Switzerland, 2018; pp. 49–59. [Google Scholar]

| Sr. No. | Biomass Type | Blending Ratio | Process and Type of Reactor | Process Condition | Outcome | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Waste epoxy resin and fir | Mixing ratio of fir:waste epoxy resin is 1:3 | Co-torrefaction Conventional heating batch-type reactor | Temperature: 120 °C–180 °C, time: 10 min–40 min | Solid yield 76.86%. Enhancement in HHV 1.12 Energy yield 85.79% Improved evaporation of volatile compounds. Solid yield adversely affected | Improvement of biochar | [23] |
| 2 | Sewage sludge and Leucaena | Mixing ratio of sewage sludge:Leucaena is (75:25%) | Co-torrefaction Microwave heating | Microwave power level 100 W, time: 30 min, temperature: 170 °C–390 °C | Bio-char made from pure Leucaena wood has a CO2 adsorption capacity of 53 mg/g | Solves waste-water problem. Production of biofuels | [44] |
| 3 | Biomass and coal | Blending ratio of biomass:coal is (30:70%) | Vertical tubular furnace | Temperature: 300 °C, time: 60 min | Produced mass yield: (57.0–63.8%), energy yield: (77.0–89.0%), (18.1–22.2%) reduction in CO2 emissions | Enhances the quality of coal | [45] |
| 4 | Microalgae and Lignocellulosic biomass | - | Co-torrefaction A gas chromatographic furnace with a glass reactor | Temperature: 250 °C, time: 60 min | Better temperatures (92.6%) result in higher energy efficiency, but the moisture content of the feed mixture quickly decreases this efficiency (16.9 to 57.3% for 70% moisture) | High production of bio-char with high calorific value | [35] |
| 5 | Mango seed and passion shell with optoelectronic sludge | Blending optoelectronic sludge with mango seed in a 25/75 ratio | Wet co-torrefaction Microwave reactor | Temperature from 120 °C to 180 °C), reaction duration from 10–40 min | Higher heating value of 19.0 MJ/kg, 92.1% of energy yield, fuel ratios of 1.60–1.82, and an energy return on investment of 14.7% | The production of fuel of the highest grade | [46] |
| 6 | Food sludge and lignocellulosic biowaste | Mixing macadamia husk and sludge in a (25/75%) ratio (db%) | Wet co-torrefaction Microwave reactor | Temperature: 150 °C, duration: 20 min | HHV:19.6 MJ/kg; decreased ash content; first-order kinetics; increased thermal stability and combustion efficiency of biochar; 7.4 energy return on investment; 45.2% reduction in carbon gas emissions | Production of bio-solid and nutrient recovery | [41] |
| 7 | Empty fruit bunch pellet, used cooking oil, and waste engine oil | - | Co-torrefaction Microwave reactor | Temperature: 200, 250 °C and 300 °C, heating rate: 50–65 °C/min, time: 5–8 min | There is an 85.5 wt% mass yield Fuel ratio: 1.8. Carbon content: 68.3%. Fixed carbon: 62.3%. HHV: 28.0 MJ/kg. | Production of solid fuel with greater improvement | [30] |
| 10 | Hemicellulose, cellulose, lignin, xylan, dextran, xylose, and glucose | Weight ratio (1:1:1) | Co-torrefaction Conventional heating thermogravimetry | Temperature: 230 °C, 260 °C and 290 °C | There is no synergistic effect of co-torrefaction on weight loss of the blend | - | [47] |
| 11 | Textile sludge and lignocellulose biowaste (macadamia husk) | - | Wet co-torrefaction | Temperature: 120 °C–180 °C, time: 10–30 min | Amount of fixed carbon: 29.8%, HHV: 19.7 MJ/kg | Production of biofuel | [41] |
| 12 | Mango branches (MBr), waste newspaper (Np), and low-density polyethylene (LDPE) | Three binary mixtures prepared, with a mass ratio of 1:1 | Bench-scale tubular reactor | Temperature: 300 °C | (MBr-LDPE) carbon content: 71.94% HHV: 35.84 MJ/kg | Improved fuel characteristics that allow co-firing | [48] |
| 13 | Food sludge and six widely produced lignocellulose bi-wastes | Blending ratios of 0/100, 25/75, 50/50, and 100/0 | Microwave heating system | Torrefaction temperature (120, 150, and 180 °C), reaction time (10, 20, and 30 min) | Food sludge blended with macadamia husk (25/75 db%) highest fixed carbon content (25%) HHV: (19.6 MJ/kg) | Renewable energy resource. | [41] |
| Biomass/Torrefied Biomass | Temp (°C) | Time (min) | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Oxygen (%) | Sulphur (%) | Moisture (%) | Volatile Matter (%) | Fixed Carbon (%) | Ash (%) | HHV (MJ/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | _ | _ | 43.89 | 4.80 | 6.38 | 43.48 | 1.45 | 99.00 | 64.89 | 9.30 | 25.81 | 13.57 | [46] |
| Mangifera indica seed (MIse) | _ | _ | 46.11 | 5.54 | 0.89 | 47.20 | 0.27 | 4.97 | 96.38 | 2.24 | 1.38 | 18.90 | [46] |
| OS and MIse (25/75%) | 150 | 30 | 45.1 | 9.8 | 4.6 | 39.6 | 0.9 | _ | _ | _ | 3.0 | 19.0 | [46] |
| EFB | _ | _ | 43 | 6 | 1.2 | 49.8 | 0 | 15 | 62 | 15 | 8 | 18.5 | [42] |
| EFB pellet with UCO | 300 | _ | 68.2 | 8.0 | 0.7 | 23.1 | 0 | 1 | 33 | 63 | 3 | 26.4 | [42] |
| Cv | _ | _ | 51.29 ± 0.09 | 7.31 ± 0.42 | 9.05 ± 0.00 | 32.11 ± 0.10 | 0.24 ± 0.04 | 6.35 ± 0.52 | 86.46 ± 0.74 | 6.01 ± 0.73 | 7.53 ±0.09 | 15.54 | [35] |
| Lc | _ | _ | 50.10 ± 0.16 | 6.21 ± 0.09 | 1.10 ± 0.08 | 42.59 ± 0.04 | 0.00 ± 0.00 | 9.28 ± 0.84 | 78.41 ± 3.89 | 19.06 ± 3.97 | 2.53 ± 0.08 | 18.94 | [35] |
| Lc 100% | 300 | 45 | 70.2 | 5.1 | 1.5 | 21.9 | 1.2 | 5 | 58.6 | 34.9 | 6.5 | 21.4 | [35] |
| Lc 50% | 300 | 30 | 61.2 | 6.0 | 5 | 27.6 | 0.2 | 30 | 63.0 | 26.6 | 10.5 | 19.1 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waheed, A.; Naqvi, S.R.; Ali, I. Co-Torrefaction Progress of Biomass Residue/Waste Obtained for High-Value Bio-Solid Products. Energies 2022, 15, 8297. https://doi.org/10.3390/en15218297
Waheed A, Naqvi SR, Ali I. Co-Torrefaction Progress of Biomass Residue/Waste Obtained for High-Value Bio-Solid Products. Energies. 2022; 15(21):8297. https://doi.org/10.3390/en15218297
Chicago/Turabian StyleWaheed, Abdul, Salman Raza Naqvi, and Imtiaz Ali. 2022. "Co-Torrefaction Progress of Biomass Residue/Waste Obtained for High-Value Bio-Solid Products" Energies 15, no. 21: 8297. https://doi.org/10.3390/en15218297





