Discharging Behavior of a Fixed-Bed Thermochemical Reactor under Different Charging Conditions: Modelling and Experimental Validation
Abstract
1. Introduction
- To investigate the effect of charging levels on the discharging performance of the reactor and optimize the operation mode.
- To investigate the effect of temperature and humidity of the inlet air on the reactor discharging performance and to obtain an accurate forecast correlation.
- To investigate the effect of charging flow rates on the reactor performance at specific charging termination temperatures for increasing the discharging temperature.
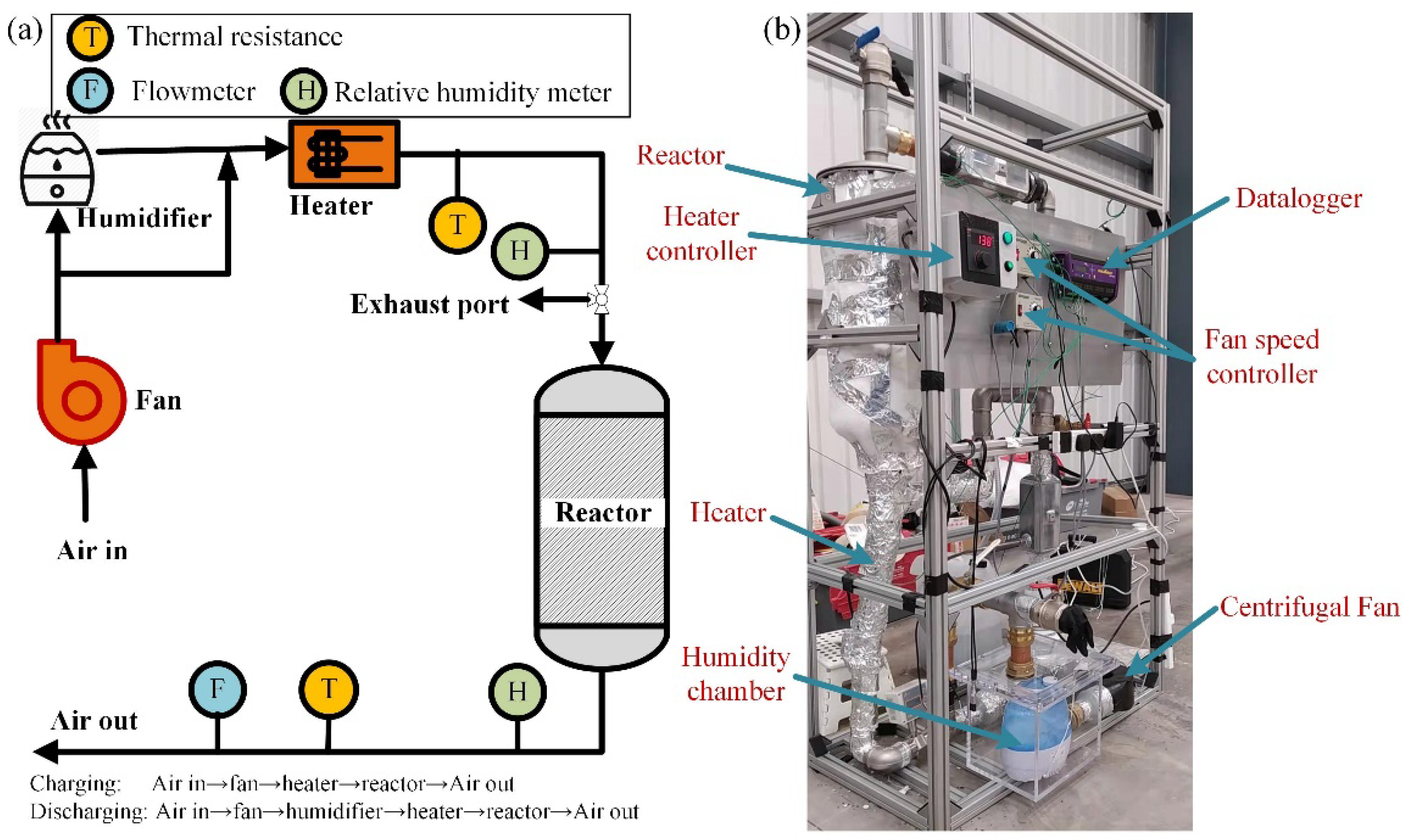
2. Experiments
3. Numerical Simulations
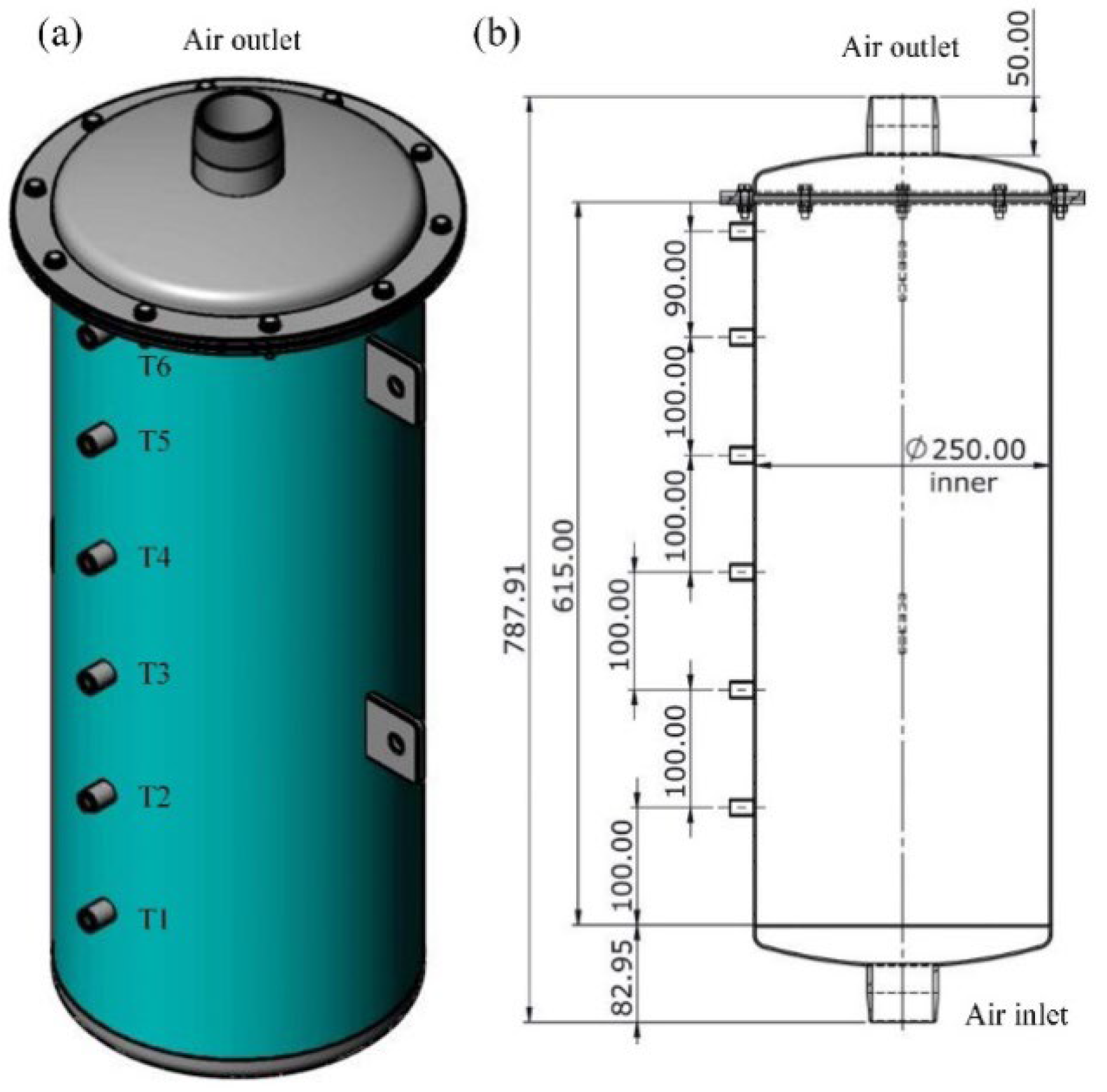
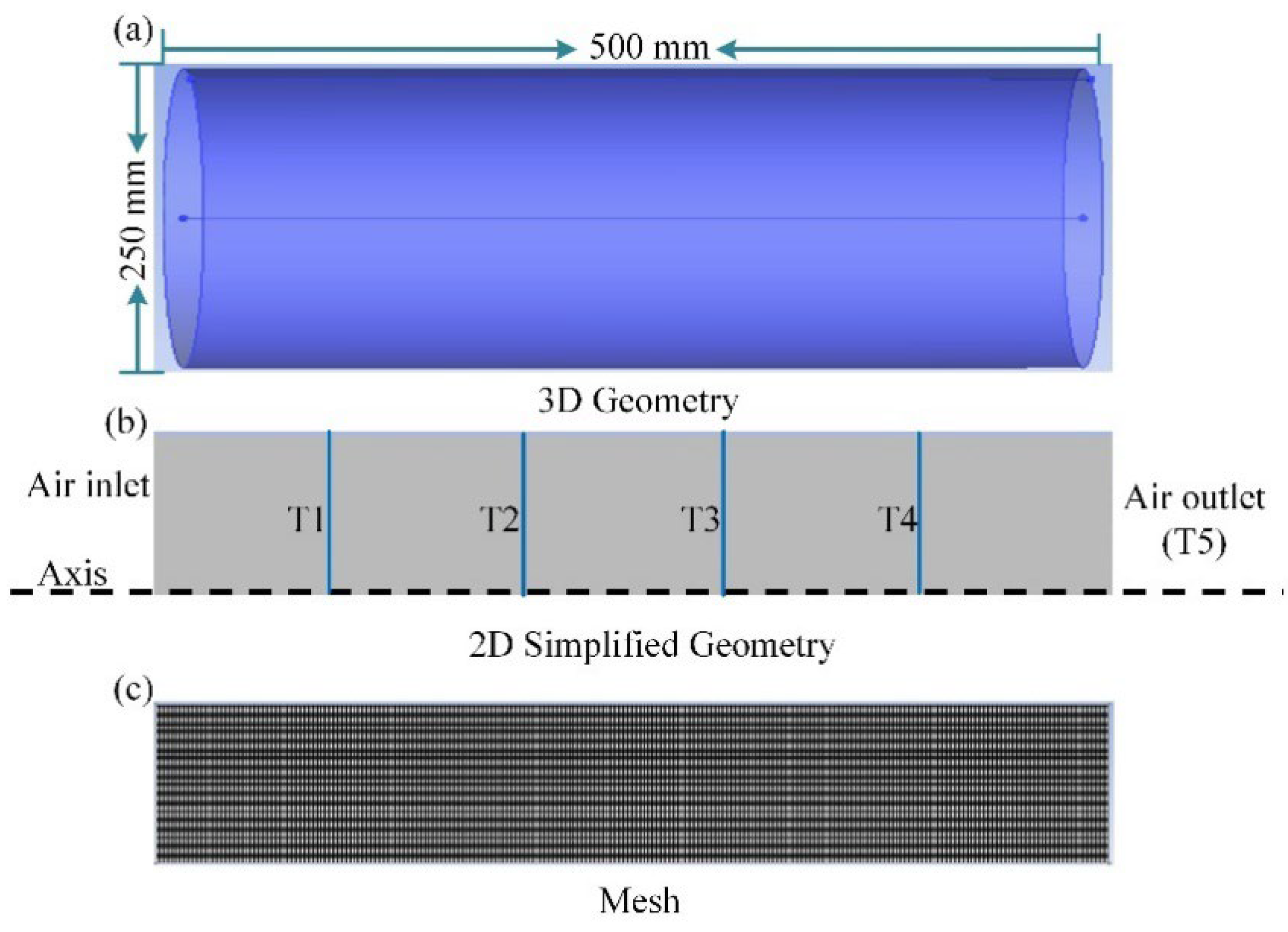
3.1. Geometric Model
3.2. Model Assumptions
- The size of the silica gels and bed porosity in the reactor are uniform and do not change with time [23].
- The thermophysical parameters of heat storage materials and air do not change with time, which is true in actual practice for the low-temperature range [24].
- Because of the low charging and discharging temperature, the radiative heat transfer in the reactor is neglected and the thermal equilibrium between gas and solid in the reactor is assumed [25].
- Water vapor in humid air is also considered an ideal gas due to its extremely low content [15].
- Because of the thicker insulation layer and the lower charging and discharging temperature, it is assumed that the reactor wall is insulated [26].
3.3. Numerical Method and Boundary Conditions
3.4. Simulation Parameters
3.5. Validation of Numerical Simulation
4. Result and Discussion
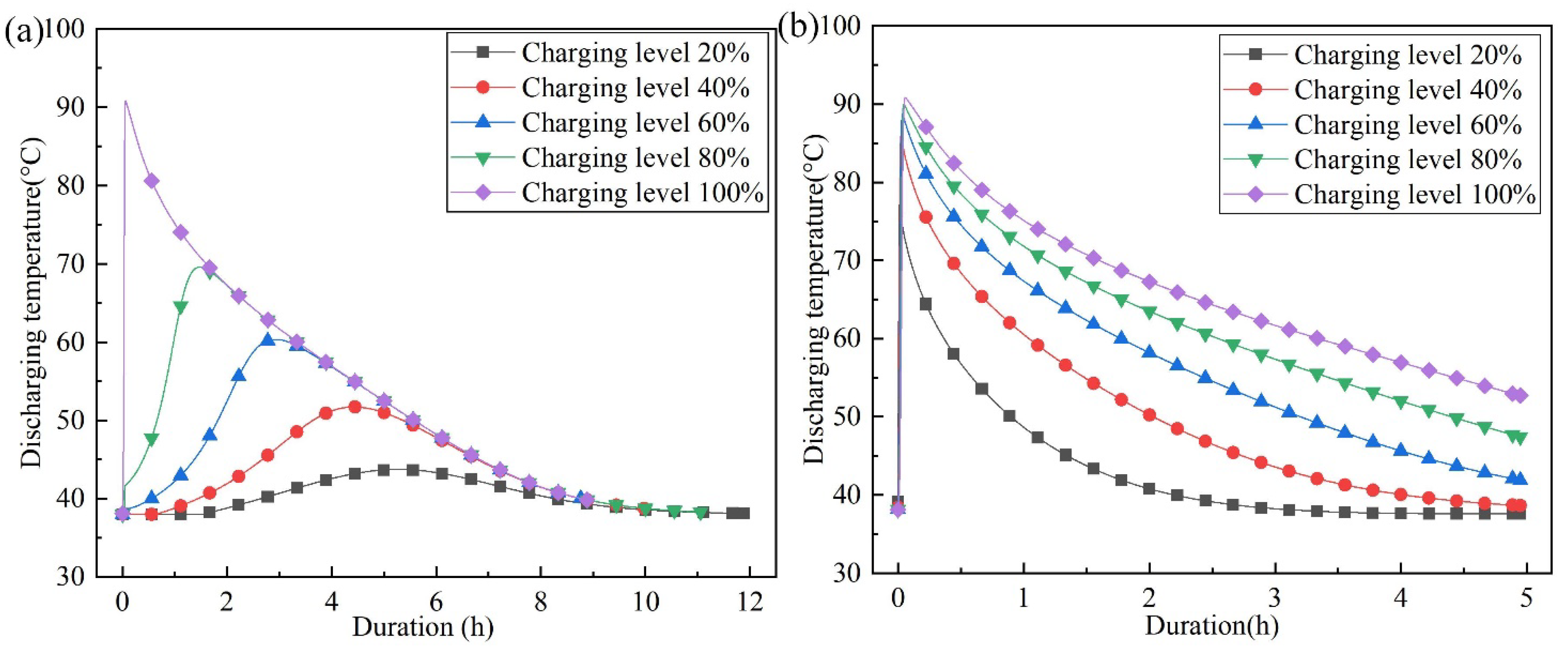
4.1. The Effect of Charging Level on Outlet Temperature of Discharging
4.2. The Effect of Charging Air Velocity on Outlet Temperature of Discharging Reaction
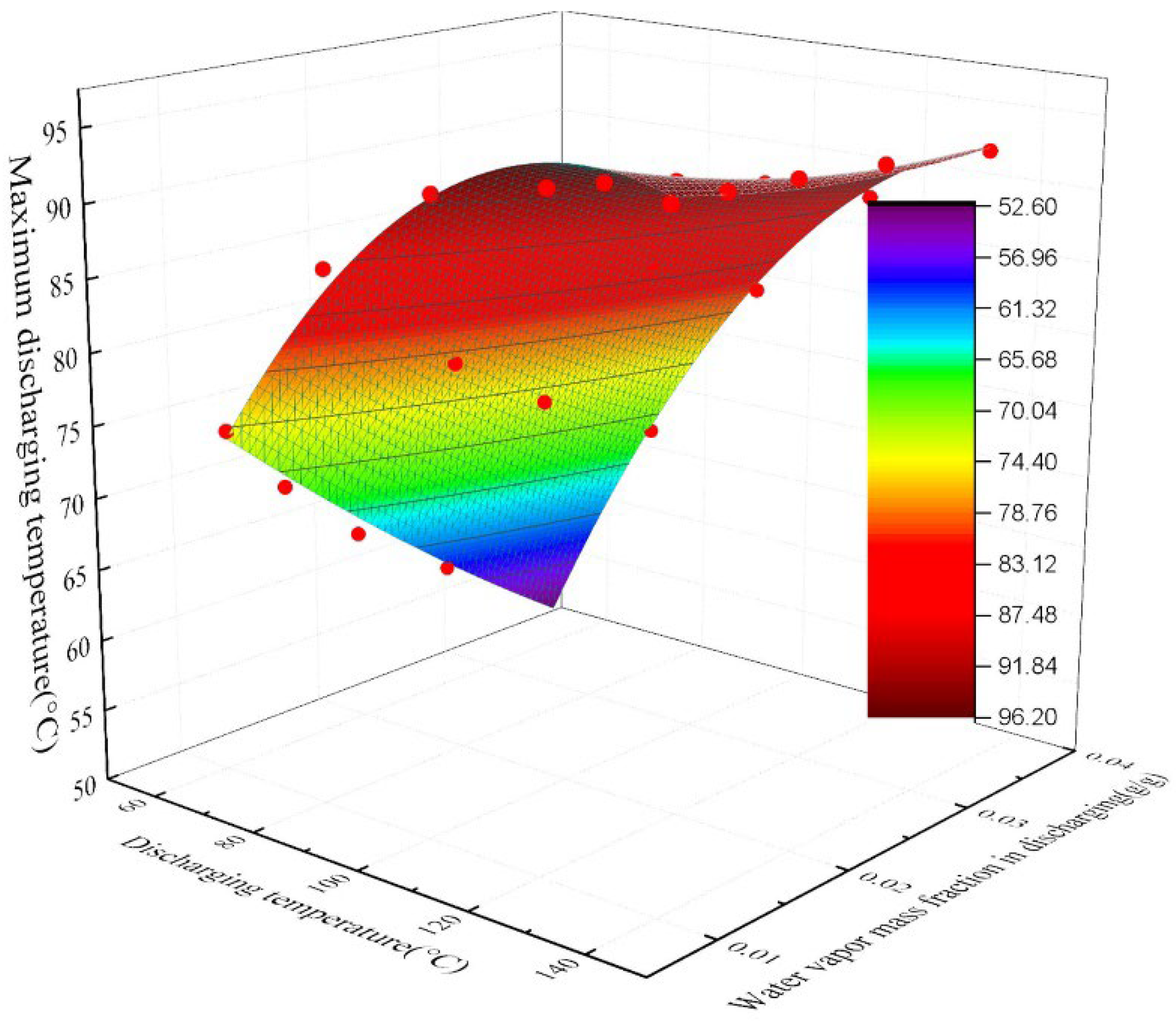
4.3. The Effect of Charging Temperature and Water Vapor Mass Fraction on the Outlet Temperature of the Discharging Reaction
5. Conclusions
- Under the condition of incomplete charging, higher discharging temperatures can be achieved when the air flow direction of the discharging process is opposite to that of the charging process. When the charging level is 20%, the maximum temperature lift of the air outlet increases from 5.73 °C to 39.22 °C, which is 6.84 times the original temperature lift.
- At the same charging termination temperature, a lower charging flow velocity can achieve a higher charging level. This leads to higher discharging temperatures. The discharging temperature at 0.214 m/s is 2.35 °C higher than that at 0.642 m/s when the charging termination temperature is 51.25 °C.
- To achieve the same discharging result, higher charging temperatures are required in areas with high water vapor content in the air. A heat source with a lower temperature can be used for charging in areas with low water vapor content in the air. The numerical simulation data shows that the relationship between the maximum discharging temperature and the charging temperature and the water vapor content in the air is fitted.
- Increasing the charging temperature and decreasing the water vapor content in the air increases the maximum outlet temperature lift to 3.37 and 1.89 times during the discharging process, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Renewables. 2021. Available online: https://www.iea.org/reports/renewables-2021 (accessed on 11 June 2022).
- Renewable and Non-Renewable Heat Consumption and Heat-Related CO2 Emissions in Buildings, 2010–2020. Available online: https://www.iea.org/data-and-statistics/charts/renewable-and-non-renewable-heat-consumption-and-heat-related-CO2-emissions-in-buildings-2010-2020 (accessed on 24 December 2021).
- Global Energy Review. 2021. Available online: https://www.iea.org/reports/global-energy-review-2021 (accessed on 11 June 2022).
- van Soest, H.L.; den Elzen, M.G.J.; van Vuuren, D.P. Net-zero emission targets for major emitting countries consistent with the Paris Agreement. Nat. Commun. 2021, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Allen-Dumas, M.R.; Rose, A.N.; New, J.R.; Omitaomu, O.A.; Yuan, J.; Branstetter, M.L.; Sylvester, L.M.; Seals, M.B.; Carvalhaes, T.M.; Adams, M.B.; et al. impacts of the morphology of new neighborhoods on microclimate and building energy. Renew. Sustain. Energy Rev. 2020, 133, 110030. [Google Scholar] [CrossRef]
- Miliozzi, A.; Dominici, F.; Candelori, M.; Veca, E.; Liberatore, R.; Nicolini, D.; Torre, L. Development and Characterization of Concrete/PCM/Diatomite Composites for Thermal Energy Storage in CSP/CST Applications. Energies 2021, 14, 4410. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, C.; Feng, L.; Li, S.; Gong, Y. Preparation and Characterization of Microencapsulated Phase Change Materials for Solar Heat Collection. Energies 2022, 15, 5354. [Google Scholar] [CrossRef]
- Critoph, R.E.; Pacho, A.M.R. District Heating of Buildings by Renewable Energy Using Thermochemical Heat Transmission. Energies 2022, 15, 1449. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Yan, X.; Zhao, J.; Zhang, C. Development of Thermochemical Heat Storage Based on CaO/CaCO3 Cycles: A Review. Energies 2021, 14, 6847. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. Closed and open thermochemical energy storage: Energy- and exergy-based comparisons. Energy 2012, 41, 83–92. [Google Scholar] [CrossRef]
- van Alebeek, R.; Scapino, L.; Beving, M.A.J.M.; Gaeini, M.; Rindt, C.C.M.; Zondag, H.A. Investigation of a household-scale open sorption energy storage system based on the zeolite 13X/water reacting pair. Appl. Therm. Eng. 2018, 139, 325–333. [Google Scholar] [CrossRef]
- Helaly, H.; El-Sharkawy, I.; Kandel, A.; Awad, M. A study on thermal energy storage using open adsorption system. MEJ. Mansoura Eng. J. 2020, 43, 34–43. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Chen, X.; Riffat, S. Novel “open-sorption pipe” reactor for solar thermal energy storage. Energy Convers. Manag. 2016, 121, 321–334. [Google Scholar] [CrossRef]
- Li, W.; Guo, H.; Zeng, M.; Wang, Q. Performance of SrBr2·6H2O based seasonal thermochemical heat storage in a novel multilayered sieve reactor. Energy Convers. Manag. 2019, 198, 111843. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Shukla, A.; Zou, Y.; Han, X.; Shen, Y.; Yang, L.; Zhang, P.; Kusakana, K. Thermal performance study of thermochemical reactor using net-packed method. Renew. Energy 2022, 182, 483–493. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Nie, B.; Zou, B.; Li, Z.; Han, J.; Tong, L.; Wang, L.; Ding, Y. Discharging behavior of a shell-and-tube based thermochemical reactor for thermal energy storage: Modeling and experimental validation. Int. J. Heat Mass Transf. 2022, 183, 122160. [Google Scholar] [CrossRef]
- Bouché, M.; Richter, M.; Linder, M. Heat transformation based on CaCl2/H2O—Part B: Open operation principle. Appl. Therm. Eng. 2016, 102, 641–647. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Calderón, A.; Svobodova-Sedlackova, A.; Fernández, A.I.; Barreneche, C. The relevance of thermochemical energy storage in the last two decades: The analysis of research evolution. J. Energy Storage 2022, 51, 104377. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.Y.; Markides, C.N.; Wang, H.; Li, W. Medium- and high-temperature latent and thermochemical heat storage using metals and metallic compounds as heat storage media: A technical review. Appl. Energy 2020, 280, 115950. [Google Scholar] [CrossRef]
- National Meteorological Science Data Center. Available online: http://data.cma.cn/ (accessed on 25 June 2022).
- Han, X.C.; Xu, H.J.; Zhao, C.Y. Design and performance evaluation of multi-layered reactor for calcium-based thermochemical heat storage with multi-physics coupling. Renew. Energy 2022, 195, 1324–1340. [Google Scholar] [CrossRef]
- Manila, M.R.; Mitra, S.; Dutta, P. Studies on dynamics of two-stage air cooled water/silica gel adsorption system. Appl. Therm. Eng. 2020, 178, 115552. [Google Scholar] [CrossRef]
- Farcot, L.; le Pierrès, N.; Michel, B.; Fourmigué, J.-F.; Papillon, P. Numerical investigations of a continuous thermochemical heat storage reactor. J. Energy Storage 2018, 20, 109–119. [Google Scholar] [CrossRef]
- Michel, B.; Neveu, P.; Mazet, N. Comparison of closed and open thermochemical processes, for long-term thermal energy storage applications. Energy 2014, 72, 702–716. [Google Scholar] [CrossRef]
- Xu, C.; Xie, Y.; Liao, Z.; Ren, Y.; Ye, F. Numerical study on the desorption process of a thermochemical reactor filled with MgCl2·6H2O for seasonal heat storage. Appl. Therm. Eng. 2019, 146, 785–794. [Google Scholar] [CrossRef]
- Mohammed, R.H.; Mesalhy, O.; Elsayed, M.L.; Hou, S.; Su, M.; Chow, L.C. Physical properties and adsorption kinetics of silica-gel/water for adsorption chillers. Appl. Therm. Eng. 2018, 137, 368–376. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.I.; Kabeel, A.E.; Uddin, K.; Miyazaki, T.; Saha, B.B. Characterization of silica gel-based composites for adsorption cooling applications. Int. J. Refrig. 2020, 118, 345–353. [Google Scholar] [CrossRef]
- Sircar, S.; Hufton, J. Why does the linear driving force model for adsorption kinetics work? Adsorption 2000, 6, 137–147. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, S.; Yang, L.; Han, X.; Song, M.; Shukla, A. Investigation of a three-phase thermochemical reactor through an experimentally validated numerical modelling. Appl. Therm. Eng. 2019, 162, 114223. [Google Scholar] [CrossRef]
- Wang, F.-L.; He, Y.-L.; Tang, S.-Z.; Kulacki, F.A.; Tao, Y.-B. Particle filtration characteristics of typical packing granular filters used in hot gas clean-up. Fuel 2018, 234, 9–19. [Google Scholar] [CrossRef]
- Yu, Y.; Tao, Y.; He, Y.; He, Y.-L. Structure optimization of granular bed filter for industrial flue gas filtration containing coagulative particles: An experimental and numerical study. Adv. Powder Technol. 2020, 31, 2244–2256. [Google Scholar] [CrossRef]
- Bellia, L.; Błaszczak, U.; Fragliasso, F.; Gryko, L. Matching CIE illuminants to measured spectral power distributions: A method to evaluate non-visual potential of daylight in two European cities. Sol. Energy 2020, 208, 830–858. [Google Scholar] [CrossRef]
- DeLurgio, S.A. Forecasting Principles and Applications; McGraw-Hill/Irwin: Boston, MA, USA, 1998. [Google Scholar]









| Parameter | Unit | Charging | Discharging |
|---|---|---|---|
| Temperature at air inlet | °C | 100 | 38 |
| Relative humidity at air inlet | - | 75% (25 °C) | 80% |
| Air inlet velocity | m/s | 0.428 | 0.428 |
| Initial temperature in reactor | °C | 20 | 38 |
| Initial water adsorption of silica gel | g/g | 0.346 | - |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Bed porosity | 0.438 | - | |
| Silica gel conductivity | 0.35 | ||
| Silica gel density | 1.2 × 103 | ||
| Activation energy | 4.15 × 104 | ||
| Pre-exponential factor | 1.3 × 10−3 | ||
| Maximum adsorption capacity | 0.346 | ||
| Characteristic energy of adsorption | E | 3800 | (J/mol) |
| Heterogeneity parameter | n | 1.6 | - |
| Silica gel particle diameter | 4 × 10−3 | m |
| Grid Type | Number of Grids | Charging Completion Time | The Total Energy Output |
|---|---|---|---|
| Rougher grid | 1288 | 10,687 s | 9847.53 kJ |
| Medium grid | 2576 | 9802 s | 9032.15 kJ |
| Finer grid | 5152 | 9934 s | 8723.97 kJ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ma, H.; Ahmad, A.; Yang, H.; Ji, M.; Zou, B.; Nie, B.; Chen, J.; Tong, L.; Wang, L.; et al. Discharging Behavior of a Fixed-Bed Thermochemical Reactor under Different Charging Conditions: Modelling and Experimental Validation. Energies 2022, 15, 8377. https://doi.org/10.3390/en15228377
Wang C, Ma H, Ahmad A, Yang H, Ji M, Zou B, Nie B, Chen J, Tong L, Wang L, et al. Discharging Behavior of a Fixed-Bed Thermochemical Reactor under Different Charging Conditions: Modelling and Experimental Validation. Energies. 2022; 15(22):8377. https://doi.org/10.3390/en15228377
Chicago/Turabian StyleWang, Chengcheng, Hongkun Ma, Abdalqader Ahmad, Hui Yang, Mingxi Ji, Boyang Zou, Binjian Nie, Jie Chen, Lige Tong, Li Wang, and et al. 2022. "Discharging Behavior of a Fixed-Bed Thermochemical Reactor under Different Charging Conditions: Modelling and Experimental Validation" Energies 15, no. 22: 8377. https://doi.org/10.3390/en15228377
APA StyleWang, C., Ma, H., Ahmad, A., Yang, H., Ji, M., Zou, B., Nie, B., Chen, J., Tong, L., Wang, L., & Ding, Y. (2022). Discharging Behavior of a Fixed-Bed Thermochemical Reactor under Different Charging Conditions: Modelling and Experimental Validation. Energies, 15(22), 8377. https://doi.org/10.3390/en15228377







