Effects of Quenching on Corrosion and Hardness of Aluminum Alloy 7075-T6
Abstract
:1. Introduction
2. Theoretical Background
3. Experimental Work
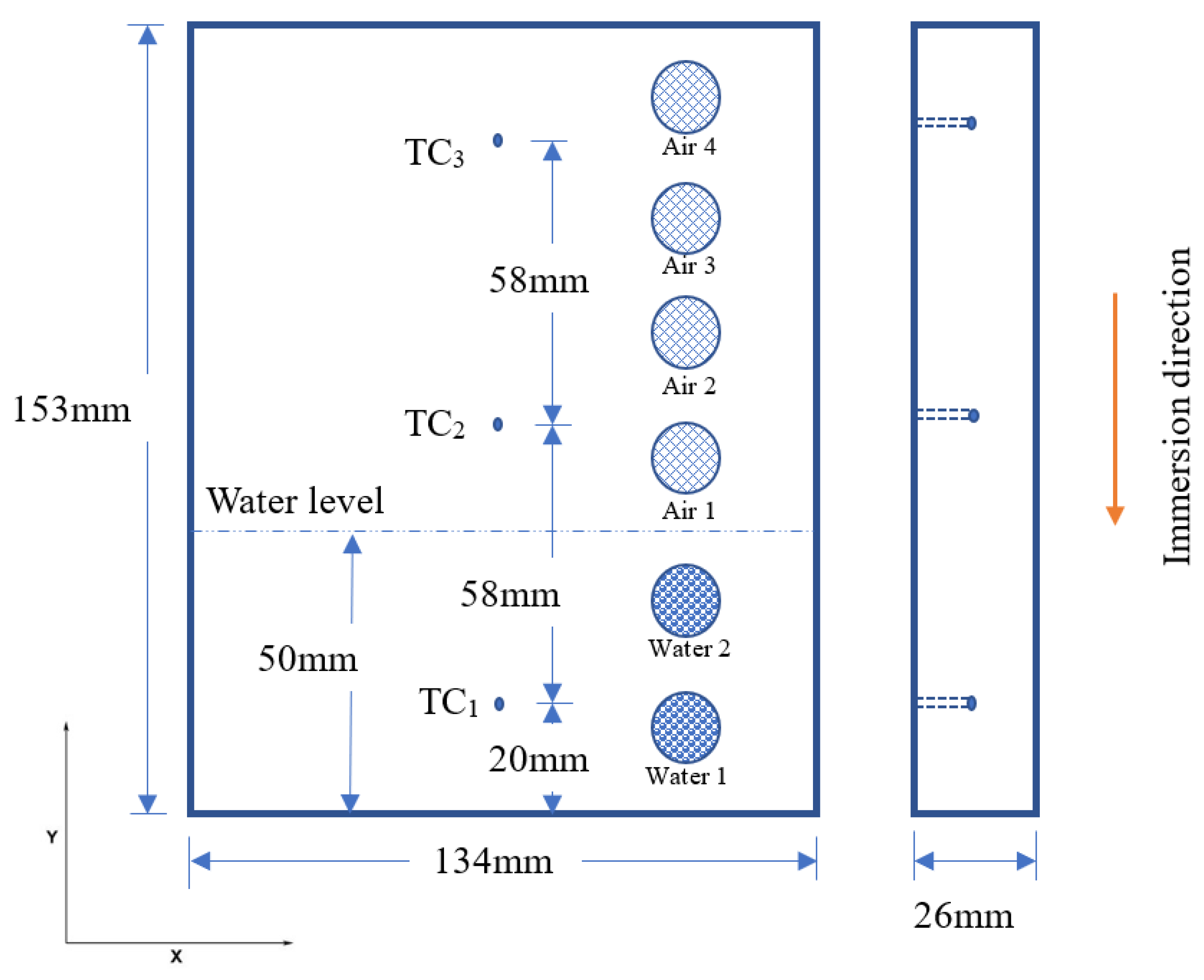
3.1. Quench Test
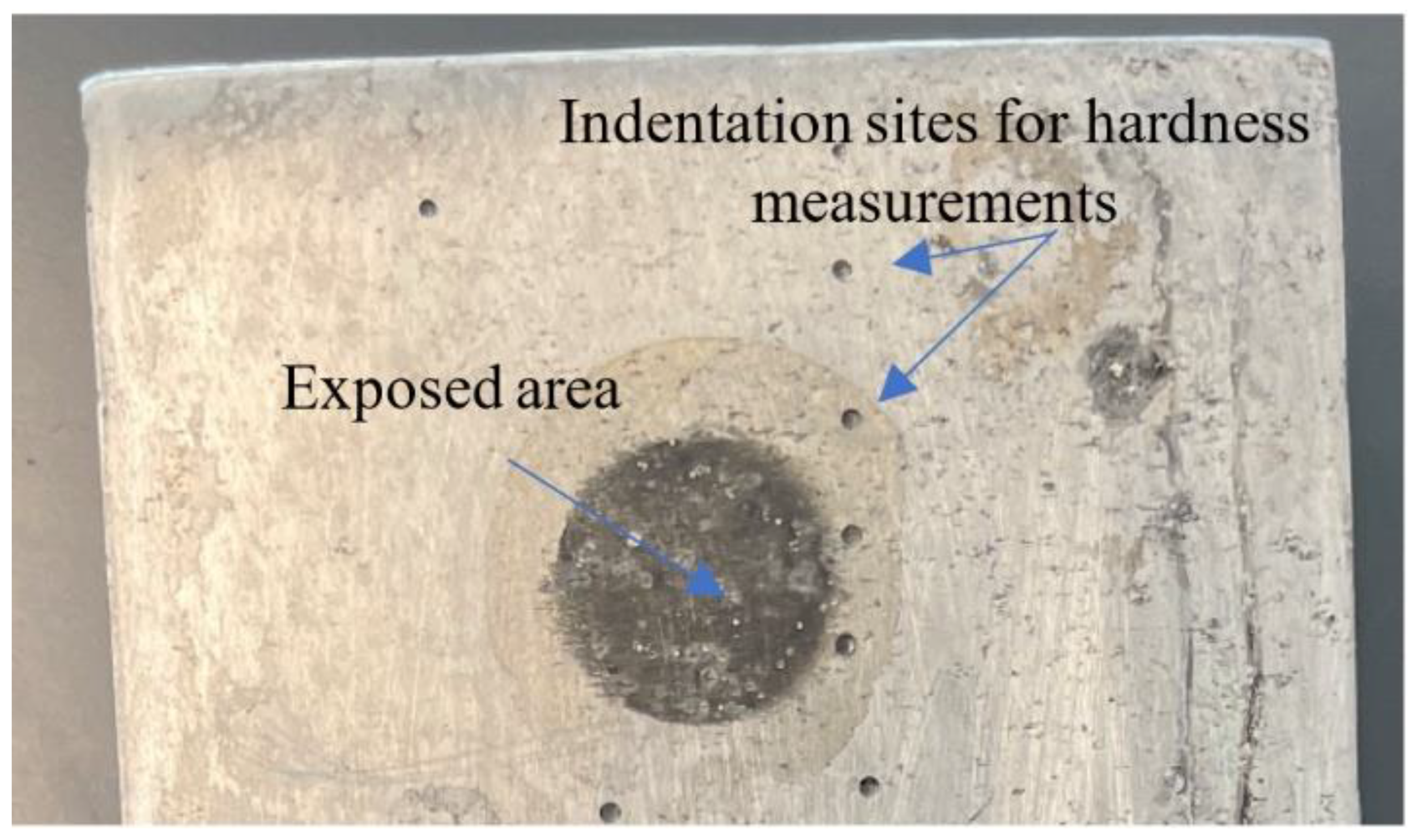
3.2. Hardness Test
3.3. Corrosion Test
4. Finite Element Analysis (FEA)
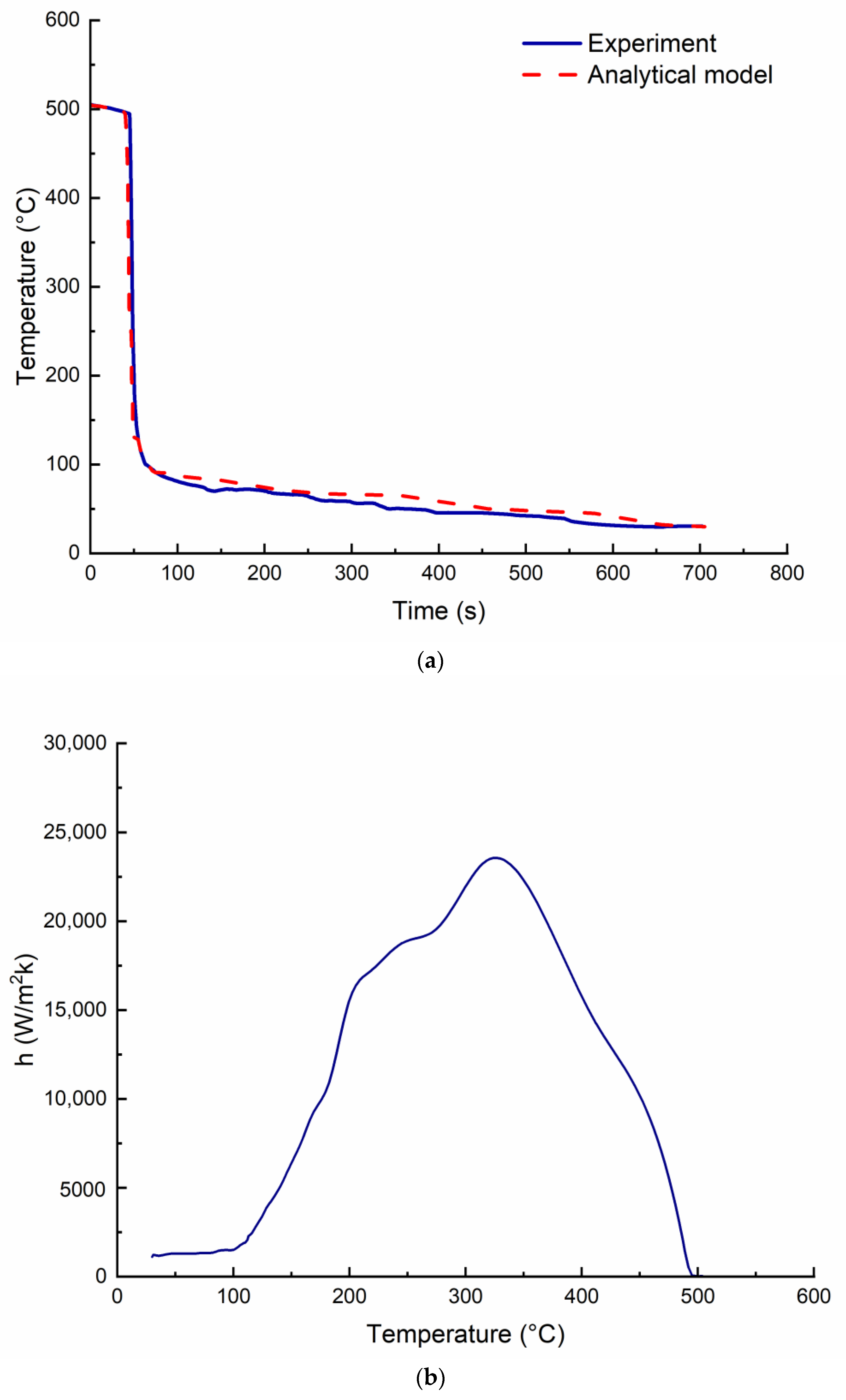
5. Results and Discussion
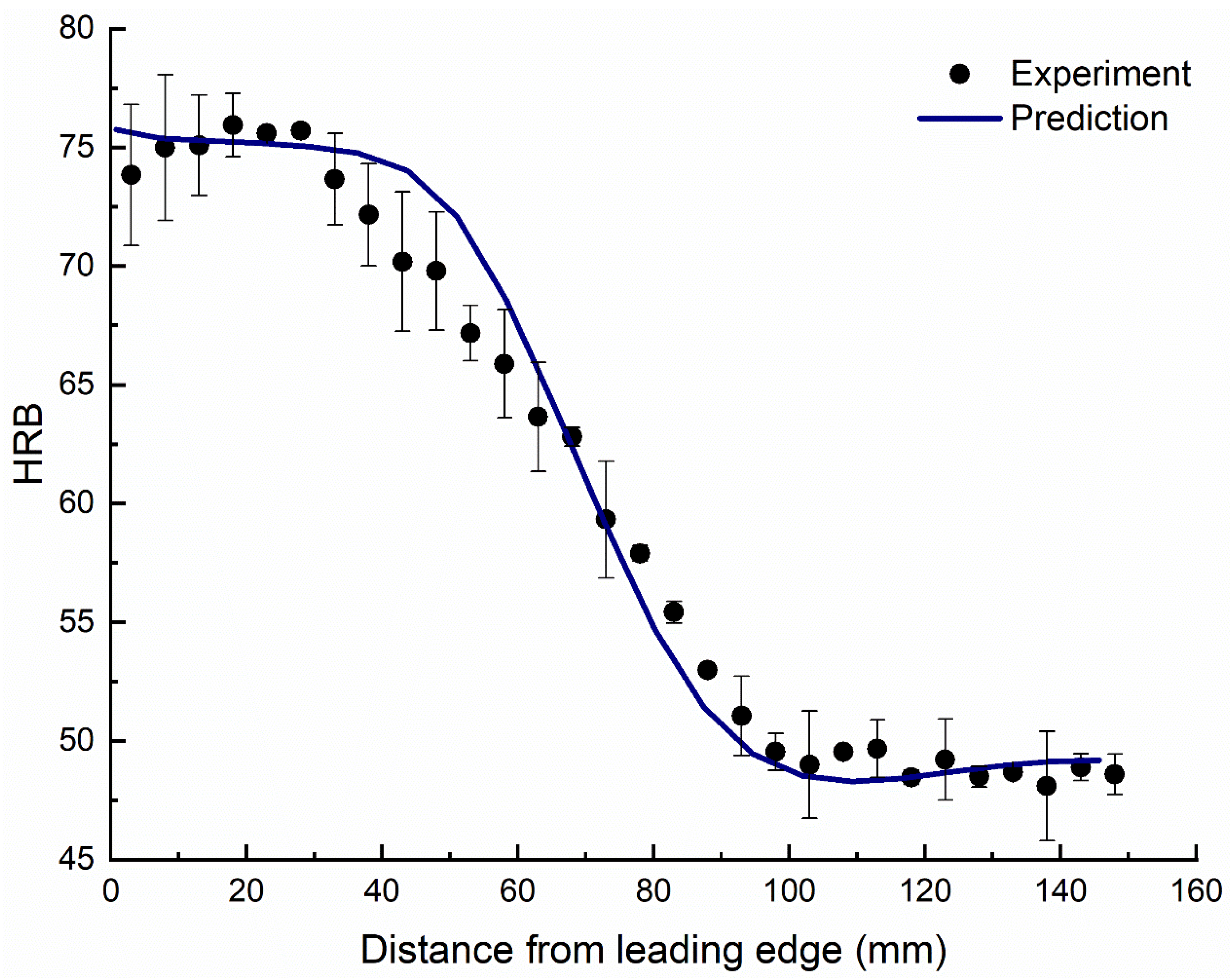
5.1. Hardness
5.2. Corrosion
5.2.1. EIS Results
5.2.2. CP Results
5.3. Corrosion Modeling
6. Conclusions
- The hardness and the corrosion potential of the quenched block decrease as the distance from the leading-edge increases. This trend is opposite for the charge transfer resistance.
- The EIS results suggest the existence of a corrosion product layer resistor, Rf, for the water-quenched part of the sample. This was not observed for the air-cooled part.
- The values of vortex potential are higher for the water-quenched parts than the air-cooled parts. This may be due to the fact that the corrosion product layer grows thicker at higher potentials and becomes protective. This observation is in agreement with the existence of a corrosion product layer resistor for the water-quenched part when compared to the air-cooled part for which the corrosion product layer resistor was not realized from the EIS data.
- A new empirical model is developed that predicts the charge transfer resistance and corrosion potential of a quenched sample fairly well. It is based on the quench factor analysis method. The model only requires cooling curves as inputs.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Zhong, Q.; Zhang, Y.; Liu, W.; Zhang, X.; Deng, Y. Investigation of Quench Sensitivity of High Strength Al–Zn–Mg–Cu Alloys by Time–Temperature-Properties Diagrams. Mater. Des. 2010, 31, 3116–3120. [Google Scholar] [CrossRef]
- Li, S.; Huang, Z.; Chen, W.; Liu, Z.; Qi, W. Quench Sensitivity of 6351 Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 46–52. [Google Scholar] [CrossRef]
- Staley, J.T. Quench Factor Analysis of Aluminium Alloys. Am. Institue Min. Met. Eng. 1948, 175, 414–427. [Google Scholar] [CrossRef]
- Dolan, G.P.; Robinson, J.S. Residual Stress Reduction in 7175-T73, 6061-T6 and 2017A-T4 Aluminium Alloys Using Quench Factor Analysis. J. Mater. Process. Technol. 2004, 153, 346–351. [Google Scholar] [CrossRef]
- FInk, W.L.; Willey, L.A. Quenching of 75S Aluminum Alloy. Mater. Sci. Eng. 1948, 43, 1–6. [Google Scholar]
- Li, H.-Y.; Cui-Ting, Z.; Han, M.-S.; Liu, J.-J.; Lu, X.-C. Time–Temperature–Property Curves for Quench Sensitivity of 6063 Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 38–45. [Google Scholar] [CrossRef]
- The Jominy End Quench Test. Standard Test Methods for Determining Hardenability of Steel; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Bryant, A.J. The Effect of Composition upon the Quecnh-Sensitivity of Some Al-Zn-Mg Alloys. Inst. Met. J. 1996, 94, 94–99. [Google Scholar]
- t’Hart, W.G.J.; Kolkman, H.J.; Schra, L. Effect of Cooling Rate on Corrosion on Properties and Microstruture of High Strength Aluminum Alloys; National Lucht-En Ruimtevaartlaboratorium: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Kolkman, H.J.; t’Hart, W.G.J.; Schra, L. Quench Sensitivity of Airframe Aluminium Alloys. In Strength of Metals and Alloys (ICSMA 8); Pergamon: Oxford, UK, 1989; pp. 597–602. [Google Scholar]
- Rometsch, P.A.; Starink, M.J.; Gregson, P.J. Improvements in Quench Factor Modelling. Mater. Sci. Eng. A 2003, 339, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Bernardin, J.D.; Issam, M. Validation of the Quench Factor Technique in Predicting Hardness in Heat Treatable Aluminum Alloys. Int. J. Heat Mass Transf. 1995, 38, 863–873. [Google Scholar] [CrossRef]
- Archambault, P.; Chevrier, J.C.; Beck, G.; Bouvaist, J. A Contribution to the Optimization of the 7075 Heat Treatment. Materials Science and Engineering. Mater. Sci. Eng. 1980, 43, 1–6. [Google Scholar] [CrossRef]
- Bates, C.E.; Landing, T.; Seitanakis, G. Quench Factor Analysis: A Powerful Tool Comes of Age. Heat Treat. 1985, 85, 13–17. [Google Scholar]
- Yazdi, A.Z.; Sajjadi, S.A.; Zebarjad, S.M.; SM Moosavi, N. Prediction of Hardness at Different Points of Jominy Specimen Using Quench Factor Analysis Method. J. Mater. Process. Technol. 2008, 199, 124–129. [Google Scholar] [CrossRef]
- Newkirk, J.W.; Ganapati, K.; MacKenzie, D.S. Faster Methods of Studying Quenching Aluminum through Jominy End Quench. In Heat Treating 1998: Proceedings of the 18th Conference of Heat Treating Including the Liu Dai Memorial Symposium, Cincinatti, OH, USA, 12–15 October 1998; ASM International: Ohio, OH, USA, 1998; pp. 143–150. [Google Scholar]
- Dolan, G.P.; Flynn, R.J.; Tanner, D.A.; Robinson, J.S. Quench Factor Analysis of Aluminium Alloys Using the Jominy End Quench Technique. Mater. Sci. Technol. 2005, 21, 687–692. [Google Scholar] [CrossRef]
- Xie, P.; Chen, K.; Chen, S.; Ye, S.; Jiao, H.; Hunag, L. Study on Quenching Sensitivity of 7097 Aluminum Alloy. Mater. Res. Express 2019, 7, 016505. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Peng, H.; Zhao, J. Quench Sensitivity of 7475 Aluminum Alloy Using End-Quenching Technique and TTP Diagrams. JOM 2021, 73, 1135–1143. [Google Scholar] [CrossRef]
- Li, P.Y.; Xiong, B.; Zhang, Y.; Li, Z.-H.; Zhu, B.; Wang, F.; Liu, H. Quench Sensitivity and Microstructure Character of High Strength AA7050. Trans. Nonferrous Met. Soc. China 2012, 22, 268–274. [Google Scholar] [CrossRef]
- Ruth Fransisca, C.; Soegijono, B. The Effect of Quench Delay on Structure and Corrosion Properties of Aluminum Lithium Alloy 2091. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 694, p. 0102030. [Google Scholar]
- Chen, Z.; Kaunitz, L.; Wu, C. Quench Rate Study on AA7075 with Advanced Aging and T6. SAE Int. J. Mater. Manuf. 2020, 13, 209–216. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, L.; Zeng, Q.; Pan, Q.; Li, S.; Liang, J.; Yang, H. The Microstructure Evolution and Electrochemical Corrosion Behavior of 7A46 Aluminum Alloy in Different Quenching Conditions. Materials 2022, 15, 477. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Wang, X.; Liu, Z. Quenching Sensitivity of Al-Zn-Mg Alloy after Non-Isothermal Heat Treatment. Materials 2019, 12, 1595. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.; Chen, K.; Zhou, L.; Chang, J.; Huang, L.; Yi, Y. Enhancing Stress Corrosion Cracking Resistance of Low Cu-Containing Al-Zn-Mg-Cu Alloys by Slow Quench Rate. Mater. Des. 2019, 164, 107558. [Google Scholar] [CrossRef]
- Song, F.; Zhang, X.; Liu, S.; Tan, Q.; Li, D. The Effect of Quench Rate and Overageing Temper on the Corrosion Behaviour of AA7050. Corrosion Sci. 2014, 78, 276–286. [Google Scholar] [CrossRef]
- Marlaud, T.; Malki, B.; Henon, C.; Deschamps, A.; Baroux, B. Relationship between Alloy Composition, Microstructure and Exfoliation Corrosion in Al–Zn–Mg–Cu Alloys. Corros. Sci. 2011, 53, 3139–3149. [Google Scholar] [CrossRef]
- Deschamps, A.; Brechet, Y.; Guyot, P.; Livet, F. On the Influence of Dislocations on Precipitation in an Al–Zn–Mg Alloy. Int. J. Mater. Res. 1997, 88, 601–606. [Google Scholar]
- Han, N.; XinMing, Z.; Liu, S.; Ke, B.; Xin, X. Effects of Pre-Stretching and Ageing on the Strength and Fracture Toughness of Aluminum Alloy 7050. Mater. Sci. Eng. A 2011, 528, 3714–3721. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Z.; Zhuang, W.; Peng, H. Effects of Pre-Strain on the Surface Residual Stress and Corrosion Behavior of an Al-Zn-Mg-Cu Alloy Plate. Mater. Charact. 2020, 160, 110129. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Z.Y. Effect of Pre-Strain on Microstructure and Stress Corrosion Cracking of over-Aged 7050 Aluminum Alloy. J. Alloys Compd. 2009, 469, 445–450. [Google Scholar] [CrossRef]
- Liu, S.D.; Zhang, X.M.; You, J.H.; Huang, Z.B.; Zhou, Z.P. TTP Diagrams for 7050 Aluminum Alloy. Mater. Sci. Technol. 2008, 24, 1419–1421. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys’, Part 1; Book of Phase Transformation in Metals and Alloys; Pergamon Press: Oxford, UK, 2002; pp. 797–1096. [Google Scholar]
- Totten, G.E.; Webster, G.M.; Jarvis, L.M.; Bates, E. Effect of Section Size, Quenchant Concentration and Agitation on the Physical Properties of Type I Polymer Quenched Aluminum Alloys. In Proceedings of the 1st International Non-Ferrous Processing and Technology Conference, St. Louis, MO, USA, 10–12 March 1997; ASM International: St. Louis, MO, USA, 1997; pp. 7–16. [Google Scholar]
- American Society for Testing and Materials. Committee E-28 on Mechanical Testing. In Standard Test Methods for Rockwell Hardness of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Daghbouj, N.; Sen, H.S.; Callisti, M.; Vronka, M.; Karlik, M.; Duchoň, J.; Čech, J.; Havránek, V.; Polcar, T. Revealing Nanoscale Strain Mechanisms in Ion-Irradiated Multilayers. Acta Mater. 2022, 229, 117807. [Google Scholar] [CrossRef]
- Ge, F.F.; Sen, H.S.; Daghbouj, N.; Callisti, M.; Feng, Y.J.; Li, B.S.; Zhu, P.; Li, P.; Meng, F.P.; Polcar, T.; et al. Toughening Mechanisms in V-Si-N Coatings. Mater. Des. 2021, 209, 109961. [Google Scholar] [CrossRef]
- MacDonald, J.R. Impedence Spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef]
- Sundaram, S.K.; Bharath, A.G.; Aravind, B. Influence of Target Dynamics and Number of Impacts on Ballistic Performance of 6061-T6 and 7075-T6 Aluminum Alloy Targets. Mech. Based Des. Struct. Mach. 2022, 50, 993–1011. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of Spray Cooling—Part 2: High Temperature Boiling Regimes and Quenching Applications. Int. J. Heat Mass Transf. 2017, 115, 1206–1222. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic Phases in Aluminum Alloys and Their Roles in Localized Corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Barmi, M.J.; Manickam, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Shahriari, A.; Khaksar, L.; Nasiri, A.; Hadadzadeh, A.; Shalchi Amirkhiz, B.; Mohammadi, M. Microstructure and Corrosion Behavior of a Novel Additively Manufactured Maraging Stainless Steel. Electrochim. Acta 2020, 339, 135925. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Ma, J.; Wen, J.; Li, Q.; Zhang, Q. Effects of Acidity and Alkalinity on Corrosion Behaviour of Al–Zn–Mg Based Anode Alloy. J. Power Sources 2013, 226, 156–161. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of Corrosion Rates Measured by the Tafel Extrapolation Method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, G.; Chen, M.-S.; Zhang, J.-L.; Chen, Z.-G.; Jiang, Y.-Q.J.; Li, J. Corrosion Resistance of a Two-Stage Stress-Aged AleCueMg Alloy: Effects of External Stress. J. Alloys Compd. 2016, 661, 221–230. [Google Scholar] [CrossRef]
- Mosayebi, A.; Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Tempering Kinetics and Corrosion Resistance of Quenched and Tempered AISI 4130 Medium Carbon Steel. Mater. Corros. 2021, 72, 1808–1812. [Google Scholar] [CrossRef]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Trueba, M.; Trasatti, S.P. Study of Al Alloy Corrosion in Neutral NaCl by the Pitting Scan Technique. Mater. Chem. Phys. 2010, 121, 523–533. [Google Scholar] [CrossRef]
- Incropera, F.P.; Dewitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rapp, B.E. Chapter 9: Fluids. In Microfluidics: Modeling, Mechanics and Mathematics; William Andrew: Norwich, NY, USA, 2016; p. 260. [Google Scholar]
- Cui, X.; Wan, M.; Ma, B.; Wu, X.; Han, J. Quenching by Immersion Considering Boiling Heat Transfer. Int. J. Therm. Sci. 2019, 139, 303–311. [Google Scholar] [CrossRef]
- Nukiyama, S. The Maximum and Minimum Values of the Heat Q Transmitted from Metal to Boiling Water under Atmospheric Pressure. Int. J. Heat Mass Transf. 1966, 9, 1419–1433. [Google Scholar] [CrossRef]
- Kopun, R.; Skerget, L.; Hriber sek, M.; Zhang, D.; Bernhard, S.; Greif, D. Numerical Simulation of Immersion Quenching Process for Cast Aluminium Part at Different Pool Temperatures. Appl. Therm. Eng. 2014, 65, 74–84. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Klein, S.; Beckman, W. Heat Transfer: A Practical Approach; WBC McGraw-Hill: Boston, MA, USA, 1998; Volume 141. [Google Scholar]
- Jan, J.; MacKenzie, S. On the Development of Parametrical Water Quenching Heat Transfer Model Using Cooling Curves by ASTM D6200 Quenchometer. J. Mater. Eng. Perform. 2020, 29, 3612–3625. [Google Scholar] [CrossRef]
- Srinivasan, V.; Kil-Min, M.; Greif, D.; Wang, D.M.; Kim, M. Numerical Simulation of Immersion Quench Cooling Process Using an Eulerian Multi-Fluid Approach. Appl. Therm. Eng. 2010, 30, 499–509. [Google Scholar] [CrossRef]
- Churchill, S.W.; Chu, H.H. Correlating Equations for Laminar and Turbulent Free Convection from a Horizontal Cylinder. Int. J. Heat Mass Transf. 1975, 18, 1049–1053. [Google Scholar] [CrossRef]





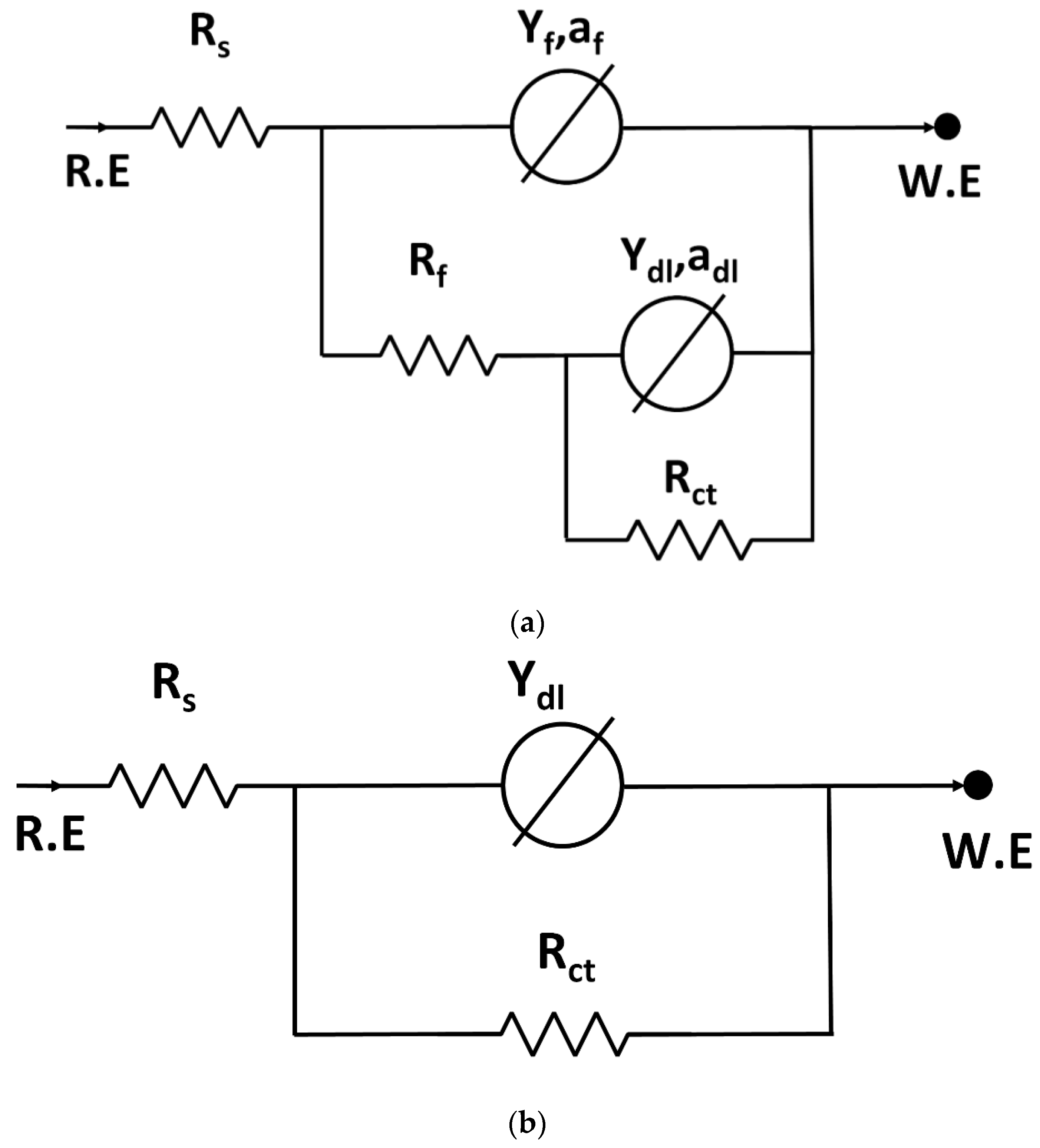
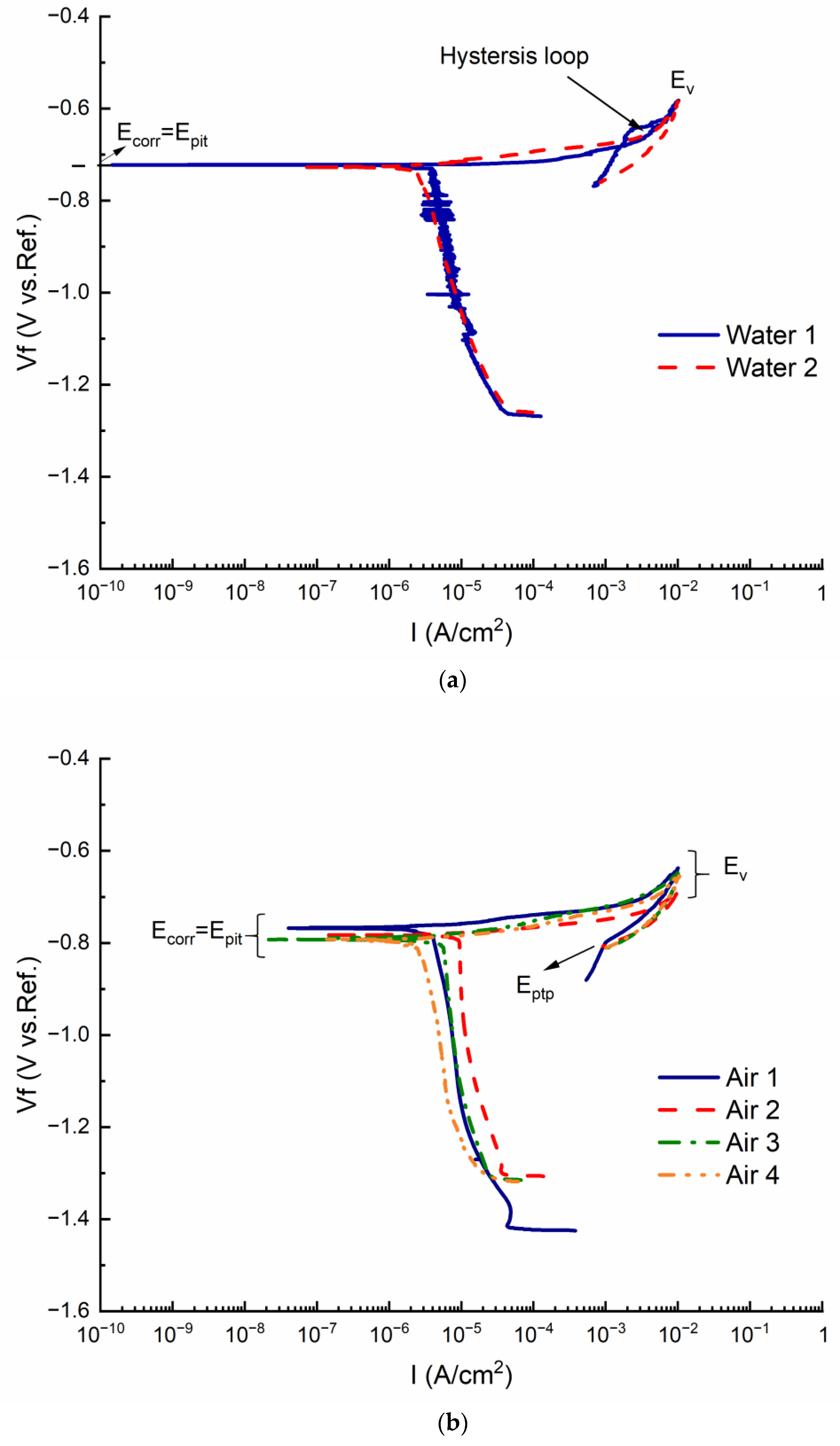
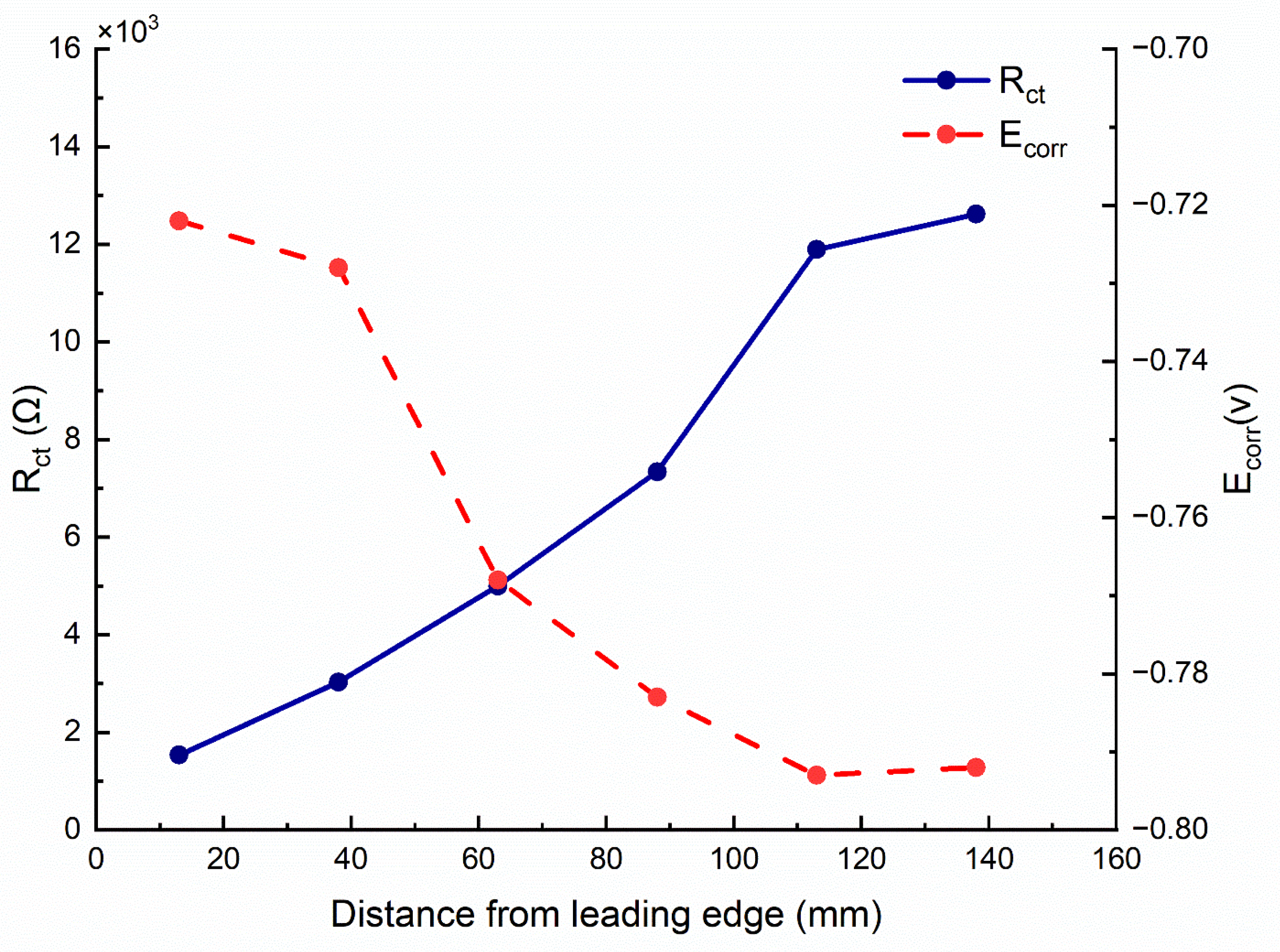

| (s) | (J/mol) | (K) | (J/mol) | R (J/Kmol) |
| 1050 | 780 | 8.3143 |
| Al | Zn | Mg | Cu | Fe | Si | Mn | Cr | Ti |
|---|---|---|---|---|---|---|---|---|
| Bal. | 5.6 | 2.5 | 1.6 | 0.5 | 0.4 | 0.3 | 0.23 | 0.2 |
| Element | Water 1 | Water 2 | Air 1 | Air 2 | Air 3 | Air 4 |
|---|---|---|---|---|---|---|
| Rs (Ω) | 10.12 | 12.29 | 11.30 | 11.76 | 11.88 | 11.72 |
| Yf (F) | 20.23 | 11.03 | - | - | - | - |
| af | 0.85 | 1 | - | - | - | - |
| Rf (Ω) | 19.13 | 47.48 | - | - | - | - |
| Ydl (F) | 11.1 | 0.33 | 8 | 2.18 | 5.13 | 1.98 |
| adl | 0.97 | 0.84 | 0.62 | 0.77 | 0.67 | 0.80 |
| Rct (Ω) | 1534 | 3030 | 4995 | 7339 | 11,891 | 12,617 |
| 1.72 | 4.69 | 3.14 | 1.33 | 1.14 | 1.01 |
| Elements | Water 1 | Water 2 | Air 1 | Air 2 | Air 3 | Air 4 |
|---|---|---|---|---|---|---|
| Ecorr (V) and Epit (V) | −0.722 ± 0.003 | −0.728 ± 0.005 | −0.768 ± 0.012 | −0.783 ± 0.009 | −0.793 ± 0.005 | −0.792 ± 0.003 |
| Icorr (µA) | 1.911 ± 0.199 | 1.612 ± 0.205 | 2.411 ± 0.594 | 7.633 ± 2.013 | 3.620 ± 0.987 | 0.953 ± 0.184 |
| Ev (V) | −0.583 ± 0.005 | −0.591 ± 0.006 | −0.638 ± 0.037 | −0.697 ± 0.042 | −0.652 ± 0.037 | −0.655 ± 0.009 |
| For Ecorr | −0.4939 | 3.1833 |
| For Rct | −0.5435 | 6.1828 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi, L.; Alfred, S.O.; Amiri, M. Effects of Quenching on Corrosion and Hardness of Aluminum Alloy 7075-T6. Energies 2022, 15, 8391. https://doi.org/10.3390/en15228391
Saberi L, Alfred SO, Amiri M. Effects of Quenching on Corrosion and Hardness of Aluminum Alloy 7075-T6. Energies. 2022; 15(22):8391. https://doi.org/10.3390/en15228391
Chicago/Turabian StyleSaberi, Leila, Samuel Onimpa Alfred, and Mehdi Amiri. 2022. "Effects of Quenching on Corrosion and Hardness of Aluminum Alloy 7075-T6" Energies 15, no. 22: 8391. https://doi.org/10.3390/en15228391
APA StyleSaberi, L., Alfred, S. O., & Amiri, M. (2022). Effects of Quenching on Corrosion and Hardness of Aluminum Alloy 7075-T6. Energies, 15(22), 8391. https://doi.org/10.3390/en15228391







