The Methods and Stands for Testing Fixed Sorbent and Sorbent Polymer Composite Materials for the Removal of Mercury from Flue Gases
Abstract
1. Introduction
2. Materials and Methods
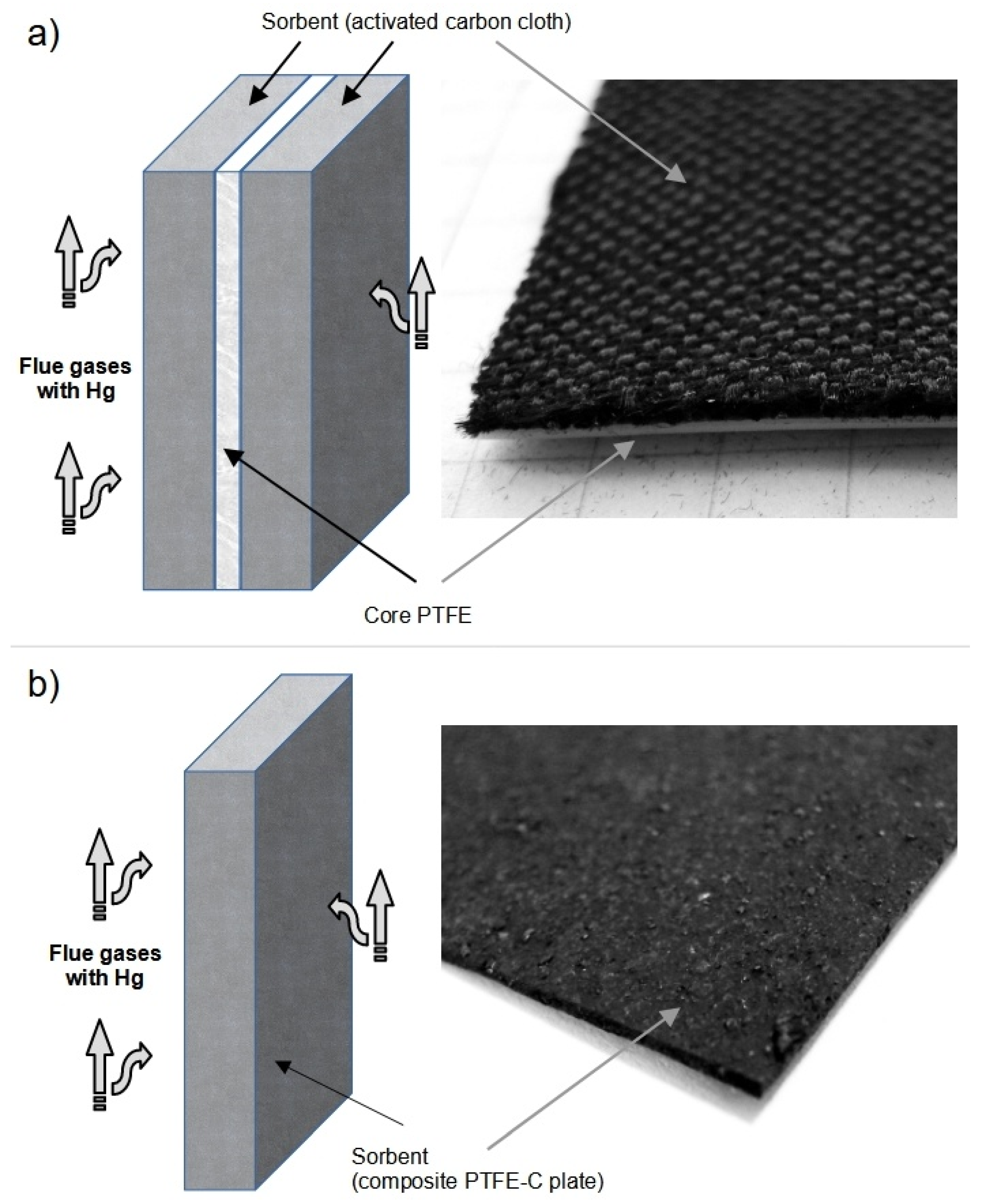
2.1. Materials
2.2. Produced Apparatus
- -
- a system for laboratory tests of materials in the flue gas,
- -
- a mobile system for preliminary industrial tests,
- -
- a larger scale system for final industrial tests.
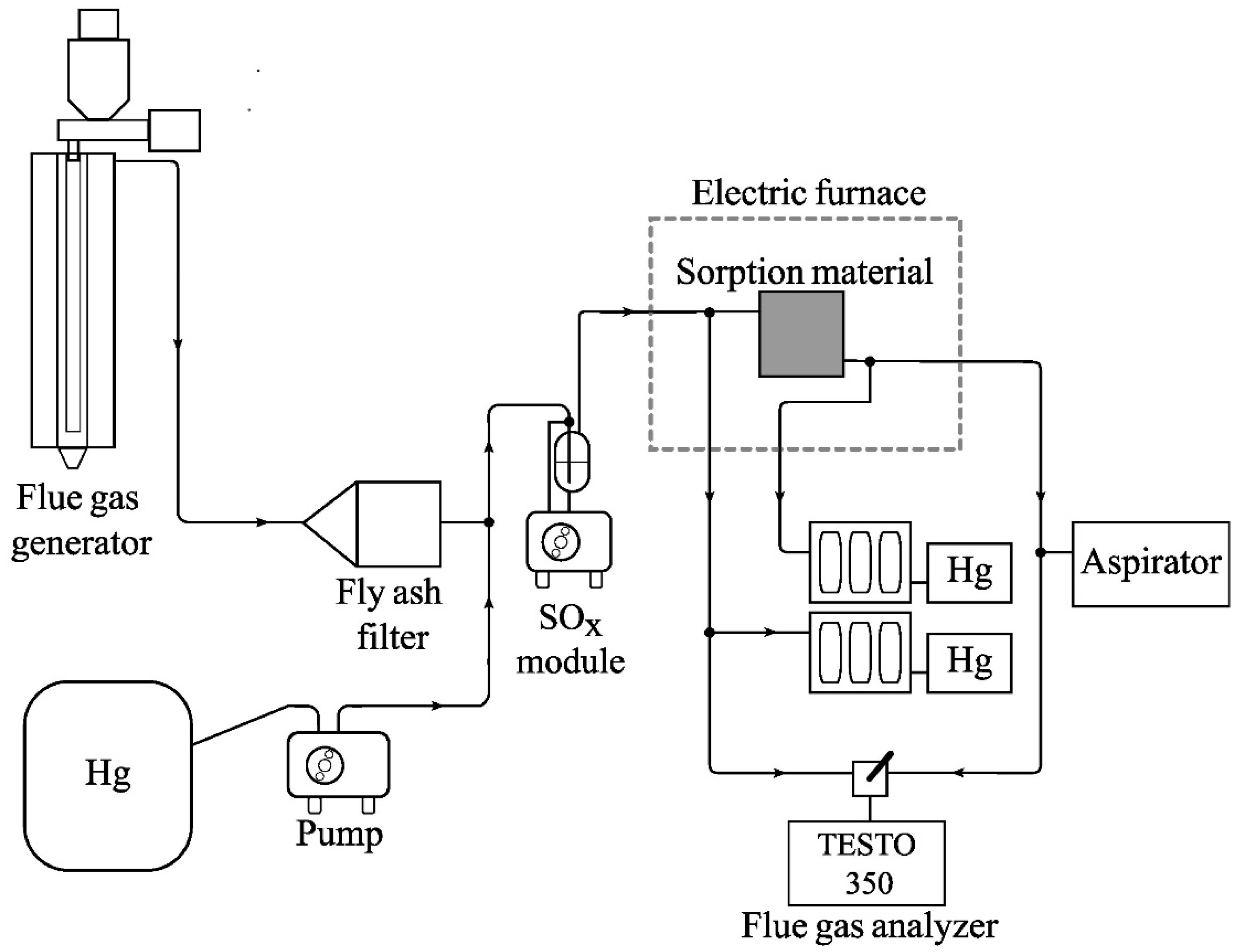
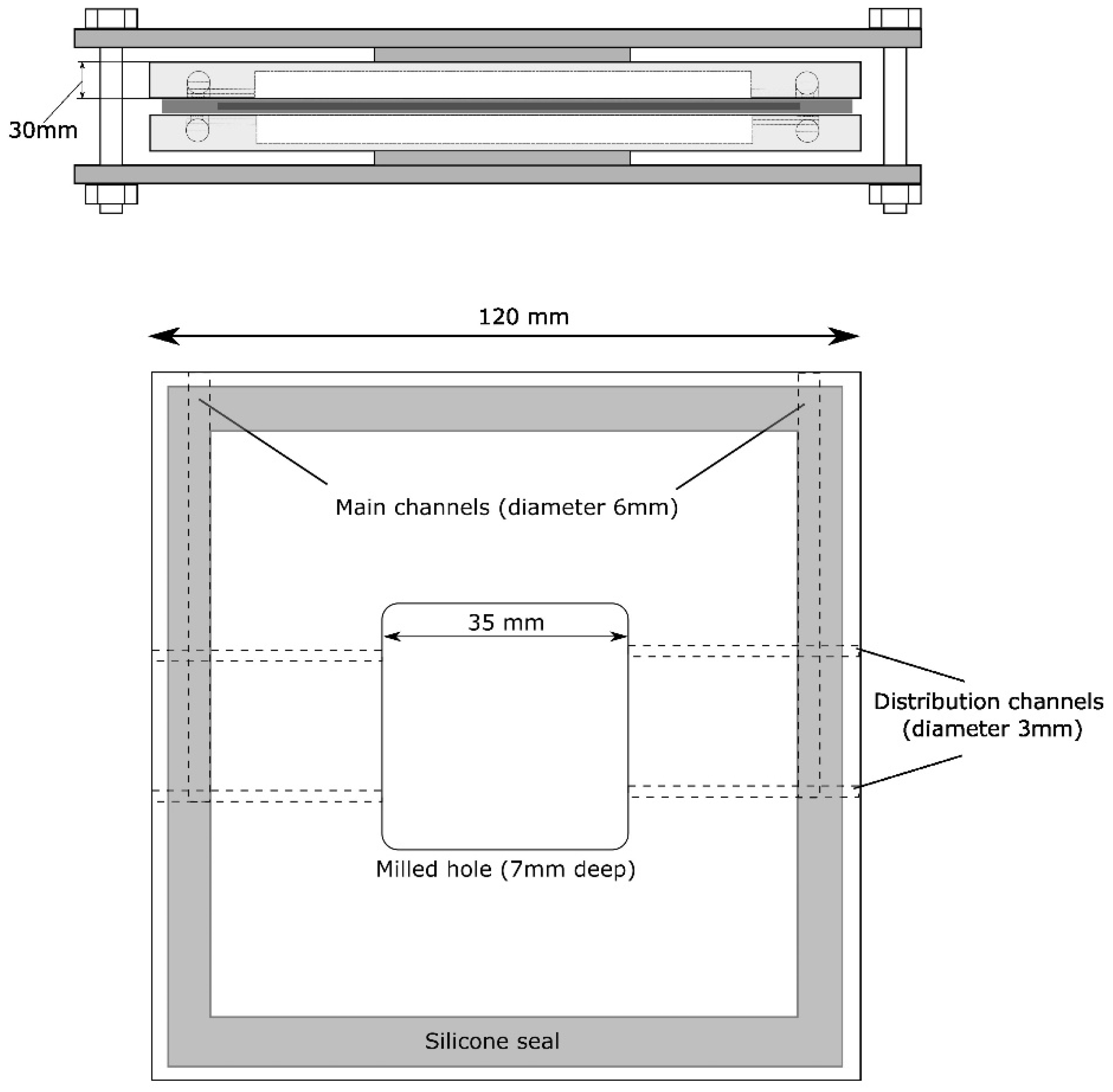
2.2.1. The Laboratory Stand for Testing FSM and SPC in Flue Gas
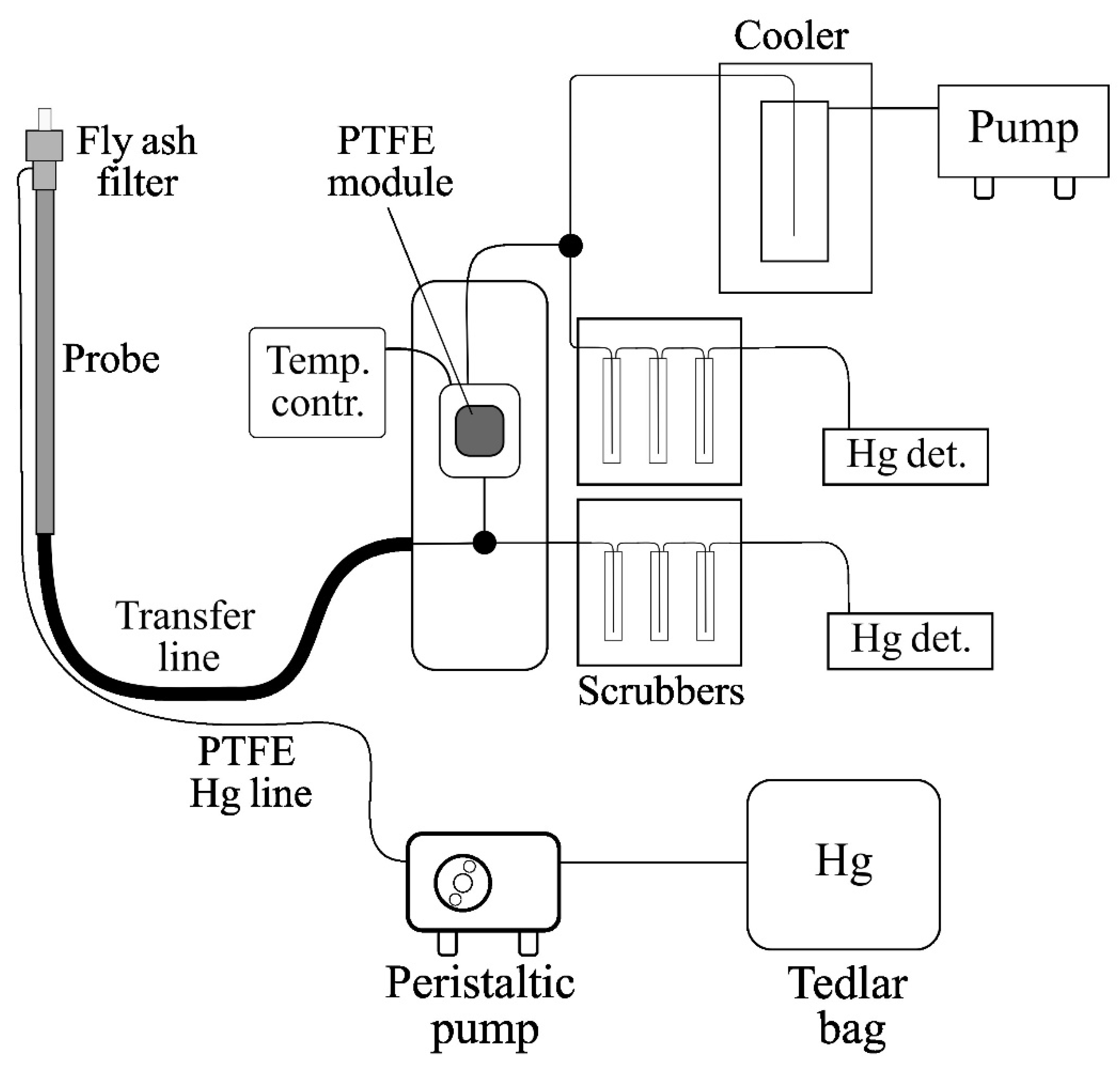
2.2.2. The Industrial System for Preliminary Testing of FSM and SPC Materials
2.2.3. The Larger Scale Industrial System for Testing Composite Materials
2.3. Procedures
2.3.1. The Procedure of Laboratory Tests in an Inert Gas
- A sample of the test FSM was cleaned by blowing off loose AC dust with a jet of air from a compressor (3 bar). The PTFE V1 module was cleaned with 10% nitric acid forced into the module by a peristaltic pump (10 min of cleaning) and distilled water and air blown from an oil-free compressor.
- An FSM sample of 6 × 6 cm was inserted between the elements of the PTFE V1 module. The module was sealed (silicone gaskets) and compressed with a steel clamp. Then, the elements of the test system were connected using 6 mm Teflon tubes.
- A Tedlar® bag with a test gas was connected behind the fly ash filter (Figure 2).
- System sorption control. The tests were performed for all gas streams: 0.4; 1.5; 3.5; 5.5; 7.5; and 9.5 dm3/min.
- Tests of mercury sorption on FSM (successively for temperatures 25 °C, 60 °C, 120 °C). The PTFE V1 module was heated to the appropriate temperature (measurement of gas temperature inside the module chamber). Then, mercury sorption measurements were performed, successively at the flow rates of 0.4, 1.5, 3.5, 5.5, 7.5, and 9.5 dm3/min.
- Cleaning the measuring system. After disassembling the module and removing sorption materials, the channels of both module elements were blown with air from an oil-free compressor (pressure 8 bar), then the elements were rinsed with distilled water, washed with 10% nitric acid, and rinsed again with deionized water. After drying, the Teflon module was assembled and the mercury sorption was monitored. In the case of Hg sorption greater than 10–15%, the washing procedure was repeated.
2.3.2. The Procedure of Laboratory Tests in Flue Gas
- A sample of the tested FSM was cleaned by blowing off loose AC dust with a jet of air from a compressor (3 bar). The PTFE V1 module was cleaned with 10% nitric acid forced into the module by a peristaltic pump (10 min of cleaning) and distilled water and air blown from an oil-free compressor.
- A sample of 6 × 6 cm was inserted between the elements of the PTFE V1 module. The module was sealed (silicone gaskets) and compressed with a steel clamp.
- Heating the module to a temperature 30 degrees higher than the operating temperature.
- Stabilization of the background mercury concentration level (max. background 1.5 µg/m3N).
- Lowering the furnace chamber temperature to the operating temperature.
- Starting the mercury and flue gas sources. Determination of mercury concentration at the level of 20–40 µg/m3N.
- Optional activation of the flue gas desulfurization laboratory unit.
- Measurement of mercury sorption with the flue gas flow of 1.5 dm3/min. Every 20 min, control of SO2 concentration and possible adjustment of desulfurization system—SO2 concentration at the level of about 100 mg/m3N.
- Turning off the flue gas generator and turning off the mercury source.
- Turning off the system and visual inspection of the cell and composite material after cooling.
- System cleaning. After disassembling the module and removing the fixed sorbent material, the channels of both module elements were blown with air from an oil-free compressor (pressure 8 bar). Then, the elements were rinsed with distilled water, washed with 10% nitric acid, and rinsed again with deionized water. After drying, the Tarflen module was assembled and the mercury sorption was monitored. If the Hg sorption of the empty module was greater than 10–15%, the washing procedure was repeated.
2.3.3. The Procedure of Final Industrial Tests
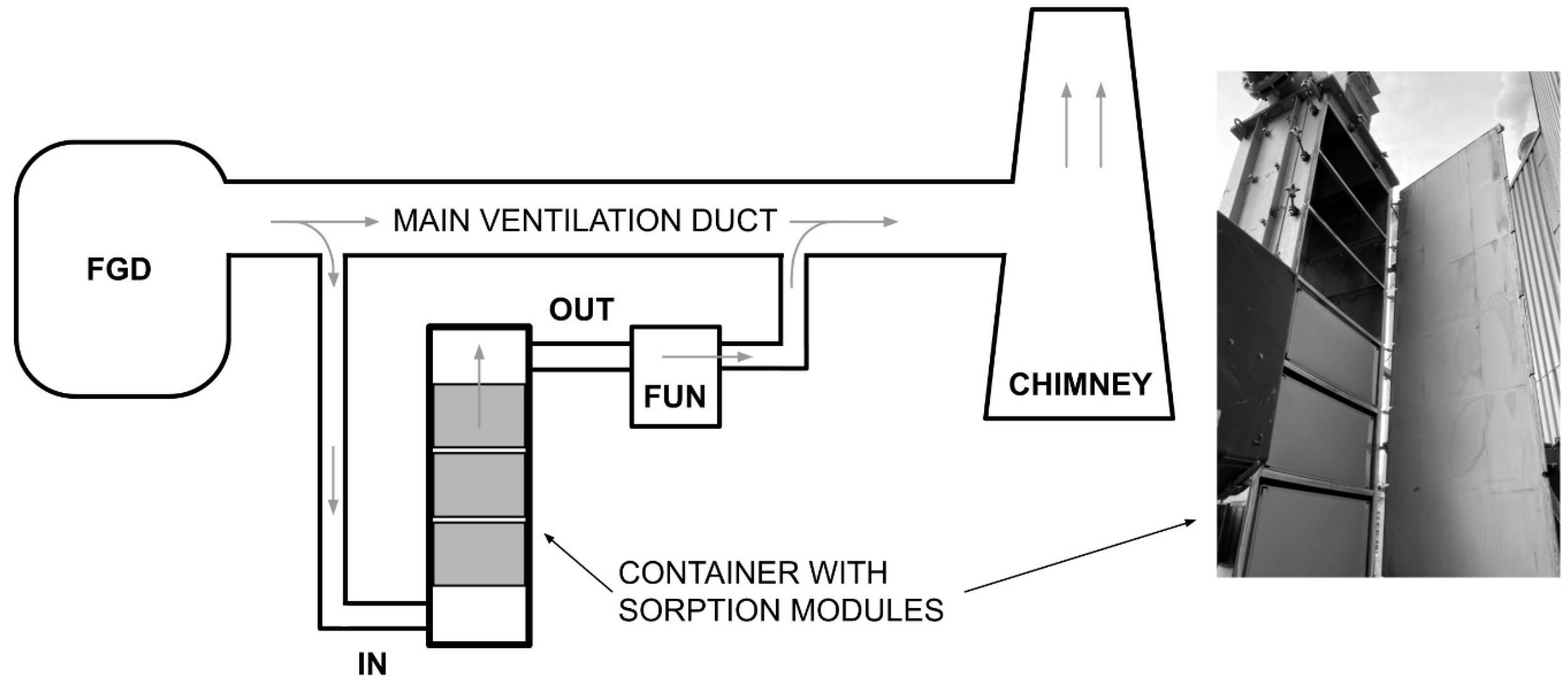
- A container with three sorbent modules made of the same type of sorbent material (FSM or SPC) was mounted in the bypass of the ventilation duct (Figure 6).
- With the use of an additional draft fan, the flue gas flow rate in the bypass was set to 120 m3/min.
- Before starting the actual tests, the modules were conditioned in a working industrial installation for 14 days.
- After the conditioning period, the following parameters were measured every 3 min: mercury concentration (with speciation) in the flue gas before and behind the container with modules (inlet/outlet), oxygen concentration in the flue gas, external temperature, temperature before and behind the container with the tested modules and behind the fan, flue gas flow rate and pressure before and behind the container.
- The container test with FSM modules lasted over 42 days, while the test of the container with SPC modules lasted 34 days.
3. Results and Discussion
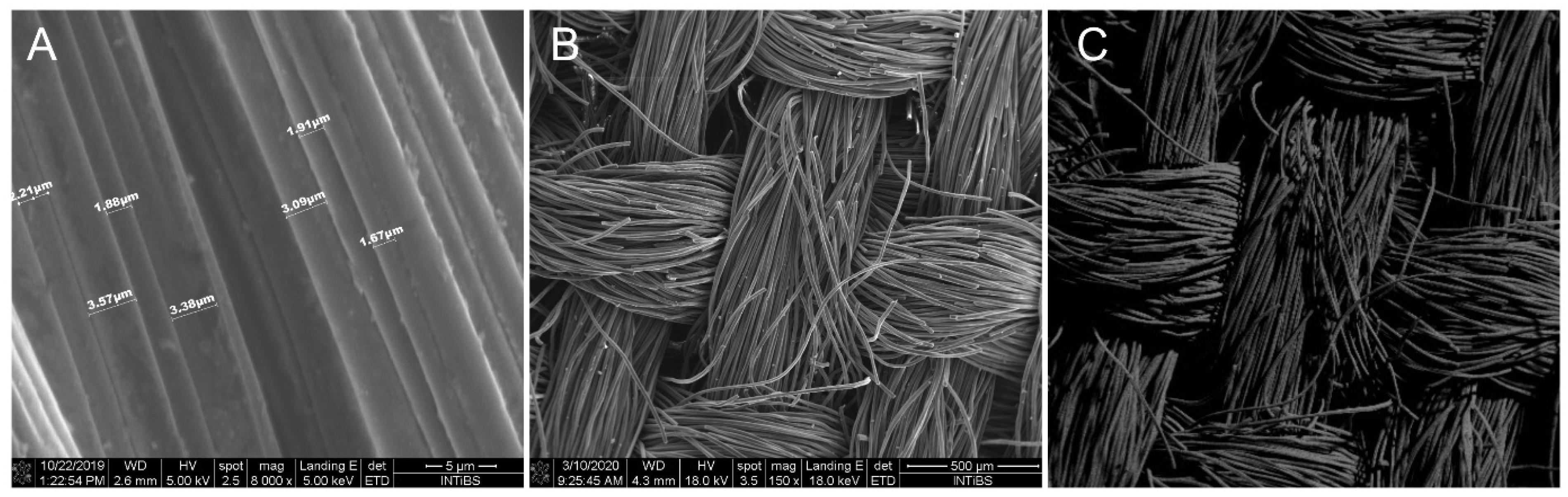
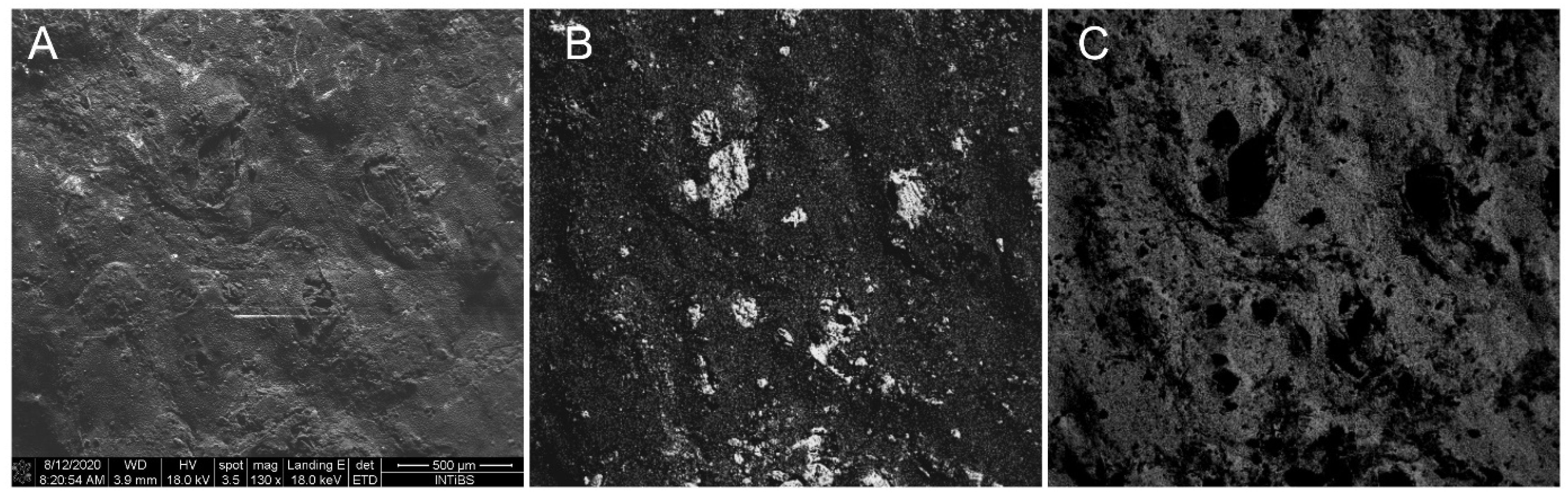
3.1. Basic Tests of FSM and SPC Materials Used for Testing Stands
3.2. Tests of the Laboratory System
3.2.1. Analytical Problems Related to the FSM Used during the Tests of the Systems
3.2.2. The Effect of the Shape of the PTFE V1 Module and Material Area on the Effectiveness of Mercury Removal
3.2.3. The Effect of Test Gas Velocity on Mercury Removal Efficiency
3.2.4. The Influence of Temperature on Mercury Removal Efficiency
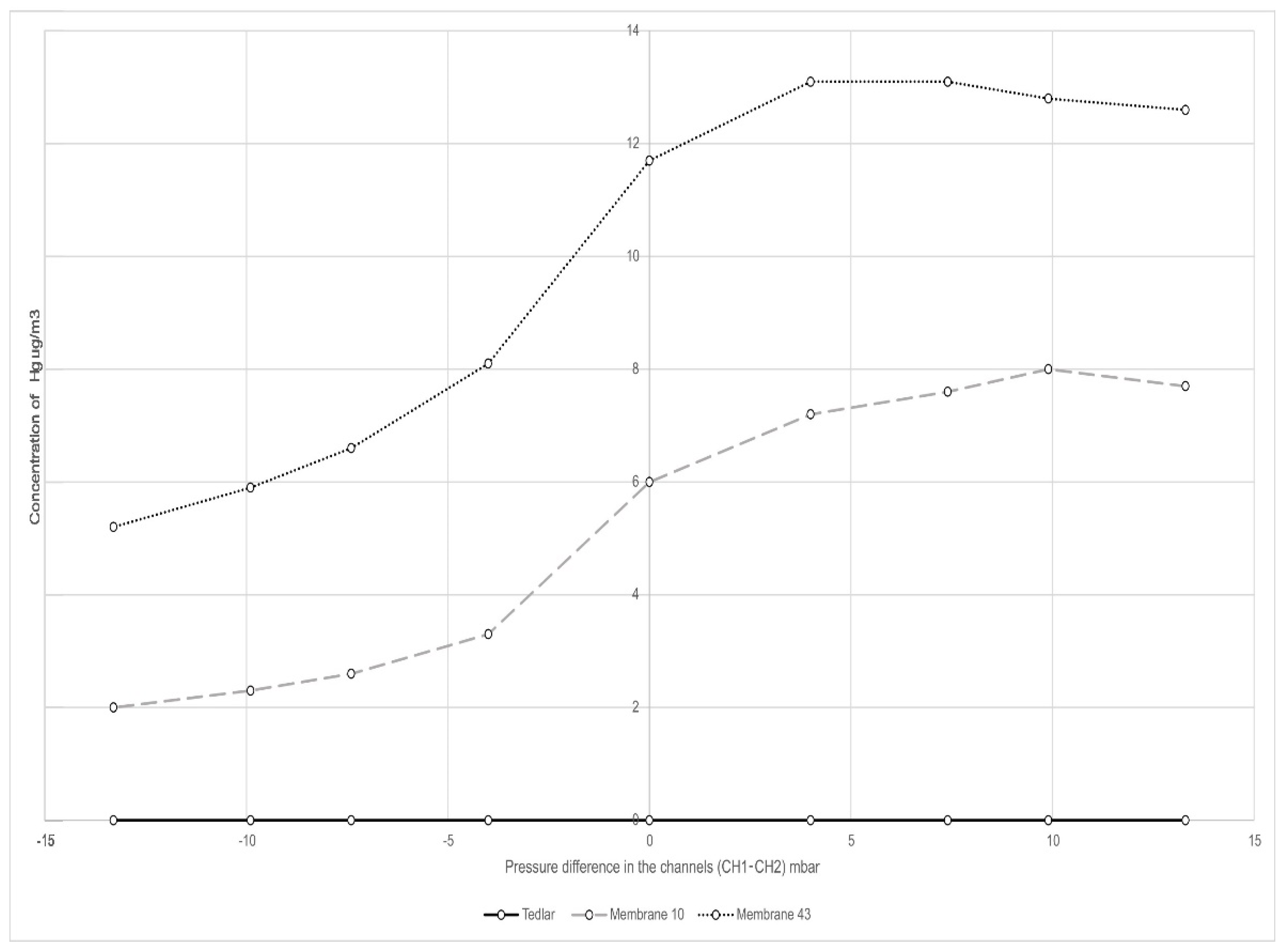
3.2.5. PTFE Membrane Permeability Tests
3.2.6. Laboratory Tests of FSM in Flue Gas
3.3. Results of Tests of Mobile Industrial System
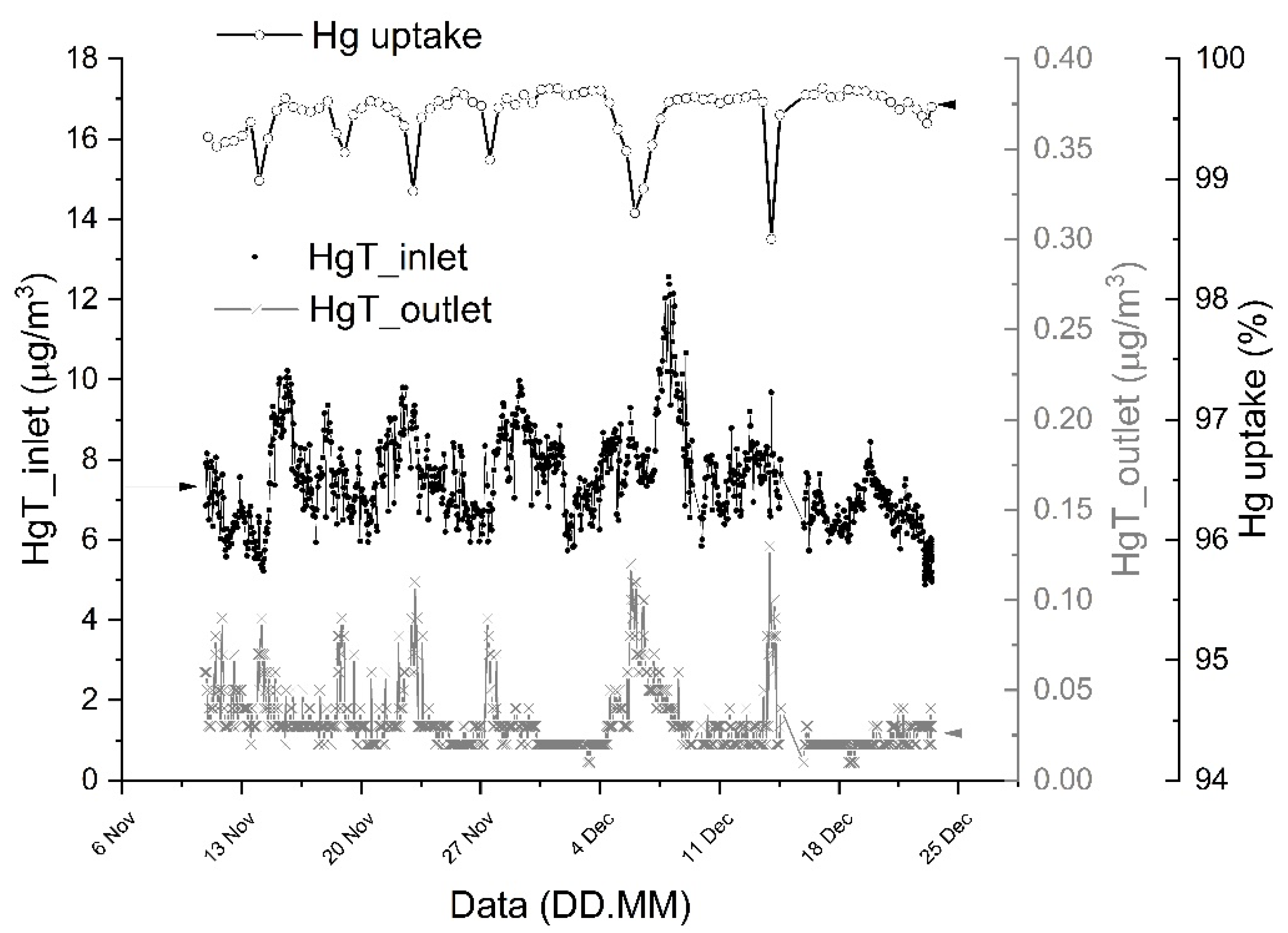
3.4. Final Industrial System Tests
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalisinska, E.; Gorecki, J.; Okonska, A.; Pilarczyk, B.; Tomza-Marciniak, A.; Budis, H.; Lanocha, N.; Kosik-Bogacka, D.I.; Kavetska, K.M.; Macherzynski, M.; et al. Hepatic and Nephric Mercury and Selenium Concentrations in Common Mergansers, Mergus Merganser, from Baltic Region, Europe. Environ. Toxicol. Chem. 2014, 33, 421–430. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environmental Programme. Global Mercury Assessment 2018: Sources, Emissions, Releases and Environmental Transport; UN Environment Programme: Geneva, Switzerland, 2018; pp. 1–60. [Google Scholar]
- Kamata, H.; Ueno, S.; Naito, T.; Yukimura, A. Mercury Oxidation over the V2O5(WO3)/TiO2 Commercial SCR Catalys. Ind. Eng. Chem. Res. 2008, 5, 8136–8141. [Google Scholar] [CrossRef]
- Yang, W.; Adewuyi, Y.G.; Hussain, A.; Liu, Y. Recent developments on gas–solid heterogeneous oxidation removal of Elemental mercury from flue gas. Environ. Chem. Lett. 2019, 17, 19–47. [Google Scholar] [CrossRef]
- Niu, Q.; Luo, J.; Sun, S.; Chen, Q.; Lu, J. Effects of Flue Gas Components on the Oxidation of Gaseous Hg0 by Dielectric Barrier Discharge Plasma. Fuel 2015, 150, 619–624. [Google Scholar] [CrossRef]
- Chang, L.; Zhao, Y.; Li, H.; Tian, C.; Zhang, Y.; Yu, X.; Zhang, J. Effect of sulfite on divalent mercury reduction and re-emission in a simulated desulfurization aqueous solution. Fuel Process. Technol. 2017, 165, 138–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yu, X.; Sun, P.; Zhao, Y.; Zhang, J.; Chen, G.; Yao, H.; Zheng, C. Migration and emission characteristics of Hg in coal-fired power plant of China with ultra low emission air pollution control devices. Fuel Process. Technol. 2017, 158, 272–280. [Google Scholar] [CrossRef]
- Górecki, J.; Łoś, A.; Macherzyński, M.; Gołaś, J.; Burmistrz, P.; Borovec, K. A portable, continuous system for mercury speciation in flue gas and process gases. Fuel Process. Technol. 2016, 154, 44–51. [Google Scholar] [CrossRef]
- Pilar, L.; Borovec, K.; Szeliga, Z.; Górecki, J. Mercury emission from three lignite-fired power plants in the Czech Republic. Fuel Process. Technol. 2021, 212, 106628. [Google Scholar] [CrossRef]
- Górecki, J.; Burmistrz, P.; Trzaskowska, M.; Sołtys, B.; Gołaś, J. Method development and validation for total mercury determination in coke oven gas combining a trap sampling method with CVAAS detection. Talanta 2018, 188, 293–298. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Di, G.; Shang, Y.; Lu, P.; Xu, G.; Liu, M.; Zheng, Y.; Dong, L. Elemental mercury capture from flue gas by magnetic recyclable Fe6Mn1-XCexOy sorbent. Part 1. Performance evaluation and regeneration. Fuel 2021, 304, 120723. [Google Scholar] [CrossRef]
- Wu, S.; Yan, P.; Yu, W.; Cheng, K.; Wang, H.; Yang, W.; Zhou, J.; Xi, J.; Qiu, J.; Zhu, S.; et al. Efficient removal of mercury from flue gases by regenerable cerium-doped functional activated carbon derived from resin made by in situ ion exchange method. Fuel Process. Technol. 2019, 196, 106167. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Chen, M.; Zhang, Q.; Du, L.; Luo, G. Simultaneous removal of NO and elemental mercury from coal-fired flue gas using natural ferruginous manganese ore at low temperature. Fuel 2022, 326, 125118. [Google Scholar] [CrossRef]
- Coker, A.K. Industrial and Laboratory Reactors—Chemical Reaction Hazards and Process Integration of Reactors; John Wiley and Sons: Hoboken, NJ, USA, 2015; ISBN 9780750685245. [Google Scholar]
- Zhou, Q.; Duan, Y.-F.; Zhao, S.-L.; Zhu, C.; She, M.; Zhang, J.; Wang, S.-Q. Modeling and experimental studies of in-duct mercury capture by activated carbon injection in an entrained flow reactor. Fuel Process. Technol. 2015, 140, 304–311. [Google Scholar] [CrossRef]
- Kogut, K.; Górecki, J.; Burmistrz, P. Opportunities for reducing mercury emissions in the cement industry. J. Clean. Prod. 2021, 293, 126053. [Google Scholar] [CrossRef]
- Czarna, D.; Baran, P.; Kunecki, P.; Panek, R.; Żmuda, R.; Wdowin, M. Synthetic zeolites as potential sorbents of mercury from wastewater occurring during wet FGD processes of flue gas. J. Clean. Prod. 2018, 172, 2636–2645. [Google Scholar] [CrossRef]
- Wiatros-Motyka, M.M.; Sun, C.; Stevens, L.A.; Snape, C.E. High capacity co-precipitated manganese oxides sorbents for oxidative mercury capture. Fuel 2013, 109, 559–562. [Google Scholar] [CrossRef]
- Marczak-Grzesik, M.; Budzyń, S.; Tora, B.; Szufa, S.; Kogut, K.; Burmistrz, P. Low-cost organic adsorbents for elemental mercury removal from lignite flue Gas. Energies 2021, 14, 2174. [Google Scholar] [CrossRef]
- Xu, W.; Adewuyi, Y.G.; Liu, Y.; Wang, Y. Removal of Elemental Mercury from Flue Gas Using CuOx and CeO2 Modified Rice Straw Chars Enhanced by Ultrasound. Fuel Process. Technol. 2018, 170, 21–31. [Google Scholar] [CrossRef]
- Wdowin, M.; Macherzyński, M.; Panek, R.; Górecki, J.; Franus, W. Investigation of the sorption of mercury vapour from exhaust gas by an Ag-X zeolite. Clay Miner. 2015, 50, 31–40. [Google Scholar] [CrossRef]
- Tauanov, Z.; Tsakiridis, P.E.; Mikhalovsky, S.V.; Inglezakis, V.J. Synthetic coal fly ash-derived zeolites doped with silver nanoparticles for mercury(II) removal from water. J. Environ. Manag. 2018, 224, 164–171. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Chand, D.; Kótai, L.; Homonnay, Z.; Sajó, I.E.; Váczi, T. Carbon microspheres decorated with iron sulfide nanoparticles for mercury(II) removal from water. J. Mater. Sci. 2020, 55, 1425–1435. [Google Scholar] [CrossRef]
- Budihardjo, M.A.; Wibowo, Y.G.; Ramadan, B.S.; Serunting, M.A.; Yohana, E. Syafrudin mercury removal using modified activated carbon of peat soil and coal in simulated landfill leachate. Environ. Technol. Innov. 2021, 24, 102022. [Google Scholar] [CrossRef]
- Wejkowski, R.; Kalisz, S.; Tymoszuk, M.; Ciukaj, S.; Maj, I. Full-scale investigation of dry sorbent injection for NOx emission control and mercury retention. Energies 2021, 14, 7787. [Google Scholar] [CrossRef]
- Cheng, Y.; Asaoka, Y.; Hachiya, Y.; Moriuchi, N.; Shiota, K.; Oshita, K.; Takaoka, M. Mercury emission profile for the torrefaction of sewage sludge at a full-scale plant and application of polymer sorbent. J. Hazard. Mater. 2022, 423, 127186. [Google Scholar] [CrossRef] [PubMed]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.M.; Mihai, M. An overview on composite sorbents based on polyelectrolytes used in advanced wastewater treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef] [PubMed]
- Knotts, J.; Guenioui, K. A Complete Mercury Control System. Available online: https://www.gore.com/sites/default/files/2017-07/IEEE-article-July-2017.pdf (accessed on 17 November 2022).
- Peng, Y.; Shi, N.; Wang, J.; Wang, T.; Pan, W.-P. Mercury speciation and size-specific distribution in filterable and condensable particulate matter from coal combustion. Sci. Total Environ. 2021, 787, 147597. [Google Scholar] [CrossRef]
- Tong, L.; Yue, T.; Zuo, P.; Zhang, X.; Wang, C.; Gao, J.; Wang, K. Effect of characteristics of KI-impregnated activated carbon and flue gas components on Hg0 removal. Fuel 2017, 197, 1–7. [Google Scholar] [CrossRef]
- Wdowin, M.; Macherzyński, M.; Panek, R.; Wałęka, M.; Górecki, J. Analysis of selected mineral and waste sorbents for the capture of elemental mercury from exhaust gases. Mineralogia 2020, 51, 17–35. [Google Scholar] [CrossRef]
- Stergaršek, A.; Horvat, M.; Kotnik, J.; Tratnik, J.; Frkal, P.; Kocman, D.; Jaćimović, R.; Fajon, V.; Ponikvar, M.; Hrastel, I.; et al. The role of flue gas desulphurisation in mercury speciation and distribution in a lignite burning power plant. Fuel 2008, 87, 3504–3512. [Google Scholar] [CrossRef]
- Commission Implementing Decision (EU) 2021/2326 of 30 November 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32021D2326&from=EN (accessed on 17 November 2022).


| Type of Fixed Sorbent Materials | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| FSM (ACC) | 1146 | 0.55 | 1.9 |
| SPC | 119 | 0.07 | 2.5 |
| Flow Rate | Gas Speed | Cref. | Cśr n = 2 | SD | Hg Removal |
|---|---|---|---|---|---|
| dm3/min | m/s | µg/m3 | µg/m3 | µg/m3 | % |
| 0.4 | 0.1 | 21.8 | 1.7 | 0.0 | 92.2% |
| 1.5 | 0.4 | 26.8 | 1.2 | 0.0 | 95.5% |
| 3.5 | 0.8 | 30.6 | 3.5 | 0.1 | 88.6% |
| 5.5 | 1.3 | 32.0 | 6.3 | 0.2 | 80.5% |
| 7.5 | 1.8 | 31.9 | 10.4 | 0.2 | 67.6% |
| 9.5 | 2.3 | 31.7 | 12.7 | 0.2 | 60.1% |
| Flow Rate | Gas Speed | Cref. | Cśr n = 2 | SD | Hg Removal |
|---|---|---|---|---|---|
| dm3/min | m/s | µg/m3 | µg/m3 | µg/m3 | % |
| 0.4 | 0.1 | 37.2 | 31.5 | 0.4 | 15.5% |
| 1.5 | 0.4 | 37.5 | 34.8 | 0.6 | 7.3% |
| 3.5 | 0.8 | 38.8 | 37.5 | 0.6 | 3.4% |
| 5.5 | 1.3 | 39.2 | 38.2 | 0.3 | 2.6% |
| 7.5 | 1.8 | 38.3 | 37.8 | 0.1 | 1.4% |
| 9.5 | 2.3 | 37.5 | 36.9 | 0.1 | 1.7% |
| Temperature | Flow Rate | Cref. | Cavg n = 2 | SD | Hg Removal |
|---|---|---|---|---|---|
| °C | dm3/min | µg/m3 | µg/m3 | µg/m3 | % |
| 25 | 0.4 | 21.8 | 1.7 | 0,0 | 92.2% |
| 60 | 0.4 | 21.8 | 1.1 | 0,1 | 95.0% |
| 120 | 0.4 | 21.8 | 0.7 | 0,1 | 97.0% |
| λ | CO2 [%] | CCO2 [%] | CCO [mg/m3N] | CSO2 [mg/m3N] | CH2S [mg/m3N] | CNOx [mg/m3N] | CNO [mg/m3N] | CNO2 [mg/m3N] | |
|---|---|---|---|---|---|---|---|---|---|
| WFGD unit ON | 1.65 | 8.3 | 12.4 | 109 | 90–311 | 273 | 1370 | 858 | 57 |
| WFGD unit OFF | 1.63 | 8.1 | 12.6 | 152 | 870–1300 | 270 | 1352 | 850 | 52 |
| Cref. | Cśr n = 5 | SD | Hg Removal % |
|---|---|---|---|
| µg/m3N | µg/m3N | µg/m3N | % |
| 30.8 | 6.1 | 0.3 | 80.2 |
| 71.7 | 14.3 | 0.7 | 80.1 |
| 116.7 | 22.5 | 0.6 | 80.7 |
| Flow Rate | Speed | Conc. | Avg. Conc | SD | Hg Remov. |
|---|---|---|---|---|---|
| dm3/min | m/s | µg/m3N | µg/m3N | µg/m3N | % |
| 0.4 | 0.1 | 35.5 | 5.3 | 0.5 | 85.07% |
| 1.5 | 0.4 | 36.6 | 8.9 | 0.1 | 75.68% |
| 5.5 | 1.3 | 34.7 | 9.2 | 0.1 | 73.49% |
| 9.5 | 2.3 | 31.0 | 13.8 | 0.3 | 55.48% |
| Flow Rate | Speed | Conc. | Avg. Conc | SD | Hg Remov. |
|---|---|---|---|---|---|
| dm3/min | m/s | µg/m3N | µg/m3N | µg/m3N | % |
| 0.4 | 0.1 | 31.2 | 3.8 | 0.2 | 87.8% |
| 1.5 | 0.4 | 33.6 | 6.8 | 0.4 | 79.7% |
| 5.5 | 1.3 | 26.7 | 6.5 | 0.6 | 75.7% |
| 9.5 | 2.3 | 37.7 | 14.8 | 3.0 | 60.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorecki, J.; Macherzynski, M.; Chmielowiec, J.; Borovec, K.; Wałeka, M.; Deng, Y.; Sarbinowski, J.; Pasciak, G. The Methods and Stands for Testing Fixed Sorbent and Sorbent Polymer Composite Materials for the Removal of Mercury from Flue Gases. Energies 2022, 15, 8891. https://doi.org/10.3390/en15238891
Gorecki J, Macherzynski M, Chmielowiec J, Borovec K, Wałeka M, Deng Y, Sarbinowski J, Pasciak G. The Methods and Stands for Testing Fixed Sorbent and Sorbent Polymer Composite Materials for the Removal of Mercury from Flue Gases. Energies. 2022; 15(23):8891. https://doi.org/10.3390/en15238891
Chicago/Turabian StyleGorecki, Jerzy, Mariusz Macherzynski, Jacek Chmielowiec, Karel Borovec, Mateusz Wałeka, Yinyou Deng, Janusz Sarbinowski, and Grzegorz Pasciak. 2022. "The Methods and Stands for Testing Fixed Sorbent and Sorbent Polymer Composite Materials for the Removal of Mercury from Flue Gases" Energies 15, no. 23: 8891. https://doi.org/10.3390/en15238891
APA StyleGorecki, J., Macherzynski, M., Chmielowiec, J., Borovec, K., Wałeka, M., Deng, Y., Sarbinowski, J., & Pasciak, G. (2022). The Methods and Stands for Testing Fixed Sorbent and Sorbent Polymer Composite Materials for the Removal of Mercury from Flue Gases. Energies, 15(23), 8891. https://doi.org/10.3390/en15238891








