1. Introduction
The industries for goods, energy, and transportation rely on fossil fuels. However, society has become aware that the usage of fossil fuels negatively affects both the environment and living beings. Additionally, the fossil fuel resources will deplete since they are finite resources [
1,
2]. With the “European Green Deal”, the European Union attempts to shift from a fossil-fuel-dependent economy to a sustainable and environment-friendly economy [
3].
Scientists have proposed lignocellulosic biomass as an alternative natural resource to fossil fuels. It is an abundant and renewable resource and has the potential to substitute fossil fuels in the production of chemicals and fuels. As a result, the idea is to rebuild old crude oil refineries to biorefineries [
4]. However, the usage of lignocellulosic biomass brings problems, such as high production costs or low production yields. Some problems are not yet solved.
The hypernym lignocellulosic biomass refers to all biomass, which consists of the structural polymers cellulose, hemicellulose, and lignin. These can be woody materials, such as spruce, willow, and birch wood, or agricultural residues, such as wheat straw, or municipal green waste [
5]. Since the term covers many different types of plant biomass, it is not surprising that the processing of each biomass varies. Thus, different processing methods have been developed for different biomass, leading to different products, but none can be universally applied on every biomass [
6,
7]. However, for a biorefinery, it is necessary to be feedstock flexible so that it can adjust itself to the market situation. This is why three different biomass types were chosen for testing the feedstock flexibility of the used pretreatment method.
Lignocellulosic biomass can be converted to chemicals and fuels by thermochemical, chemical, and biological processes [
8,
9]. In this paper, the processing steps from bioethanol production were chosen. These were pretreatment, enzymatic hydrolysis, and fermentation. Pretreatment is necessary for opening up the complex structure of lignocellulosic biomass, otherwise hydrolysis and fermentation will be ineffective. As a negative side effect, lignocellulosic inhibitors can be formed during pretreatment, which impede enzymatic hydrolysis and fermentation. As a result, the biomass is often detoxified after pretreatment. In this paper, the biomass was detoxified by filtration.
The hydrolysis step is the chemical conversion of sugar polymers, such as hemicellulose and cellulose, to sugar monomers. This chemical reaction is catalyzed with enzymes or acids. Afterwards, microorganisms ferment the sugar monomers to the desired products, such as ethanol and lactic acid. After fermentation, the product is purified and concentrated. This includes steps such as filtration and distillation [
10,
11].
The whole production chain has inputs, waste, and losses. Lignin is a common waste in this production chain, and plant operators often valorize lignin for just its heating value in boilers.
Recently, lignin has received more attention as a potential source for aromatic compounds since it is a heterogeneous polymer consisting of different phenolic compounds. It has the potential to substitute the BTX hydrocarbons from crude oil, which are used for the production of different polymers and resins. Yet, the extraction of lignin from lignocellulosic biomass is still in the research stage. Additionally, the lignin after pretreatment and hydrolysis is chemically different to the native lignin. As a result, it is important to extract lignin before pretreatment in order to receive unmodified lignin. The extraction of lignin with ionic liquids is one example for gaining lignin in its original form [
12,
13,
14,
15,
16].
During the processing of lignocellulosic biomass, wastewater is generated, containing inhibitors and sugars. This wastewater needs to be treated before it can be released into the environment. An economical treatment would be the extraction of chemicals from wastewater or the anaerobic digestion of wastewater [
17].
Product losses occur during pretreatment, detoxification, and fermentation. As a result, it is important to keep losses as low as possible in order to maximize the production of desired chemicals, therefore making the overall processing more economically feasible.
The most important inputs in the processing of lignocellulosic biomass are energy in the form of electricity and heating and chemicals, such as enzymes, water, and feedstock. All those inputs are expenses. Therefore, an optimum between high product output and low material input should be targeted [
18,
19].
While keeping the process parameters, waste, inputs, and product losses in mind, a two-step pretreatment process was developed. The process is meant to be easily applicable in industry, creating high product yields and avoiding the formation of inhibitors. As a pretreatment method, the “nitrogen explosive decompression” from [
20] was chosen. The pretreatment method is chemical-free, except for water and nitrogen. Additionally, the method is efficient in solubilizing hemicellulose of barley straw at 175 °C [
21]. This is why, for the first pretreatment, the incubation temperature was set to 175 °C. For the second pretreatment, a temperature of 200 °C was chosen since the glucose yields from aspen wood were the highest at this temperature [
22]. With solubilizing hemicellulose in the first pretreatment, the formation of inhibitors, such as furfural and hydroxymethylfurfural, can be avoided. Additionally, the majority of sugars from hemicellulose do not degrade during pretreatment [
23]. For detoxifying pretreated biomass, a solid–liquid filtration with a fine metal sieve was selected. This is a simple method and can be easily applied in an industrial production process, as well. In research papers, the biomass is often dried or washed with water after pretreatment before it is set up for hydrolysis. Yet, washing creates wastewater, and drying consumes energy, therefore those process steps should be avoided. This is another reason why filtration without rinsing was selected as a detoxification method.
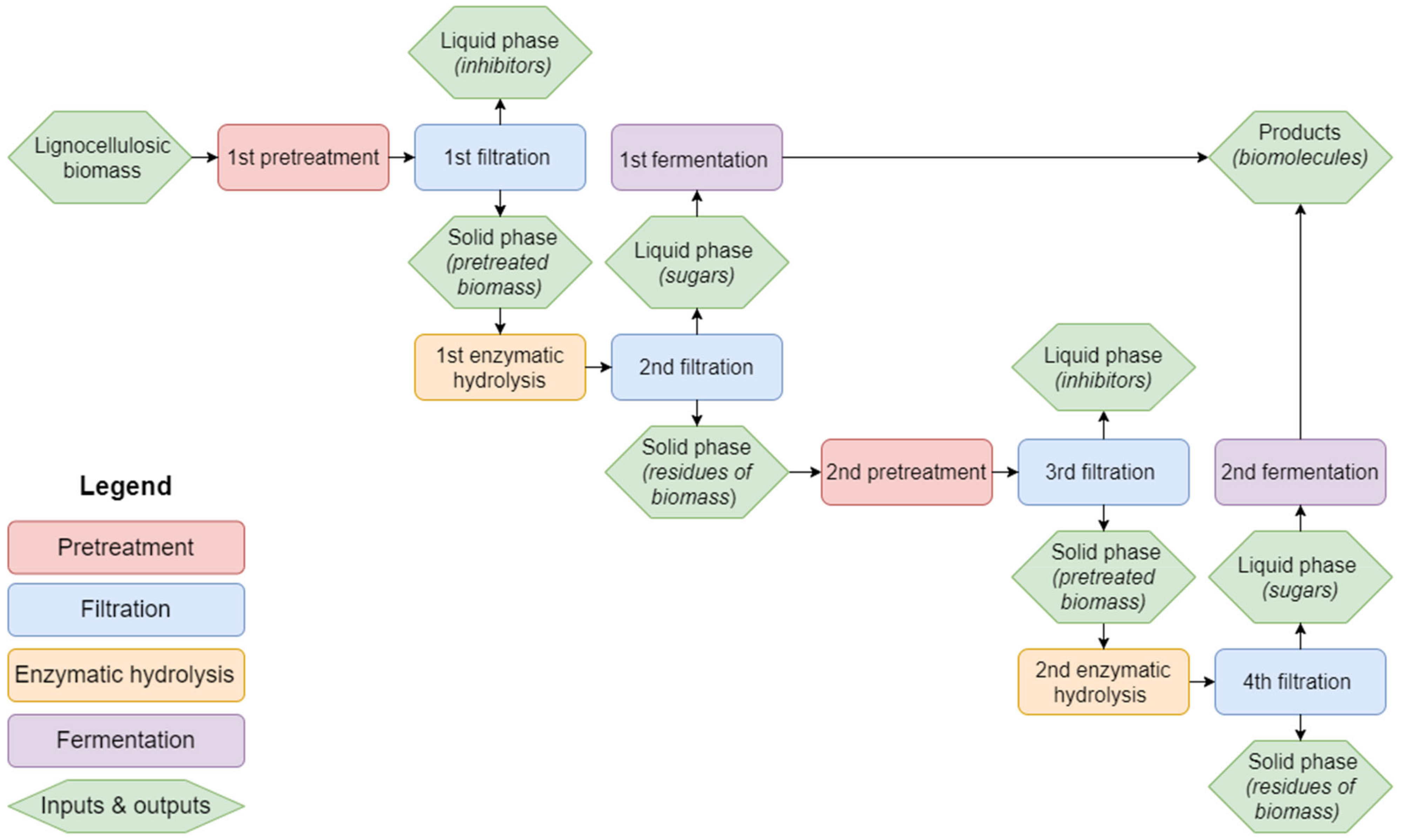
Figure 1 gives an overview of the process steps used in this experimental setup. Further details are explained in
Section 2.
2. Materials and Methods
For this experimental setup, barley straw (from Hordeum vulgare), aspen wood (from Populus tremula), and debarked pine wood (from Pinus sylvestris) were tested. All three plant species are common to northern Europe and widely used in the Estonian agricultural and forestry industry. Barley straw represents the agricultural residues or herbaceous plants, aspen wood represents the hardwoods and pine wood represents the softwoods. Since the composition of biomass varies from one plant species to another, those three materials were selected to test the feedstock flexibility of the pretreatment method.
2.1. Biomass Origin and Characterization
Fully mature barley straw was harvested from a haplic luvisol (hypereutric) near Tartu in Estonia. Wood shavings from aspen and debarked pine were collected from a forest nearby the village Maaritsa (around 30 km south of Tartu).
The moisture of the used biomass was analyzed with an infrared moisture analyzer (Kern MLS 50-3D, Balingen, Germany). The plant fractions (hemicellulose, cellulose, lignin, and extractives) were determined using an ANKOM 2000 Automated Fiber Analyzer (Macedon, NY, USA) and its connected analytical protocols [
24]. The ash content of the biomass was determined according to the NREL/TP-510-42622 protocol. All results from the fiber analysis and the ashing were recalculated on a dry-weight basis. All analyses were conducted at least in triplicates.
2.2. Two-Step Pretreatment of Biomass
The pretreatment method from [
20] was used in this experimental setup. Before pretreatment, raw aspen and pine wood shavings were dried in a drying oven at 60 °C for 48 h until the moisture content was less than 10%. Barley straw came already sun-dried from the field. Next, the biomass was milled and sieved with a Cutting Mill SM 100 comfort (Retsch GmbH, Haan, Germany) to a particle size of 3 mm or less.
For pretreatment, 60 g of dry biomass was weighted into a pressure reactor (Parr instruments, net volume 1.8 L, working volume 1 L, heated with an electrical heating jacket,
Figure 2) and mixed with 480 mL of deionized water (biomass-water ratio 1:8). The reactor was flushed with nitrogen (purity 5.0), after which it was closed and pressurized to 10 bar. After pressurization, the reactor was heated to 175 °C and incubated for 1 min at this temperature. The reactor was cooled at room temperature to 80 °C, after which the pressure was released in an explosive manner through a ball valve. The pretreated biomass was transferred to a metal filter press (volume 800 mL or 350 mL, depending on the amount of biomass). The biomass settled down for 10 min in the vessel and was then filtered. The filtrate (1st filtration) and the solid residues (1st filtration) were transferred into separate 1 L Erlenmeyer vessels. Both fractions were enzymatically hydrolyzed for 48 h (for more information see
Section 2.3). After hydrolysis, the solid residues (1st filtration) were filtered again and additionally washed with 150 mL of deionized water. Filtrate (2nd filtration) and washing solution (2nd filtration) were caught separately and set up for fermentation (more information in
Section 2.4).
The solid residues (2nd filtration) were transferred to the reactor and mixed with 100 mL of deionized water or less until a similar consistency was achieved as in the first pretreatment. The reactor was closed and pressurized with nitrogen. The pretreatment parameters were the same as in the first pretreatment, except that the set temperature was 200 °C. After pretreatment, the steps of filtration, hydrolysis, and fermentation were performed the same way as in the first pretreatment.
For each material, the experiments were conducted at least in triplicates. All the equipment in the experiments was sterilized before usage.
2.3. Enzymatic Hydrolysis of Pretreated Biomass
After the first pretreatment and the first filtration, the filtrate and solid residues were transferred into two separate 1 L Erlenmeyer vessels. The solid residues (1st filtration) were mixed with deionized water, and the pH was adjusted to 5 using 1 molar calcium carbonate (CaCO3) or 1 molar hydrochloric acid solution (HCl). The pH was measured with a pH-meter (SevenCompact pH/Ion S220 Mettler-Toledo, Greifensee, Switzerland).
Buffers, such as citric acid, were not used, as they would be too expensive for an industrial scale process. As a result, cheap chemicals were selected for pH regulation. CaCO
3 was chosen because Ca
2+ ions have shown to decrease the inhibiting effect of acetic acid on
Saccharomyces cerevisiae [
25]. Acetic acid is generated after the pretreatment of lignocellulosic biomass because the acetyl-groups of hemicellulose cleave off during pretreatment [
11]. HCl was chosen because it is a cheap chemical and often used in industry. Mostly CaCO
3 was used for the pH adjustment since after the pretreatment pH often dropped below 5.
After pH adjustment, 30 mL of commercial enzyme cocktail ACCELLERASE 1500 (Genencor, enzymes from deactivated Trichoderma reesei, containing cellulases and hemicellulases, endoglucanase activity 2200–2800 CMC U/g, beta-glucosidase activity 450–775 pNPG U/g, density (25 °C) 1.04 g/mL, solid load 108.4 mg/mL, protein content 76 mg/mL (determined by Pierce BCA Protein Assay), more information available from enzyme producer) was added to the solid residues (1st filtration), which is equivalent to 0.5 mL of enzyme solution or 38 mg enzyme protein per 1 g of dry biomass. Afterwards, the vessel was filled up to 800 mL with deionized water and sealed with aluminum foil.
The filtrate (1st filtration) was processed the same way as the solid residues (1st filtration), except that the vessel was filled up to 1000 mL with deionized water. Then, the filtrate and solid residues were hydrolyzed for 48 h at 50 °C and 250 rpm on a shaker-incubator (IKA KS 4000i control, Staufen, Germany). After hydrolysis, samples were taken from the filtrate (1st filtration), and the pH of the solution was measured. The solid residues (1st filtration) were filtered and washed (see
Section 2.2). The hydrolysis procedure after the second pretreatment was the same.
2.4. Fermentation of the Hydrolysate
The filtrate (2nd filtration) and washing solution (2nd filtration) were separately collected after filtration. The solid residues (2nd filtration) were prepared for the second pretreatment.
The pH of the filtrate (2nd filtration) and washing solution (2nd filtration) was adjusted to 5. Thereafter, the filtrate was filled up to 800 mL and the washing solution to 200 mL with deionized water. Samples were taken from both solutions. Afterwards, 2.5 g of dry yeast (Superjæst T3) was added to the filtrate. After the yeast had dissolved, the washing solution was added to the filtrate, and the vessel was closed with an airlock for anaerobic conditions. The hydrolysate was fermented for 7 days at 32 °C and 150 rpm on a shaker-incubator. After this, the pH of the fermentation broth was measured, and samples were taken. The fermentation procedure was the same after the second pretreatment. All taken samples were frozen until further analysis, if not otherwise mentioned.
2.5. Analysis of Processed Biomass
2.5.1. HPLC Analysis
The taken samples were thawed at room temperature. Following this, they were centrifuged at 10,000 rpm for 5 min twice and, each time, the supernatant was transferred to a new micro centrifuge tube. In a final step, the samples were filtered with centrifuge filters (Thermo Scientific, Waltham, MA, USA, PTFE, 0.2 μm) and diluted into vials (dilution 1:3). A HPLC system (Shimadzu Prominence-i LC-2030 3D Plus (Kyoto, Japan), equipped with a Refractive Index Detector (RID) and Photodiode Array Detector (PDA)) was used for qualification and quantification of the samples. The plant sugars glucose, mannose, arabinose, galactose, cellobiose, and xylose were separated with an Aminex HPX-87P column (300 × 7.8 mm, BIO-RAD, Hercules, CA, USA) and detected with the RID. The main column was protected by a Micro-Guard De-Ashing precolumn (BIO-RAD). The mobile phase was autoclaved-degassed-deionized water at a flow rate of 0.6 mL/min. The oven temperature was set to 80 °C, and the detector temperature was set to the highest value. The sample injection volume was 5 µL.
The inhibitors and fermentation products (formic acid, acetic acid, glycerin, ethanol, lactic acid, hydroxymethylfurfural (HMF), furfural and levulinic acid) were separated using a Rezex ROA-Organic Acid H+ column (300 × 7.8 mm). All molecules, except for HMF and furfural, were detected with the RID. Furfural and HMF were detected with the PDA at 283 nm. The mobile phase was an autoclaved-degassed-deionized 5 mM H2SO4 solution at a flow rate of 0.5 mL/min. The detector and oven temperature were set to 50 °C and the sample injection volume was 10 µL. The HPLC parameters, columns, and mobile phases are based on the NREL/TP-510-42623 protocol and modified according to our research purposes.
2.5.2. Hydrolysis Efficiency Calculations and Data Analysis
The hydrolysis efficiency
EH (%) of cellulose
mcel (g) to glucose
mglu (g) was calculated according to Equation (1).
The stoichiometric conversion factor 1.11 comes from the hydrolysis reaction of cellulose to glucose whereby water is also consumed.
All data analysis and calculations were conducted in Microsoft Excel 2016.
2.5.3. pH Measurements
Changes of the pH during each process step (pretreatment, filtration, hydrolysis, and fermentation) were measured and monitored. This includes pH adjustments before hydrolysis and fermentation.
2.5.4. Scanning Electron Microscopy of Pretreated Biomass
Surface changes of the biomass after pretreatment and enzymatic hydrolysis were analyzed with a scanning electron microscope (SEM). Samples for each biomass were prepared, as described in
Section 2.2 and
Section 2.3. Just the fermentation part was left out. After each filtration step, a sample from the solid residues was taken and dried for 24 h at 40 °C and then stored at room temperature until further use. Before acquiring the SEM pictures, the samples were coated with carbon using an Emitech K950 carbon coater (Lewes, UK), and with gold, using a Cressington 108 auto sputter coater (Watford, UK). The SEM pictures were taken with a Phenom XL G2 tabletop SEM (Waltham, MA, USA), which used an acceleration voltage of 15 kV. For detection, the backscatter and secondary electron detectors were used, from which the detection signals were superimposed.
2.5.5. Verification of Metabolites from Yeast
With the exception of ethanol, other metabolites, such as lactic acid, acetic acid, and glycerin, were found in the fermentation broth after fermentation.
Saccharomyces cerevisiae is well known to produce ethanol from glucose through the glycolysis pathway when the environmental conditions are anaerobic [
26]. Therefore, it was surprising that the used yeast strain produced three other metabolites in higher concentrations. In order to exclude a contamination during experiments, it was tested whether the yeast strain is able to produce the same metabolites in an artificial medium.
The nutrient concentrations for the artificial media were taken from the hydrolysis results of barley straw after the first pretreatment at 175 °C and 48 h of hydrolysis. The media contained 17 g/L glucose, 7 g/L xylose, 0.4 g/L arabinose, 1 g/L acetic acid, and 1 g/L peptone. The sugars in the artificial media represent hydrolysate sugars, acetic acid represents the degradation product from acetylated hemicellulose, and peptone represents the degraded enzymes after pretreatment and hydrolysis. The chemicals used for the media were either chemical or analytical grade. After preparation of the medium, it was autoclaved, and its pH was adjusted to 5 with CaCO3 as in previous experiments. Acetic acid was added after autoclaving so that acid-catalyzed reactions during the sterilization process could be avoided.
A 250 mL Erlenmeyer vessel was filled with 200 mL of media, and 1 g of dry yeast was added. The vessel was closed with an airlock and fermented on the shaker-incubator at 32 °C and 150 rpm for 7 days. After this period, the pH of the solution was measured, and samples were taken. The barley straw simulation was conducted at least in triplicates.
3. Results
3.1. Biomass Characteristics
Table 1 shows the main characteristics of untreated barley straw, aspen wood, and pine wood. It gives an overview of the biochemical composition of the used biomass and helps to understand in which quantities sugars can be gained and how recalcitrant the material is. Barley straw has the highest hemicellulose and extractive content and the lowest lignin and cellulose content of the three materials. Aspen wood has the highest cellulose content and pine wood the highest lignin content. Therefore, it can be presumed that barley straw is the most accessible for enzymes. This is due to the fact that the used pretreatment method is efficient in solubilizing hemicellulose, which is a major part of barley straw, and the extractives are easily washed out with water [
21]. Additionally, barley is an annual plant, which means it degrades naturally faster compared to woody materials.
Aspen wood is not as easily degradable as barley straw. However, it is more easily degradable than pine wood. With its high cellulose content of 50.68%, it can be expected that high glucose yields can be achieved [
10]. The material also has a lower lignin content than pine wood, which means it is more vulnerable to fungal and environmental decay and has less mechanical strength [
27].
Pine wood is the most recalcitrant material of the three materials and well protected from environmental impacts. Its high lignin content is partly responsible for this resistance. Additionally, pine wood is a preferred building material, where durability is a desired attribute. Thus, it can be concluded that the degradability of materials goes as follows: barley straw > aspen wood > pine wood.
3.2. Sugar Yields from Pretreated Biomass
Barely straw, aspen wood, and pine wood were processed, as described in
Figure 1.
Figure 3 shows the hydrolysis yields for each biomass after the first and second hydrolysis. Additionally, the sugar losses after the first and third filtration are displayed. Barley straw had the highest sugar yields. The overall glucose and xylose yields with filtration losses were 29.22 g and 13.75 g per 100 g of dry biomass (DB), respectively. The overall hydrolysis efficiency was 73.69%, which is 27.09% more than what [
21] achieved with a single pretreatment. Most of the glucose and xylose was released after the first hydrolysis and pretreatment at 175 °C, which means that the sugar losses were the highest after the first filtration.
Aspen wood released nearly the same amount of sugars as barley straw. The overall amount of glucose and xylose were 29.19 g and 12.99 g per 100 g DB, respectively. However, the overall hydrolysis efficiency of 51.90% was lower compared to barley straw. Yet, it is still 22.36% more than what [
22] achieved with a single pretreatment of aspen wood. In comparison to barley straw, most sugars were released from aspen wood after the second pretreatment at 200 °C and second hydrolysis. After the second pretreatment, the sugar losses were the highest as well. The increased sugar release after the second pretreatment indicates that the first pretreatment temperature was not high enough to properly open up the biomass structure. Higher pretreatment temperatures during first and second pretreatment could probably increase the hydrolysis efficiency. However, a temperature increase could also enhance the formation of inhibitors, which then could shatter the benefits of an improved hydrolysis. This speculation needs further investigation.
The lowest sugar yields were gained from pine wood, which were 5.19 g glucose and 1.93 g xylose per 100 g DB. Yet, the highest mannose yield, with 3.44 g per 100 g DB, was achieved from this biomass. Aspen wood also released some mannose, but only half of the amount that pine wood did. The higher mannose yield compared to the other materials can be explained by the fact that the hemicellulose of softwoods is mainly built up from mannan polysaccharides [
28]. Traces of arabinose and galactose were released as well (less than 1 g per 100 g DB). The overall cellulose conversion efficiency was 12.15%, which indicates that the enzymatic hydrolysis barely worked. Possible reasons why the enzymatic hydrolysis with this material did not work will be discussed in detail in
Section 4.2.
3.3. Lignocellulosic Inhibitors and pH
Depending on the pretreatment conditions, different lignocellulosic inhibitors can be formed, which impede the enzymatic hydrolysis and fermentation of pretreated biomass. At a pretreatment temperature of 175 °C, [
20] and [
22] showed that the fermentation of barley straw and aspen wood was impeded due to lignocellulosic inhibitors. Therefore, in this experimental setup, the biomass was filtered after pretreatment. The inhibitors, such as acetic acid, formic acid, levulinic acid, HMF, and furfural, have shown to impede fermentation processes [
11]. As a result, each taken sample was analyzed for those inhibitors. Formic acid and levulinic acid were not detected in any of the samples. Traces of furfural and HMF were found (less than 0.4 g per 100 g of DB) after pretreatment. Those traces disappeared after fermentation. The yeast probably reduced them to their corresponding alcoholic forms (furan-2,5-diyldimethanol and furan-2-ylmethanol), which are less toxic for yeast [
29]. Acetic acid was the most prevalent inhibitor after pretreatment and reached a concentration of 4.11 g per 100 g DB in the case of aspen wood. [
22] achieved, with the same pretreatment method and biomass, similar results. Overall, it can be concluded that the amount of formed and identified inhibitors was low. Therefore, it can be reconsidered, whether the filtration step after the first and second pretreatment is necessary for the detoxification of pretreated biomass since the formation of known inhibitors is already avoided with the two-step pretreatment approach. Additionally, the loss of sugars due to filtration can be prevented. However, this speculation needs further investigation since it is possible that some unidentified inhibitors were formed during pretreatment, and they still have a severe effect on the enzymatic hydrolysis and fermentation.
The pH plays a vital role in the processing of lignocellulosic biomass and affects each process step. For example, a low pH during pretreatment improves the enzymatic digestibility of lignocellulosic biomass, but also increases the formation of inhibitors [
30]. A low or high pH can deactivate enzymes or yeast cells. As a result, it is important that the pH is adjusted to the optimum for each process step.
After the first pretreatment at 175 °C, the pH ranged between 4.05 to 4.76 and, after the second pretreatment at 200 °C, it ranged between 3.63 to 3.97. The solutions of aspen wood and pine wood were more acidic after pretreatment compared to barley straw.
Before enzymatic hydrolysis, the pH of each hydrolysate was adjusted to 5. During hydrolysis the pH dropped between 4.03 and 4.81, depending on the biomass and previous pretreatment temperature. Aspen wood had the most acidic solution after hydrolysis, which indicates that the acetylated xylan backbone of aspen wood hemicellulose was hydrolyzed to acetic acid and xylose [
11]. This also explains the acetic acid concentration of 4.11 g per 100 g DB.
Before fermentation, the pH was again adjusted to 5 and dropped between 3.63 and 4.17 after seven days of fermentation. This vast pH drop indicates that the yeast also produced acids, which lowered the pH of the solution. In the fermentation broth, acetic acid, and lactic acid were detected, which explains the pH drop during fermentation.
These results show how important it is to adjust the pH during enzymatic hydrolysis and fermentation, since it can change significantly during each process step and, thus, be outside of the optimum pH range. The ACCELLERASE 1500 enzyme producer claims that the optimum pH range for their enzymes lays between 4 and 5 and that they can be deactivated if the pH is lower than 3.9 or higher than 7 [
31]. The optimum pH range for
Saccharomyces cerevisiae for maximal ethanol production lays between 5 and 5.5 [
32]. In this experimental setup, CaCO
3 without any buffer, such as citric acid, was used to adjust and maintain a stable pH. Since the pH fluctuated in each process step, it is necessary to monitor and adjust the pH constantly in an industrial process in order to keep the production stable. The usage of buffers in an industrial process is too expensive. As a result, an automated pH adjustment and monitoring system would be the best choice for keeping a stable pH in each process step.
3.4. Metabolite Yields from Fermented Sugars
3.4.1. Metabolite Yields from Hydrolyzed Biomass
After fermentation, the metabolites ethanol, acetic and lactic acid, and glycerin were found in the fermentation broth from each used biomass.
Figure 4 shows the concentrations of previously mentioned metabolites gained from barley straw (A), aspen wood (B), and pine wood (C). The amount of metabolites from barley straw and aspen wood were similar, although the ethanol yield from aspen wood (13.57 g per 100 g DB) was 35.43% higher compared to barley straw (10.02 g per 100 g DB). The highest acetic acid concentrations were gained from barley straw (9.39 g per 100 g DB) and the second highest from aspen wood (7.51 g per 100 g DB). The concentrations of lactic acid and glycerin from barley straw (lactic acid = 7.07 g per 100 g DB, glycerin = 2.56 g per 100 g DB) and aspen wood (lactic acid = 7.37 g per 100 g DB, glycerin = 2.56 g per 100 g DB) were almost the same. For barley straw, most of the metabolites were produced after the first pretreatment at 175 °C, while for aspen wood, the yields were the highest after the second pretreatment at 200 °C. A similar pattern regarding the sugar yields appeared after the enzymatic hydrolysis. Since the overall glucose and xylose yields were almost the same for barley straw and aspen wood, it is not surprising that the metabolite yields after fermentation are also nearly the same for both materials. Only the acetic acid and ethanol yields differentiate between those two materials. The reason for this difference is unknown. Possible reasons will be discussed in
Section 4.4.
The lowest metabolite yields were gained from pine wood. This can be explained by the fact that just low amounts of sugars were released after enzymatic hydrolysis. Thus, the metabolite yields were low as well.
The detected metabolites are useful chemicals in different industries. Ethanol can be used as a biofuel in combustion engines. Acetic and lactic acid are base materials for biodegradable polymers, such as cellulose acetate and polylactic acid. Glycerin has different applications in the food and personal care industry [
33,
34,
35,
36]. All four chemicals were produced by one organism and gained from one source, which shows that the biorefinery concept can be realized, and crude oil refineries can be replaced.
3.4.2. Metabolite Yields from Artificial Medium
The used yeast strain was able to convert sugars to lactic acid. It is common for genetically engineered strains to conduct this chemical conversion, but not for natural yeast strains. As a result, it was tested if the strain is able to produce the same metabolites in a simulated hydrolysate.
Table 2 shows the results of this experiment and reveals that the strain is indeed capable of producing the same metabolites, such as ethanol, glycerin, acetic acid, and lactic acid. Additionally, it can be excluded that lactic acid was produced by a bacterial contamination. At the end of the experiment, not all xylose was consumed, which indicates that the simulation hydrolysate was still different from the real barley straw hydrolysate. The possible reasons for this low consumption are unknown. It can be speculated that the real barley straw hydrolysate contained additional substances, which triggered the xylose consumption. It needs further research to determine the actual cause.
3.5. SEM-Pictures from Pretreated and Enzymatically Hydrolyzed Biomass
Figure 5 shows the SEM-pictures of barley straw (A), aspen wood (B), and pine wood (C) after the first pretreatment at 175 °C. In picture A, it is visible that the plant cells were disrupted due to the pretreatment. Before pretreatment, the barley straw particles had a smooth surface, and the cellular structure was intact (picture not shown = p.n.s.). After pretreatment and 48 h of enzymatic hydrolysis, the enzymes had degraded and smoothed the edges of the disrupted plant cells (p.n.s.). This indicates that the enzymes start hydrolyzing the biomass from the rough edges and work themselves down layer by layer.
Picture B illustrates the disrupted plant cells of aspen wood after the first pretreatment. Those disrupted plant cells appear more filamentous compared to barley straw. After 48 h of hydrolysis, the enzymes degraded the filaments, and the leftover particles (p.n.s) had the same surface morphology as the ones from barley straw (p.n.s.) after 48 h of hydrolysis.
For pine wood on picture C, the plant cells were partly disrupted after pretreatment, but the intensity of disruption is not comparable to the plant cells seen in pictures A and B from barley straw and aspen wood. This indicates that harsher pretreatment conditions are required for disrupting the pine wood cells. This can be achieved with higher pretreatment temperatures, such as 225 and 250 °C.
Picture D shows barley straw after the second pretreatment at 200 °C. On the biomass surface, small spherical droplets are present (circled red on picture D). Similar droplets were also found on the surface of aspen wood after the second pretreatment at 200 °C (p.n.s). On pine wood, no droplets occurred even after the second pretreatment (p.n.s).
The detected droplets on pretreated barley straw and aspen wood could be either repolymerized lignin or pseudo-lignin. Both kinds of lignin have been described earlier by scientists who used hydrothermal pretreatment in their experiments on lignocellulosic biomass. It is still unclear how the two kinds of lignin are exactly formed. However, it is proven that they are inhibiting the enzymatic hydrolysis of lignocellulosic biomass. Thus, it is important to avoid their formation [
37,
38,
39].
Repolymerized lignin occurs when the pretreatment temperature surpasses the melting point of lignin. The melted lignin detaches itself from the biomass and condensates on the biomass surface after cooling. For barley straw, the lignin melting point is over 175 °C because the lignin droplets only appeared after the second pretreatment at 200 °C (picture D). The same applies to aspen wood (p.n.s). For pine wood, no droplets were found on the biomass surface after both pretreatments, which indicates that the lignin melting point for pine wood is higher than 200 °C [
37,
38,
40,
41].
Pseudo-lignin is the product of degraded polysaccharides, and it is formed under harsh pretreatment conditions. It has similar characteristics as lignin but is described as a humin-like substance [
40]. It also appears on the biomass surface in the form of droplets after pretreatment. However, it is unlikely that the droplets on barley straw and aspen wood were pseudo-lignin. Firstly, the inhibitor concentrations of HMF and furfural (less than 0.4 g per 100 g of DB) were low, which are precursors of pseudo-lignin. Secondly, the used pretreatment conditions were mild, which is not in line with the formation of pseudo-lignin. [
37,
40,
41]. It requires further analysis to determine whether the droplets on the biomass surface are repolymerized lignin or pseudo-lignin.