Fuel-Water Emulsion as an Alternative Fuel for Gas Turbines in the Context of Combustion Process Properties—A Review
Abstract
:1. Introduction
2. Theoretical Background
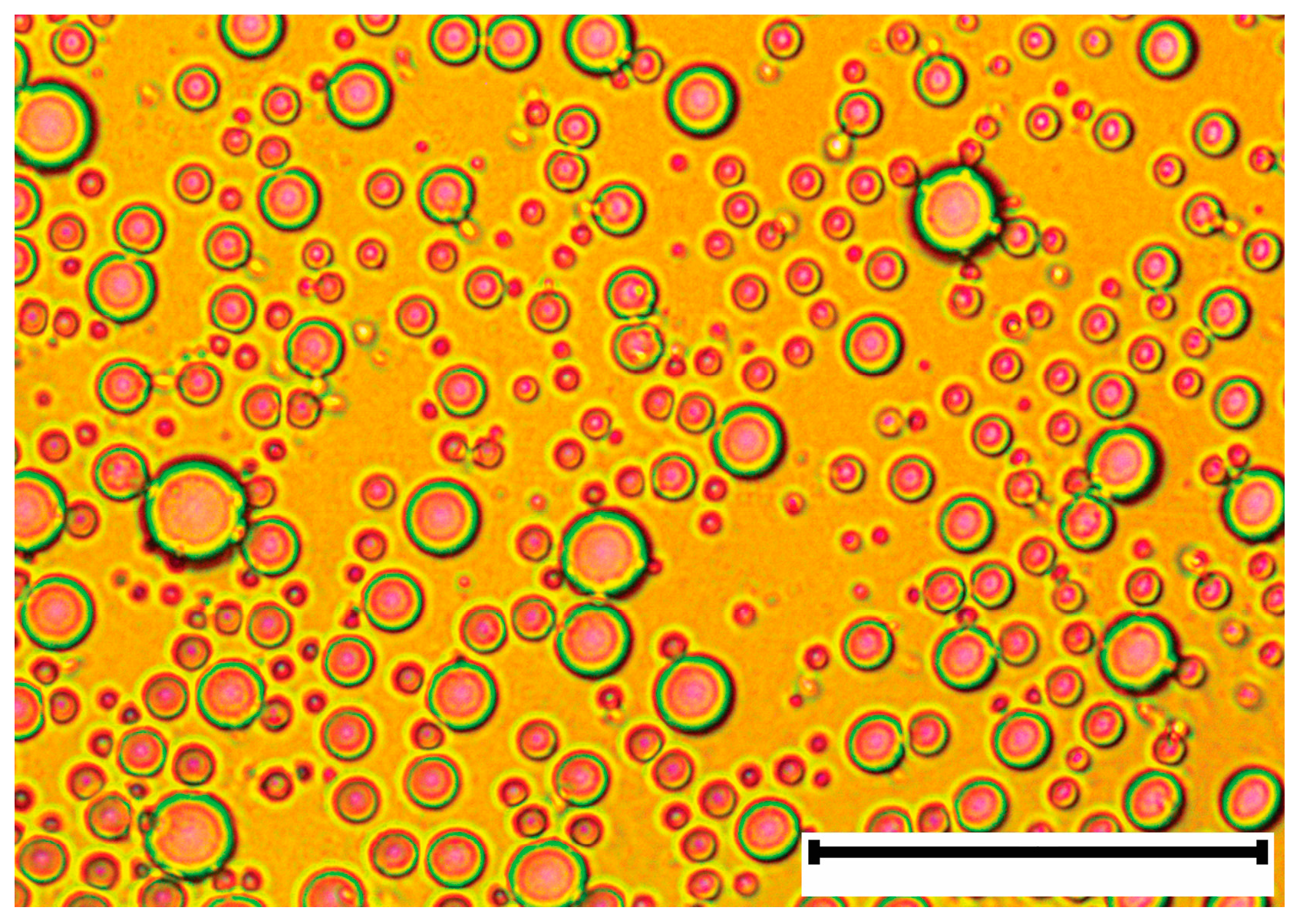
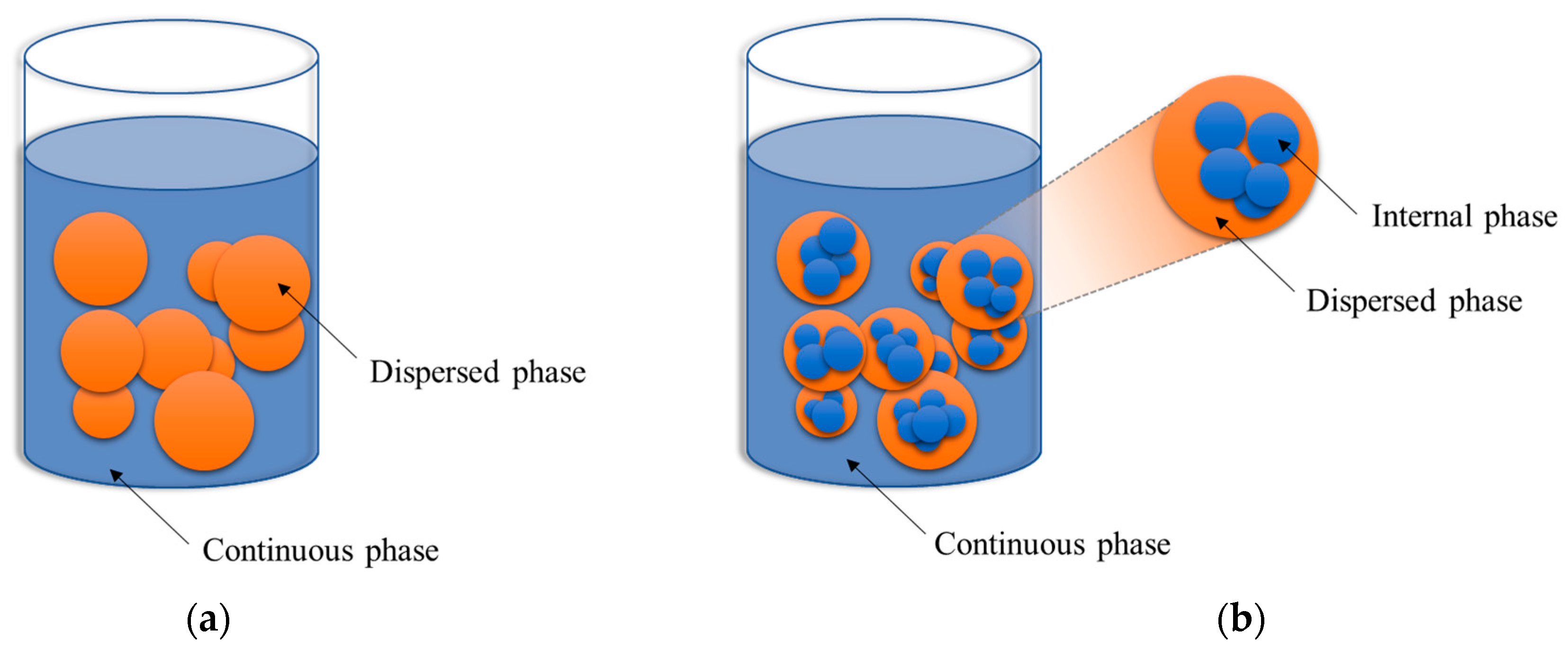
2.1. Fuel-Water Emulsion Characteristics
2.2. Surfactant
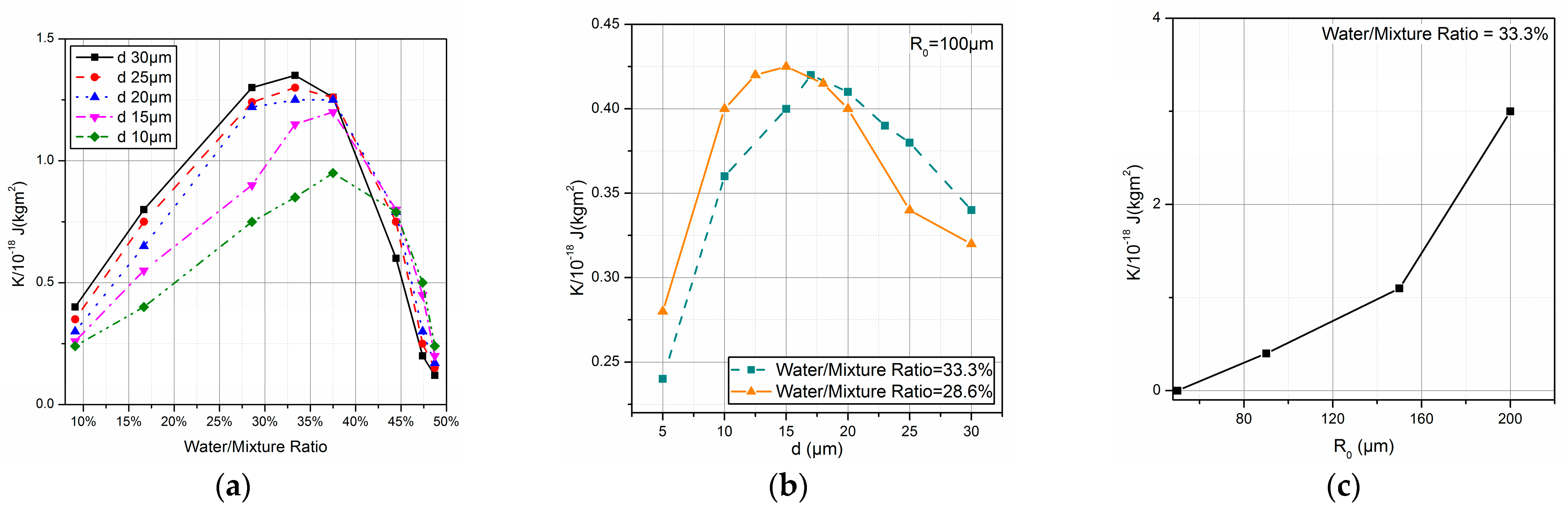
2.3. Combustion Process of the Emulsified Fuel
3. Materials and Methods
4. Literature Research
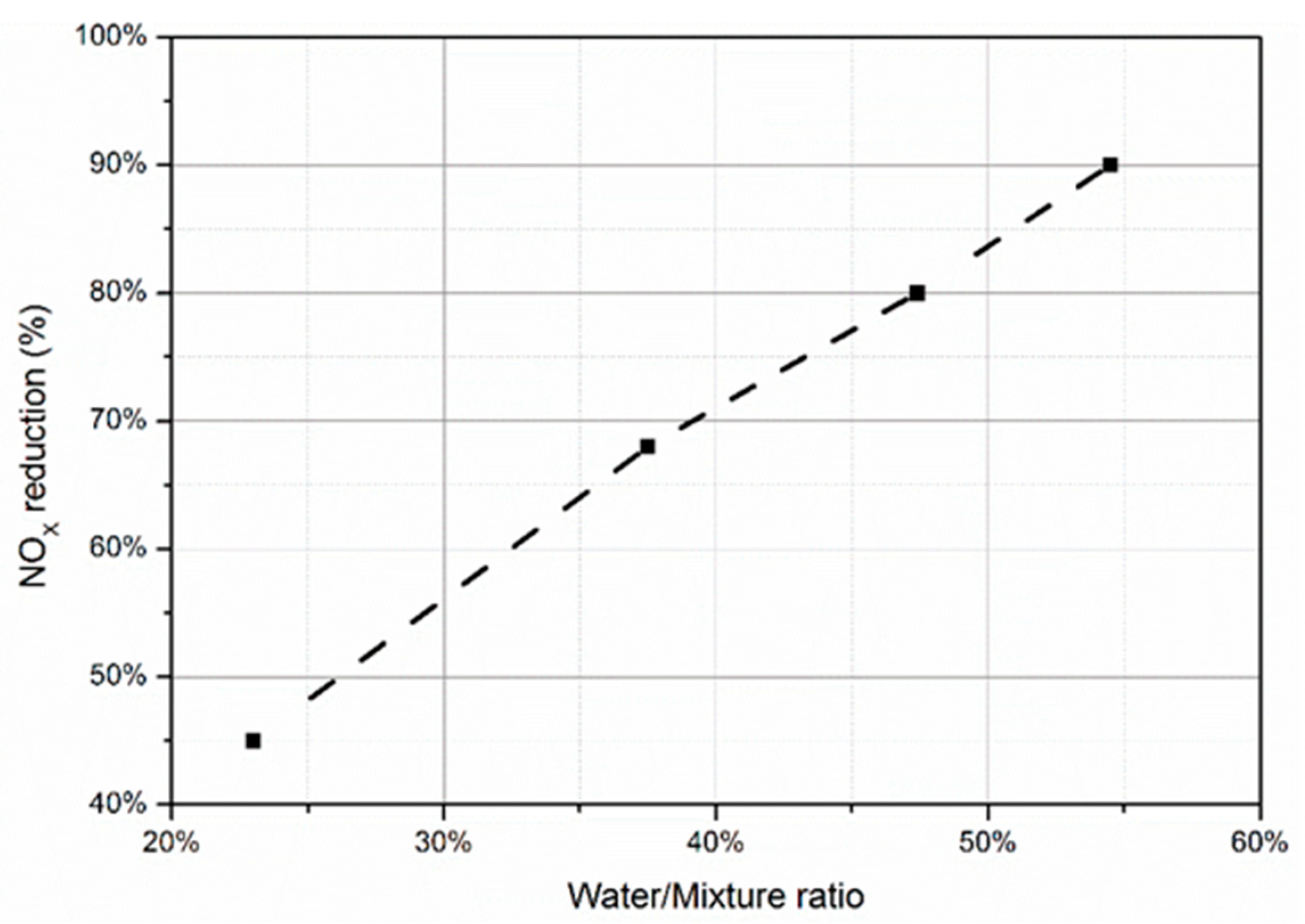
4.1. NOx Emissions
4.2. CO Emissions
4.3. Smoke in the Exhaust Gasses
5. Summary of the Literature Research
6. Conclusions
- The addition of water to the fuel in the form of a fuel-water emulsion effectively reduces emissions in fuel mixtures containing negligible amounts of nitrogen. The reduction is caused by the reduced production of NO according to the Zeldovich mechanism, which occurs as a result of the reduction of the maximum temperature in the reaction area, and it becomes more intense the more water is in the emulsion.
- Increasing the water content of an emulsion in which the base fuel contains significant amounts of nitrogen reduces the reduction efficiency. This is because the conversion of fuel nitrogen to increases as the amount of water in the emulsion increases.
- The use of a fuel-water emulsion to supply gas turbines reduces the smoke and particulate matter in the exhaust gasses.
- The use of fuel-water emulsion has little effect on CO emissions from gas turbines. The reason for the observed increases in emissions in the analyzed studies may be a change in the structure of the reaction zone in the combustion chamber, which may translate into a significantly less optimal course of this process compared to the original design conditions of the combustion chamber.
- The use of fuel-water emulsion does not cause radical changes in the consumption of the base fuel. The total mass flow of the fuel mixture applied by the gas turbine injectors in the case of using emulsified fuel is approximately equal to the mass flow of the base fuel burned in the reference case, increased by the mass flow of water and (if applicable) the emulsifier contained in the emulsion.
- In the process of analyzing the available literature, the necessity to analyze the influence of the phenomenon of micro-explosion of fuel-water emulsion droplets on the operating parameters and emission of pollutants from gas turbines was demonstrated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Capuano, D.L. Energy Information Administration; Annual Energy Outlook: Washington, DC, USA, 2020.
- Čampara, L.; Hasanspahić, N.; Vujičić, S. Overview of MARPOL ANNEX VI regulations for prevention of air pollution from marine diesel engines. In Proceedings of the GLOBMAR 2018—Global Maritime Conference, SHS Web Conference, Sopot, Poland, 19–20 April 2018; p. 01004. [Google Scholar] [CrossRef]
- van Vliet, J.; van den Berg, M.; Schaeffer, M.; van Vuuren, D.P.; Den Elzen, M.; Hof, A.F.; Beltran, A.M.; Meinshausen, M. Copenhagen Accord Pledges imply higher costs for staying below 2 °C warming. Clim. Change 2012, 113, 551–561. [Google Scholar] [CrossRef]
- Rhodes, C.J. The 2015 Paris climate change conference: COP21. Sci. Prog. 2016, 99, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Bows, A. Executing a Scharnow turn: Reconciling shipping emissions with international commitments on climate change. Carbon Manag. 2012, 3, 615–628. [Google Scholar] [CrossRef]
- Armellini, A.; Daniotti, S.; Pinamonti, P.; Reini, M. Evaluation of gas turbines as alternative energy production systems for a large cruise ship to meet new maritime regulations. Appl. Energy 2018, 211, 306–317. [Google Scholar] [CrossRef] [Green Version]
- El-Gohary, M.M. The future of natural gas as a fuel in marine gas turbine for LNG carriers. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2012, 226, 371–377. [Google Scholar] [CrossRef]
- Ammar, N.R.; Farag, A.I. CFD modeling of syngas combustion and emissions for marine gas turbine applications. Pol. Marit. Res. 2016, 3, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.; Sugimoto, T.; Imai, K.; Miyaji, H.; Nakanishi, K.; Hamachi, Y. Research and development of gas turbine for next-generation marine propulsion system (super marine gas turbine). In Proceedings of the International Gas Turbine Congress, Tokyo, Japan, 2–7 November 2003. [Google Scholar]
- Cames, M.; Graichen, J.; Pulles, H. Issues at Stake at the 10th Session of the ICAO Committee on Avitaion Environmental Protection (CAEP/10); Technical Report; European Parlament (EU): Strasbourg, France, 2016. [Google Scholar]
- National Aeronautics and Space Administration. Types of Gas Turbines. Available online: https://www.grc.nasa.gov/www/k-12/airplane/trbtyp.html (accessed on 17 July 2022).
- Owen, B.; Lee, D.S.; Lim, L. Flying into the future: Aviation emissions scenarios to 2050. Environ. Sci. Technol. 2010, 44, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, K.; Guan, K.; Gardi, A.; Sabatini, R. Review of advanced low-emission technologies for sustainable aviation. Energy 2019, 188, 115945. [Google Scholar] [CrossRef]
- Mercer, C.; Haller, W.; Tong, M. Adaptive engine technologies for aviation CO2 emissions reduction. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006. [Google Scholar] [CrossRef]
- Fulara, S.; Chmielewski, M.; Gieras, M. Variable Geometry in Miniature Gas Turbine for Improved Performance and Reduced Environmental Impact. Energies 2020, 13, 5230. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Sethi, V.; Nalianda, D.; Li, Y.G.L.; Wang, L. Review of modern low emissions combustion technologies for aero gas turbine engines. Prog. Aerosp. Sci. 2017, 94, 12–45. [Google Scholar] [CrossRef] [Green Version]
- Devi, P.B.; Joseph, D.R.; Gokulnath, R.; Manigandan, S.; Gunasekar, P.; Anand, T.P.; Venkatesh, S.; Vimal, R.M. The effect of TiO2 on engine emissions for gas turbine engine fueled with jatropha, butanol, soya and rapeseed oil. Int. J. Turbo Jet Engines 2020, 37, 85–94. [Google Scholar] [CrossRef]
- Chmielewski, M.; Gieras, M. Impact of variable geometry combustor on performance and emissions from miniature gas turbine engine. J. Energy Inst. 2017, 90, 257–264. [Google Scholar] [CrossRef]
- Renyu, F.; Man, Z. Low emission commercial aircraft engine combustor development in China: From airworthiness requirements to combustor design. Procedia Eng. 2011, 17, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Stathopoulos, P.; Paschereit, C.O. Retrofitting micro gas turbines for wet operation. A way to increase operational flexibility in distributed CHP plants. Appl. Energy 2015, 154, 438–446. [Google Scholar] [CrossRef]
- Klimenko, V.V.; Klimenko, A.V.; Kasilova, E.V.; Rekunenko, E.S.; Tereshin, A.G. Performance of gas turbines in Russia under the changing climatic conditions. Therm. Eng. 2016, 63, 690–698. [Google Scholar] [CrossRef]
- Wołowicz, M.; Kolasiński, P.; Badyda, K. Modern Small and Microcogeneration Systems—A Review. Energies 2021, 14, 785. [Google Scholar] [CrossRef]
- Harun, N.F.; Tucker, D.; Adams II, T.A. Technical challenges in operating an SOFC in fuel flexible gas turbine hybrid systems: Coupling effects of cathode air mass flow. Appl. Energy 2017, 190, 852–867. [Google Scholar] [CrossRef] [Green Version]
- Harutyunyan, A.; Badyda, K.; Wołowicz, M.; Milewski, J. Gas turbine selection for hot windbox repowering on 200 MW fossil fuel power plant. J. Power Technol. 2019, 99, 142. [Google Scholar]
- Jonsson, M.; Yan, J. Humidified gas turbines—a review of proposed and implemented cycles. Energy 2005, 30, 1013–1078. [Google Scholar] [CrossRef]
- Moschini, G.; Cui, J.; Lapan, H. Economics of biofuels: An overview of policies, impacts and prospects. Bio Based Appl. Econ. 2012, 1, 269–296. [Google Scholar]
- Caspeta, L.; Buijs, N.A.; Nielsen, J. The role of biofuels in the future energy supply. Energy Environ. Sci. 2013, 6, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Rochelle, D.; Najafi, H. A review of the effect of biodiesel on gas turbine emissions and performance. Renew Sustain. Energy Rev. 2019, 105, 129–137. [Google Scholar] [CrossRef]
- Gökalp, I.; Lebas, E. Alternative fuels for industrial gas turbines (AFTUR). Appl. Therm. Eng. 2004, 24, 1655–1663. [Google Scholar] [CrossRef]
- Starik, A.M.; Savel’ev, A.M.; Favorskii, O.N.; Titova, N.S. Analysis of emission characteristics of gas turbine engines with some alternative fuels. Int. J. Green Energy 2018, 15, 161–168. [Google Scholar] [CrossRef]
- Blakey, S.; Rye, L.; Wilson, C.W. Aviation gas turbine alternative fuels: A review. Proc. Combust. Inst. 2011, 33, 2863–2885. [Google Scholar] [CrossRef]
- Gupta, K.K.; Rehman, A.; Sarviya, R.M. Bio-fuels for the gas turbine: A review. Renew Sustain. Energy Rev. 2010, 14, 2946–2955. [Google Scholar] [CrossRef]
- Talero, G.; Bayona-Roa, C.; Muñoz, G.; Galindo, M.; Silva, V.; Pava, J.; Lopez, M. Experimental methodology and facility for the J69-engine performance and emissions evaluation using jet A1 and biodiesel blends. Energies 2019, 12, 4530. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.; Itaoka, K.; Hirose, K.; Davidson, F.T.; Nagasawa, K.; Lloyd, A.C.; Webber, M.E.; Kurban, Z.; Managi, S.; Tamaki, T.; et al. A review of four case studies assessing the potential for hydrogen penetration of the future energy system. Int. J. Hydrogen Energy 2019, 44, 6371–6382. [Google Scholar] [CrossRef]
- Juknelevicius, R.; Szwaja, S.; Pyrc, M.; Gruca, M. Influence of hydrogen co-combustion with diesel fuel on performance, smoke and combustion phases in the compression ignition engine. Int. J. Hydrogen Energy 2019, 44, 19026–19034. [Google Scholar] [CrossRef]
- Wang, S.; Ji, C.; Zhang, B.; Zhou, X. Analysis on combustion of a hydrogen-blended gasoline engine at high loads and lean conditions. Energy Procedia 2014, 61, 323–326. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G.; Mazzocchi, L. Using hydrogen as gas turbine fuel. J. Eng. Gas Turbines Power 2005, 127, 73–80. [Google Scholar] [CrossRef]
- Ayed, A.H.; Kusterer, K.; Funke, H.W.; Keinz, J.; Bohn, D. CFD based exploration of the dry-low-NOx hydrogen micromix combustion technology at increased energy densities. Propuls. Power Res. 2017, 6, 15–24. [Google Scholar] [CrossRef]
- Chiong, M.C.; Chong, C.T.; Ng, J.H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Munawer, M.E. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Jonson, J.E.; Borken-Kleefeld, J.; Simpson, D.; Nyíri, A.; Posch, M.; Heyes, C. Impact of excess NOx emissions from diesel cars on air quality, public health and eutrophication in Europe. Environ. Res. Lett. 2017, 12, 094017. [Google Scholar] [CrossRef] [Green Version]
- Łapucha, R. Komory Spalania Silników Turbinowo-Odrzutowych: Procesy, Obliczenia, Badania, 1st ed.; Wydawnictwa Naukowe Instytutu Lotnictwa: Warsaw, Poland, 2004; pp. 128–143.
- Pavri, R.; Moore, G.D. Gas Turbine Emissions and Control; GE Energy Services: Atlanta, GA, USA, 2001. [Google Scholar]
- Gieras, M. Komory Spalania Silników Turbinowych: Organizacja Procesu Spalania, 1st ed.; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, Poland, 2010; pp. 124–132. [Google Scholar]
- Yan, J.; Ji, X.; Jonsson, M. Thermodynamic property models for the simulation of advanced wet cycles. In Proceedings of the 2003 International Joint Power Generation Conference, Atlanta, GA, USA, 16–19 June 2003; Volume 3686, pp. 211–219. [Google Scholar] [CrossRef]
- Klarman, A.F.; Rollo, A.J.; Scott, H.C. Evaluation of the Water/Fuel Emulsion Concept for Test Cell Smoke Abatement; Naval Air Propulsion Center, Propulsion Technology and Project Engineering Dept: Trenton, NJ, USA, 1978. [Google Scholar]
- Aguilar Hernandez, D.; Sullivan-Lewis, E.; McDonell, V. Adaptation of a 60kW Commercial Natural Gas Fired Microturbine for Operation on Diesel and Diesel-Water Emulsions. In Proceedings of the ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition, Charlotte, NC, USA, 26–30 June 2017; Volume 50848, p. V04AT04A053. [Google Scholar] [CrossRef]
- Molière, M.; Colas, M.; Freimark, M. Des émulsions eau-fuel pour réduire les émissions de NOx de turbines à gaz. Rev. Gén. Therm. 1989, 330–331, 428–435. [Google Scholar]
- Niszczota, P.; Gieras, M. Impact of the Application of Fuel and Water Emulsion on CO and NOx Emission and Fuel Consumption in a Miniature Gas Turbine. Energies 2021, 14, 2224. [Google Scholar] [CrossRef]
- Meisl, J.; Lauer, G.; Hoffmann, S. Low NOx emission technology for the VX4. 3A gas turbine series in fuel oil operation. In Proceedings of the ASME Turbo Expo 2002: Power for Land, Sea, and Air, Amsterdam, The Netherlands, 3–6 June 2002; Volume 36061, pp. 665–671. [Google Scholar] [CrossRef]
- Encyclopedia Britannica. Available online: https://www.britannica.com/science/emulsion-chemistry (accessed on 14 September 2021).
- Sartomo, A.; Santoso, B.; Muraza, O. Recent progress on mixing technology for water-emulsion fuel: A review. Energy Convers. Manag. 2020, 213, 112817. [Google Scholar] [CrossRef]
- Basilicata, C.; Cerri, G.; Chennaoui, L.; Giovannelli, A.; Miglioli, M. Dynamics of a Prescribed Mixing Ratio Variable Fuel Flow Valve. In Proceedings of the ASME Turbo Expo 2010: Power for Land, Sea, and Air, Glasgow, UK, 14–18 June 2010; Volume 43963, pp. 527–536. [Google Scholar] [CrossRef]
- Yahaya Khan, M.; Abdul Karim, Z.A.; Hagos, F.Y.; Aziz, A.R.A.; Tan, I.M. Current Trends in Water-in-Diesel Emulsion as a Fuel. Sci. World J. 2014, 2014, 527472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Cucheval, A.; Chow, R.C.Y. A study on the emulsification of oil by power ultrasound. Ultrason. Sonochemistry 2008, 15, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, M.T.; Selim, M.Y. Stability behavior of water-in-diesel fuel emulsion. Pet. Sci. Technol. 2009, 27, 396–411. [Google Scholar] [CrossRef]
- Patil, H.; Gadhave, A.; Mane, S.; Waghmare, J. Analyzing the stability of the water-in-diesel fuel emulsion. J. Dispers. Sci. Technol. 2015, 36, 1221–1227. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, L.W. Comparison of fuel properties and emission characteristics of two-and three-phase emulsions prepared by ultrasonically vibrating and mechanically homogenizing emulsification methods. Fuel 2008, 87, 2154–2161. [Google Scholar] [CrossRef]
- Mondal, P.K.; Mandal, B.K. Experimental investigation on the combustion, performance and emission characteristics of a diesel engine using water emulsified diesel prepared by ultrasonication. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 1–17. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Sacco, N.; Bregni, C. The studies on hydrophilic-lipophilic balance (HLB): Sixty years after William C. Griffin’s pioneer work (1949-2009). Lat. Am. J. Pharm. 2009, 28, 313–317. [Google Scholar]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Davies, J.T. A quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent. In Proceedings of the International Congress of Surface Activity, London, England, January 1957. [Google Scholar]
- Schmidts, T.; Dobler, D.; Guldan, A.C.; Paulus, N.; Runkel, F. Multiple W/O/W emulsions—Using the required HLB for emulsifier evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 48–54. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Rangkuti, C.; Anuar, K.; Haq, M.R.U.; Tan, I.B.; Shah, S.S. Diesel engine performance and emission evaluation using emulsified fuels stabilized by conventional and gemini surfactants. Fuel 2006, 85, 2111–2119. [Google Scholar] [CrossRef]
- Hait, S.K.; Moulik, S.P. Gemini surfactants: A distinct class of self-assembling molecules. Curr. Sci. Bangalore 2002, 82, 1101–1111. [Google Scholar]
- Watanabe, H.; Suzuki, Y.; Harada, T.; Matsushita, Y.; Aoki, H.; Miura, T. An experimental investigation of the breakup characteristics of secondary atomization of emulsified fuel droplet. Energy 2010, 35, 806–813. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Karmakar, S.; Som, S.K. Puffing and micro-explosion behavior in combustion of butanol/Jet A-1 and acetone-butanol-ethanol (ABE)/Jet A-1 fuel droplets. Combust. Sci. Technol. 2017, 189, 1796–1812. [Google Scholar] [CrossRef] [Green Version]
- Kadota, T.; Yamasaki, H. Recent advances in the combustion of water fuel emulsion. Prog. Energy Combust. Sci. 2002, 28, 385–404. [Google Scholar] [CrossRef]
- Jeong, I.; Lee, K.H.; Kim, J. Characteristics of auto-ignition and micro-explosion behavior of a single droplet of water-in-fuel. J. Mech. Sci. Technol. 2008, 22, 148–156. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust. Flame 2011, 158, 354–368. [Google Scholar] [CrossRef]
- Kim, H.; Baek, S.W. Combustion of a single emulsion fuel droplet in a rapid compression machine. Energy 2016, 106, 422–430. [Google Scholar] [CrossRef]
- Califano, V.; Calabria, R.; Massoli, P. Experimental evaluation of the effect of emulsion stability on micro-explosion phenomena for water-in-oil emulsions. Fuel 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Ivanov, V.M.; Nefedov, P.I. Experimental Investigation of the Combustion Process of Natural and Emulsified Liquid Fuels; National Aeronautics and Space Administration: Washington, DC, USA, 1965.
- Antonov, D.V.; Strizhak, P.A.; Fedorenko, R.M.; Nissar, Z.; Sazhin, S.S. Puffing/micro-explosion in rapeseed oil/water droplets: The effects of coal micro-particles in water. Fuel 2021, 289, 119814. [Google Scholar] [CrossRef]
- Shinjo, J.; Xia, J.; Ganippa, L.C.; Megaritis, A. Physics of puffing and microexplosion of emulsion fuel droplets. Phys. Fluids 2014, 26, 103302. [Google Scholar] [CrossRef]
- Rodzewicz, R.; Gieras, M. Numerical investigation of heat transfer in fuel-water emulsion droplet. Arch. Combust. 2018, 38, 1–10. [Google Scholar]
- Moussa, O.; Tarlet, D.; Massoli, P.; Bellettre, J. Investigation on the conditions leading to the micro-explosion of emulsified fuel droplet using two colors LIF method. Exp. Therm. Fluid Sci. 2020, 116, 110106. [Google Scholar] [CrossRef]
- Mura, E.; Massoli, P.; Josset, C.; Loubar, K.; Bellettre, J. Study of the micro-explosion temperature of water in oil emulsion droplets during the Leidenfrost effect. Exp. Therm. Fluid. Sci. 2012, 43, 63–70. [Google Scholar] [CrossRef]
- Segawa, D.; Yamasaki, H.; Kadota, T.; Tanaka, H.; Enomoto, H.; Tsue, M. Water-coalescence in an oil-in-water emulsion droplet burning under microgravity. Proc. Combust. Inst. 2000, 28, 985–990. [Google Scholar] [CrossRef]
- Fu, W.B.; Hou, L.Y.; Wang, L.; Ma, F.H. A unified model for the micro-explosion of emulsified droplets of oil and water. Fuel Process. Technol. 2002, 79, 107–119. [Google Scholar] [CrossRef]
- Nissar, Z.; Rybdylova, O.; Sazhin, S.S.; Heikal, M.; Aziz, A.R.B.; Ismael, M.A. A model for puffing/microexplosions in water/fuel emulsion droplets. Int. J. Heat Mass Transf. 2020, 149, 119208. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Rybdylova, O.; Crua, C.; Heikal, M.; Ismael, M.A.; Nissar, Z.; Aziz, A.R.B. A simple model for puffing/micro-explosions in water-fuel emulsion droplets. Int. J. Heat. Mass Transf. 2019, 131, 815–821. [Google Scholar] [CrossRef]
- Le Clercq, P.; Noll, B.; Aigner, M. Modeling evaporation and microexplosion of water-in-alkane emulsion droplets. In Proceedings of the AIChE 2006 Annual Meeting, San Francisco, CA, USA, 12–17 November 2006. [Google Scholar]
- Fostiropoulos, S.; Strotos, G.; Nikolopoulos, N.; Gavaises, M. Numerical investigation of heavy fuel oil droplet breakup enhancement with water emulsions. Fuel 2020, 278, 118381. [Google Scholar] [CrossRef]
- Shaw, H. Fuel Modification for Abatement of Aircraft Turbine Engine Oxides of Nitrogen Emissions; Esso Research and Engineering Co. Linden NJ Government Research Lab.: Linden, NJ, USA, 1972. [Google Scholar]
- Spadaccini, L.J.; Pelmas, J. Evaluation of oil/water emulsions for gas turbine engines. Adv. Chem. 1978, 166, 232–244. [Google Scholar] [CrossRef]
- Moses, C.A.; Coon, C.W.; Altavilla, P.A. Reduction of Exhaust Smoke from Gas-Turbine Engines by Using Fuel Emulsions; Army Fuels and Lubricants Research Lab, Southwest Research Institute: San Antonio, TX, USA, 1980. [Google Scholar]
- Naegeli, D.W.; Moses, C.A. Fuel microemulsions for jet engine smoke reduction. J. Eng. Power 1983, 105, 18–23. [Google Scholar] [CrossRef]
- Naegeli, D.W.; Moses, C.A. Fuel Microemulsions for Jet Engine Smoke Reduction. In Proceedings of the ASME 1982 International Gas Turbine Conference and Exhibit, London, England, 18–22 April 1982; Volume 79580, p. V003T06A009. [Google Scholar] [CrossRef]
- Naegeli, D.W.; Fodor, G.E.; Moses, C.A. Fuel Microemulsions for Jet Engine Smoke Reduction; Tyndall Air Force Base: Panama City, FL, USA, 1980. [Google Scholar]
- Singh, P.P.; Mulik, P.R.; Cohn, C. Effect of Using Emulsions of High Nitrogen Containing Fuels and Water in a Gas Turbine Combustor on NOx and Other Emissions. In Proceedings of the ASME 1982 International Gas Turbine Conference and Exhibit, London, England, 18–22 April 1982; Volume 79580, p. V003T06A031. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Gollahalli, S.R. Combustion of Kerosene-Water Emulsions in a Gas Turbine Combustor. In Proceedings of the ASME 1985 Beijing International Gas Turbine Symposium and Exposition, Beijing, China, 1–7 September 1985; Volume 79436, p. V002T04A030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Gollahalli, S.R. Combustion of Kerosene-Water Emulsions in a Gas Turbine Combustor. Int. J. Turbo Jet Engines 1988, 5, 39–50. [Google Scholar] [CrossRef]
- Lee, T.S.; Yang, J.Y. An Experimental Study on the Burning of Water-in-Ol Emulsions. Trans. Aeronaut Astronaut Soc. China 1999, 31, 252–277. [Google Scholar]
- Prade, B.; Meisl, J.R.; Berenbrink, P.; Streb, H.; Hoffmann, S. Burner development for flexible engine operation of the newest Siemens gas turbines. In Proceedings of the ASME Turbo Expo 2003, collocated with the 2003 International Joint Power Generation Conference, Atlanta, GA, USA, 16–19 June 2003; Volume 36851, pp. 371–376. [Google Scholar] [CrossRef]
- Lauer, G.; Meisl, J.; Belting, C.; Hoffmann, S. Further development of low-emissions oil combustion in the Vx4. 3A hybrid burner. VGB Powertech. 2002, 82, 51–55. [Google Scholar]
- Zucca, A.; Khayrulin, S.; Vyazemskaya, N.; Shershnyov, B.; Myers, G. Development of a liquid fuel system for GE MS5002E gas turbine: Rig test validation of the combustor performance. In Proceedings of the ASME Turbo Expo 2014: Turbine Technical Conference and Exposition, Düsseldorf, Germany, 16–20 June 2014; Volume 4569, p. V04BT04A007. [Google Scholar] [CrossRef]
- De Giorgi, M.G.; Ciccarella, G.; Ficarella, A.; Fontanarosa, D.; Pescini, E. Effect of jet-A1 emulsified fuel on aero-engine performance and emissions. In Proceedings of the International Conference on Multifunctional Materials (ICMM-2019), Hyderabad, India, 19–21 December 2019. [Google Scholar] [CrossRef]
- De Giorgi, M.G.; Fontanarosa, D.; Ficarella, A.; Pescini, E. Effects on performance, combustion and pollutants of water emulsified fuel in an aeroengine combustor. Appl. Energy 2020, 260, 114263. [Google Scholar] [CrossRef]
- De Giorgi, M.G.; Pescini, E.; Campilongo, S.; Ciccarella, G.; Fontanarosa, D.; Ficarella, A. Effects of emulsified fuel on the performance and emission characteristics of aeroengine combustors. J. Eng. Gas Turbines Power 2019, 141, 101021. [Google Scholar] [CrossRef]
- Chmielewski, M.; Gieras, M.; Niszczota, P. Fuel-Water emulsion impact on miniature gas turbine pollutant emission. In Proceedings of the XIV Research & Development in Power Engineering, E3S Web of Conferences, Warsaw, Poland, 3–6 December 2019. [Google Scholar] [CrossRef] [Green Version]
- Chmielewski, M.; Niszczota, P.; Gieras, M. Combustion efficiency of fuel-water emulsion in a small gas turbine. Energy 2020, 211, 118961. [Google Scholar] [CrossRef]
- Park, J.; Nguyen, T.H.; Joung, D.; Huh, K.Y.; Lee, M.C. Prediction of NO x and CO emissions from an industrial lean-premixed gas turbine combustor using a chemical reactor network model. Energy Fuels 2013, 27, 1643–1651. [Google Scholar] [CrossRef]
- Anufriev, I.S. Review of water/steam addition in liquid-fuel combustion systems for NOx reduction: Waste-to-energy trends. Renew. Sustain. Energy Rev. 2021, 138, 110665. [Google Scholar] [CrossRef]
- Blazowski, W.S. Combustion considerations for future jet fuels. Int. Symp. Combust. 1977, 16, 1631–1639. [Google Scholar] [CrossRef]
- Niszczota, P.; Gieras, M. Effect of Adding Emulsifier to Fuel on Work Efficiency and Gas Turbine Emissions. Energies 2021, 14, 5255. [Google Scholar] [CrossRef]
- Dryer, F.L. Water addition to practical combustion systems—concepts and applications. Int. Symp. Combust. 1977, 16, 279–295. [Google Scholar] [CrossRef]
- Jhalani, A.; Sharma, D.; Soni, S.L.; Sharma, P.K.; Sharma, S. A comprehensive review on water-emulsified diesel fuel: Chemistry, engine performance and exhaust emissions. Environ. Sci. Pollut. Res. 2019, 25, 4570–4587. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zaid, M. An experimental study of the evaporation characteristics of emulsified liquid droplets. Heat. Mass. Transf. 2004, 40, 737–741. [Google Scholar] [CrossRef]




| Ref. | Base Fuel | Water Content (max) | Emulsified Content | Emulsifier Type | Research Object | Pollutant’s Emission: FWE vs. Pure Fuel | ||
|---|---|---|---|---|---|---|---|---|
| NOx | CO | Smoke | ||||||
| [87] | Jet A | 32.3% (2) | 0.77% ÷ 0.97% (2) | Tech Mul-2/ TWEEN20 SPAN80 (Petrolite/ Atlas Chemical Industries) | High Pressure Cannular (6) | no change | no change | N/A (4) |
| [88] | Redwood 650 oil | 10% (2) | 0% | - | FT4/FT12 (6) (Pratt & Whitney) | increased | no change | reduced |
| [46] | JP-5 | 38.2% (2) | 2% (3) | SPAN80 (90%) TWEEN80 (10%) (ICI America, Incorporated, Wilmington, Delaware, USA) | J79—GE—10 (7) (General Electric Company) | reduced | increased | reduced |
| [89] | JP-5 | 33.3% (1) | up to 2% (3) | SPAN80 (90%) TWEEN80 (10%) (ICI America, Incorporated, Wilmington, Delaware, USA) | T-63 (6) (Allison Engine Company) | reduced | increased | reduced |
| [90,91,92] | JP-4, JP-8 | 20% (1) | 5 ÷ 20% (1) | Clindrol 100CG (Clintwood Chemical Company) | T-63 (6) (Allison Engine Company) | increased | increased | reduced |
| [93] | No 2 oil, Paraho shale oil, H-Coal®, No 2 oil doped with quinoline | 55% (2) | Undisclosed | - | Westinghouse (6) | reduced | increased | reduced |
| [94,95] | Commercial kerosene | 10% (1) | 1% (1) | SPAN80 (75%) TWEEN85 (25%) | TF-41 (6) (Allison Engine Company) | reduced | increased | reduced |
| [46] | Diesel | 33.3% (2) | less than 0.01% (1) | Undisclosed | 9000B (7) (Alstom) | reduced | no change | no change |
| [96] | No 6 oil | 9.09% (5) | Undisclosed | - | test ring (6) | reduced | reduced | reduced |
| [50,97,98] | Naphtha, Condensate, Distillate Fuel No 2 | 50% (5) | Undisclosed | - | V94.V3 GT (7) V84.V3 GT (7) (Siemens) | reduced | no change | no change |
| [99] | No 2 oil | 54.5% (5) | Undisclosed | - | GE MS5002E (6) (General Electric Company) | reduced | no change | N/A (4) |
| [47] | Diesel | 50% (2) | 0% | - | C60 CHP 60 kW (7) (Capstone Green Energy Corporation) | Initial increase with declining trend | increased | N/A 4) |
| [100,101,102] | Jet-A1 | 68.3% (2) | 0% | - | Swirl 300 kW (6) | reduced | Initial decrease with upward trend | N/A (4) |
| [49,103,104] | Jat-A1 (95%) + AeroShell Turbine Oil 500 (5%) | 12% (2) | 2% (2) | Rokwin 80 (50%) + Rokanol RZ4P11 (25%) + Rokanol DB3 (22.5%) + Rokafenol N8 (1.67%) + Water (0.83%) (PCC SE) | GTM-120 (7) (JETPOL, Poznań, Poland) | reduced | reduced | N/A (4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niszczota, P.; Chmielewski, M.; Gieras, M. Fuel-Water Emulsion as an Alternative Fuel for Gas Turbines in the Context of Combustion Process Properties—A Review. Energies 2022, 15, 8979. https://doi.org/10.3390/en15238979
Niszczota P, Chmielewski M, Gieras M. Fuel-Water Emulsion as an Alternative Fuel for Gas Turbines in the Context of Combustion Process Properties—A Review. Energies. 2022; 15(23):8979. https://doi.org/10.3390/en15238979
Chicago/Turabian StyleNiszczota, Paweł, Maciej Chmielewski, and Marian Gieras. 2022. "Fuel-Water Emulsion as an Alternative Fuel for Gas Turbines in the Context of Combustion Process Properties—A Review" Energies 15, no. 23: 8979. https://doi.org/10.3390/en15238979





