Analysis of Temperature Influence on Precipitation of Secondary Sediments during Water Injection into an Absorptive Well
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Temperature Impact on Water Injection Process
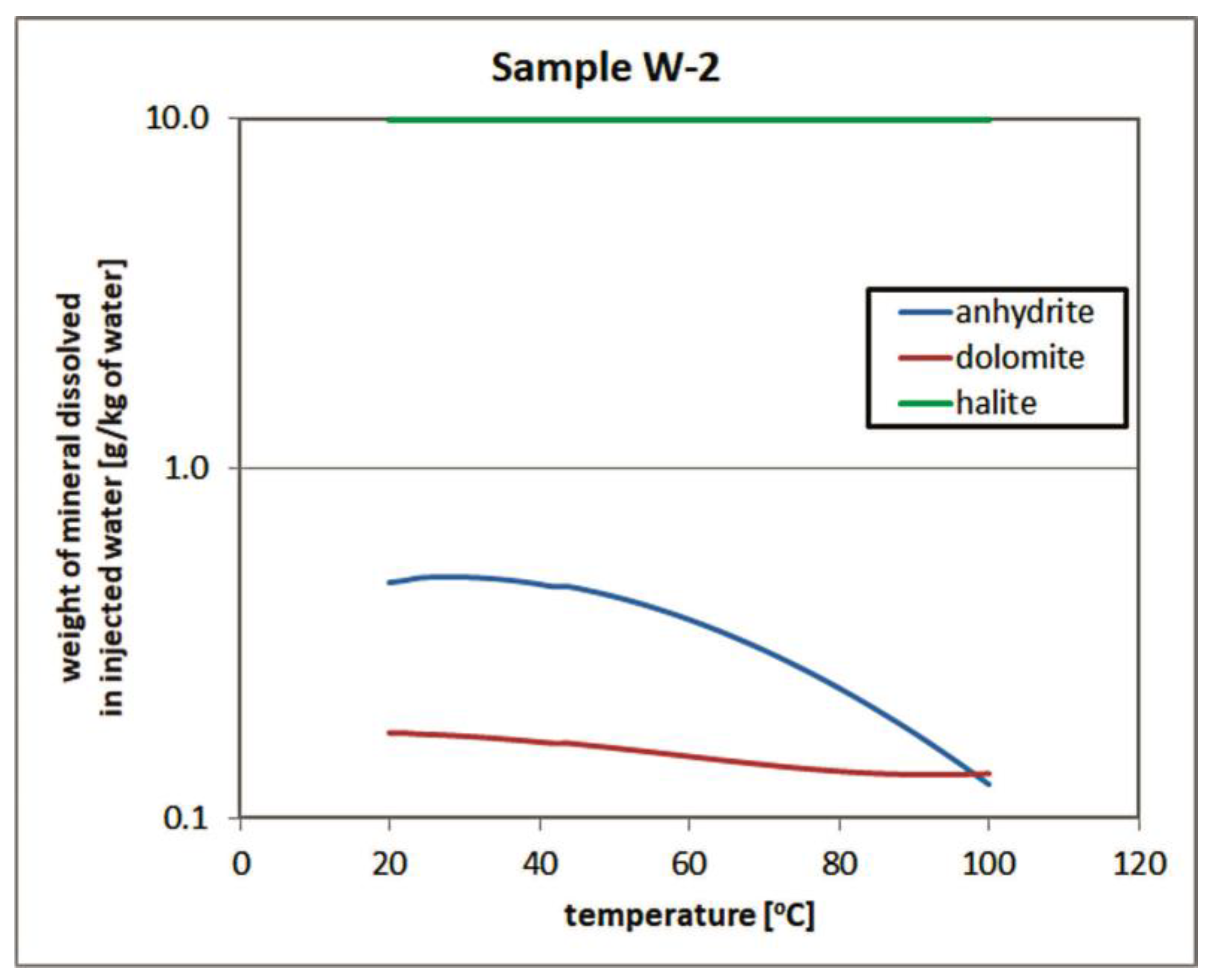
3.2. Temperature Influence on Solubility of Rock Matrix Materials in Injected Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steliga, T.; Jakubowicz, P.; Kapusta, P. Changes in toxicity during treatment of wastewater from oil plant contaminated with petroleum hydrocarbons. JCTB 2015, 90, 1408–1418. [Google Scholar] [CrossRef]
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Phil. Trans. R. Soc. A 2014, 372, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, J.L.; Mahani, A.B. Myths and Facts on Wastewater Injection, Hydraulic Fracturing, Enhanced Oil Recovery and Induced Seismicity. Seismol. Res. Lett. 2015, 86, 1060–1067. [Google Scholar] [CrossRef]
- Lubaś, J.; Stopa, J.; Warnecki, M.; Wojnicki, M. Możliwości zastosowania zaawansowanych metod wspomagania wydobycia ropy naftowej ze złóż dojrzałych. Nafta-Gaz 2019, 1, 24–28. [Google Scholar] [CrossRef]
- Purnima, M.; Paul, T.; Pakshirajan, K.; Pugazhenthi, G. Onshore oilfield produced water treatment by hybrid microfiltration-biological process using kaolin based ceramic membrane and oleaginous Rhodococcus opacus. Chem. Eng. J. 2023, 453, 139850. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Louvisse, A.M.T.; Borges, C.P.; Meabe, E.; Izquierdo, J.; Campos, J.C. Evaluation of ceramic membranes for oilfield produced water treatment aiming reinjection in offshore units. J. Pet. Sci. Eng. 2015, 131, 51–57. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Janocha, A.; Kluk, D. Research on the possibility of using loading materials to support the treatment of reservoir waters. Nafta-Gaz 2021, 4, 255–263. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Characterization of ballasted flocs in water treatment using microscopy. Water Res. 2016, 90, 119–127. [Google Scholar] [CrossRef]
- Zafisah, N.S.; Ang, W.L.; Mohammad, A.W.; Hilal, N.; Johnson, D.J. Interaction between ballasting agent and flocs in ballasted flocculation for the removal of suspended solids in water. J. Water Process Eng. 2020, 33, 101028. [Google Scholar] [CrossRef]
- Jakubowicz, P. Wybrane problemy zagospodarowania odpadowych wód kopalnianych. Nafta-Gaz 2010, 5, 383–389. [Google Scholar]
- The, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Jakubowicz, P.; Steliga, T. Efektywność działania nowoczesnych koagulantów glinowych w warunkach obniżonego pH wód z formacji łupkowych. Nafta-Gaz 2017, 3, 169–176. [Google Scholar] [CrossRef]
- He, W.; Xie, Z.; Lu, W.; Huang, M.; Ma, J. Comparative analysis on floc growth behaviors during ballasted flocculation by using aluminum sulphate (AS) and polyaluminum chloride (PACl) as coagulants. Sep. Purif. Technol. 2019, 213, 176–185. [Google Scholar] [CrossRef]
- Jakubowicz, P.; Steliga, T. Assessment of the Main Threats to Injection Well Damage Caused by Reservoir Waters Using Aquachem Software as Well as Laboratory Tests Application. Nafta-Gaz 2012, 10, 655–660. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Kluk, D. Badania procesu mieszania wód zatłaczanych z wodami złożowymi o zróżnicowanych potencjałach elektrochemicznych. Nafta-Gaz 2011, 2, 98–106. [Google Scholar]
- Abbasi, P.; Abbasi, S.; Moghadasi, J. Experimental investigation of mixed-salt precipitation during smart water injection in the carbonate formation. J. Mol. Liq. 2020, 299, 112131. [Google Scholar] [CrossRef]
- Dobrzyński, D. Modelowanie geochemiczne narzędziem poznania geochemii systemów wód podziemnych. Przykłady zastosowań, aktualny stan w Polsce. Przegląd Geol. 2006, 54, 976–981. [Google Scholar]
- Li, X.; He, X.; Yang, G.; Zhao, L.; Chen, S.; Wang, C.; Chen, J.; Yang, M. Study of groundwater using visual MODFLOW in the Manas River Basin, China. Water Policy 2016, 18, 1139–1154. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Q.; Yan, Z.; Zhao, N.; Duan, C.; Cheng, X.; Wang, H. Fine Prediction for Mine Water Inflow on Basis of Visual Modflow. OGCE 2019, 7, 52–59. [Google Scholar]
- Li, L.; Wu, Y.; Chong, S.; Wen, Q. The application of TOUGHREACT in the field of energy and environment. IOP Conf. Ser. Earth Environ. Sci. 2020, 569, 012093. [Google Scholar] [CrossRef]
- Pruess, K.; Oldenburg, C.; Moridis, G. TOUGH2 User’s Guide, Version 2.0. LBL-43134; Lawrence Berkeley Laboratory Report: Berkeley, CA, USA, 1999. [Google Scholar]
- Wanner, C.; Eichinger, F.; Jahrfeld, T.; Diamond, L.W. Unraveling the formation of large amounts of calcite scaling in geothermal wells in the Bavarian Molasse Basin: A reactive transport modeling approach. Procedia Earth Planet. Sci. 2017, 17, 344–347. [Google Scholar] [CrossRef][Green Version]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. TOUGHREACT User’s Guide: A Simulation Program for Nonisothermal Multiphase Reactive Geochemical Transport in Variably Saturated Geologic Media. Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2004. [Google Scholar]
- Roberts-Ashly, T.L.; Berger, P.M.; Cunningham, J.A. Modeling geologic sequestration of carbon dioxide in a deep saline carbonate reservoir with TOUGH2–ChemPlugin, a new tool for reactive transport modelling. Environ. Geosci. 2020, 27, 103–116. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey Water-Resources Investigations: New Cumberland, PA, USA, 1999; Volume 312, pp. 99–4259.
- Abdelaziz, R.; Merkel, B.J.; Zambrano-Bigiarini, M.; Nair, S. Particle swarm optimization for the estimation of surface complexation constants with the geochemical model PHREEQC-3.1.2. Geosci. Model Dev. 2019, 12, 167–177. [Google Scholar] [CrossRef]
- Huber, P.; Neyret, C.; Fourest, E. Implementation of the anaerobic digestion model (ADM1)in the PHREEQC chemistry engine. Water Sci. Technol. 2017, 76, 1090–1103. [Google Scholar] [CrossRef]
- Ren, J.M.; Yang, Y.; Hu, X.W. Application of GIS and FEFLOW in Forecasting Groundwater Flow Field of Minqin Basin. Adv. Mater. Res. 2011, 368–373, 2128–2131. [Google Scholar] [CrossRef]
- Gao, Y.; Pu, S.; Zheng, C.; Yi, S. An improved method for the calculation of unsaturated–saturated water flow by coupling the FEM and FDM. Sci. Rep. 2019, 9, 14995. [Google Scholar] [CrossRef]
- Lewkiewicz-Małysa, A.; Winid, B. Geologiczne i geochemiczne aspekty chłonności otworów wykorzystywanych do zatłaczania wód złożowych. Srod.-Pomor. Tow. Nauk. Ochr. Sr. Rocz. Ochr. Sr. 2011, 13, 1985–1999. [Google Scholar]
- Hu, Y.; Mackay, E. Modelling of geochemical reactions during smart water injection in carbonate reservoirs. Eur. Assoc. Geosci. Eng. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Klunk, M.A.; Damiani, L.H.; Feller, G.; Conceição, R.V.; Abel, M.; De Ros, L.F. Geochemical modeling of diagenetic reactions in Snorre Field reservoir sandstones: A comparative study of computer codes. Braz. J. Geol. 2015, 45, 29–40. [Google Scholar] [CrossRef]
- Jakóbczyk, S.; Kowalczyk, A. Zastosowanie modelowania geochemicznego do oceny warunków kształtowania się składu chemicznego wód podziemnych w rejonie ujęcia Gliwice Łabędy. Biul. Państwowego Inst. Geol. 2011, 445, 217–226. [Google Scholar]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources 2018, 7, 54. [Google Scholar] [CrossRef]
- Lassin, A.; Andre, L.; Lach, A. Considerations about the building of a thermodynamic database for the chemical description of highly saline systems. Procedia Earth Planet. Sci. 2017, 17, 304–307. [Google Scholar] [CrossRef]
- Heredia, D.J. Improvement of the Numerical Capacities of Simulation Tools for Reactive Trans-Port Modelling in Porous Media. Ph.D. Thesis, Université Rennes 1, Rennes, France, 2017. [Google Scholar]
- Uliasz-Misiak, B.; Chruszcz-Lipska, K. Aspekty hydrogeochemiczne związane z mieszaniem wód złożowych zatłaczanych do złoża węglowodorów. Gospod. Surowcami Miner. 2017, 33, 69–80. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, C.; Sheikhi, S. Role of ion exchange in the brine-rock interaction systems: A detailed geochemical modeling study. Chem. Geol. 2021, 559, 119992. [Google Scholar] [CrossRef]
- Tranter, M.; De Lucia, M.; Kühn, M. Barite Scaling Potential Modelled for Fractured-Porous Geothermal Reservoirs. Minerals 2021, 11, 1198. [Google Scholar] [CrossRef]
- Soulaine, C.; Pavuluri, S.; Claret, F.; Tournassat, C. porousMedia4Foam: Multi-scale open-source platform for hydro-geochemical simulations with OpenFOAM®. Environ. Model. Softw. 2021, 145, 105199. [Google Scholar] [CrossRef]
- Bagrezaie, M.A.; Dabir, B.; Rashidi, F. A novel approach for pore-scale study of fines migration mechanism in porous media. J. Pet. Sci. Eng. 2022, 216, 110761. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, X.; Hu, A.; Fan, C.; Shabani, A. A novel approach for modeling permeability decline due to mineral scaling: Coupling geochemistry and deep bed filtration theory. J. Pet. Sci. Eng. 2021, 205, 108995. [Google Scholar] [CrossRef]
- Kalantariasl, A.; Tale, F.; Parsaei, R.; Keshavarz, A.; Jahanbakhsh, A.; Maroto-Valer, M.M.; Mosallanezhad, A. Optimum salinity/composition for low salinity water injection incarbonate rocks: A geochemical modelling approach. J. Mol. Liq. 2022, 362, 119754. [Google Scholar] [CrossRef]
- Chen, Q.; Abu-Al-Saud, M.O.; Ayirala, S.C.; AlYousef, A.A. Propagation of mineral dissolution waves driven by cation exchange in low salinity waterflooding. Fuel 2022, 328, 125350. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, G.; Apps, J.; Zhu, C. Comparison of thermodynamic data files for PHREEQC. Earth-Sci. Rev. 2022, 225, 103888. [Google Scholar] [CrossRef]
- Doubra, P.; Kamran-Pirzaman, A.; Mohammadi, A.H.; Hassanalizadeh, R. Thermodynamic modelling of scale (Calcite, Barite, Anhydrite and Gypsum) deposition from brine. J. Mol. Liq. 2017, 230, 96–103. [Google Scholar] [CrossRef]
- Skupio, R.; Kubik, B.; Drabik, K.; Przelaskowska, A. Comprehensive interpretation of the borehole profile including anhydrite, carbonate and mudstone rocks based on non-destructive core tests. Nafta-Gaz 2022, 9, 641–653. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Thorstenson, D.C.; Plummer, L.N. PHREEQE—A Computer Program for Geochemical Calculations; US Geological Survey Water-Resources Investigations: New Cumberland, PA, USA, 1980; Volume 195, pp. 80–96.
- Pitzer, K.S. Thermodynamics of electrolytes—1. Theoretical basis and general equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Plummer, L.N.; Parkhurst, D.L.; Fleming, G.W.; Dunkle, S.A. A Computer Program Incorporating Pitzer’s Equations for Calculation of Geochemical Reactions in Brines; US Geological Survey Water-Resources Investigations: New Cumberland, PA, USA, 1988; Volume 310, pp. 88–4153.
- Ball, J.W.; Nordstrom, D.K. WATEQ4F—User’s Manual with Revised Thermodynamic Data Base and Test Cases for Calculating Speciation of Major, Trace and Redox Elements in Natural Waters; U.S. Geological Survey Open-File Report: Washington, DC, USA, 1991; Volume 185, pp. 90–129.
- Pitzer, K.S. Theory—Ion interaction apoproach. In Activity Coefficients in Electrolyte Solutions; Pytkowicz, R.M., Ed.; Boca Raton: Florida, CA, USA; CRC Press: Florida, CA, USA, 1979; pp. 157–208. [Google Scholar]
- Jakubowicz, P.; Steliga, T.; Kluk, D.; Wojtowicz, K. The possibility of application of computer simulation to predict the direction of the reaction during injection of reservoir waters. Nafta-Gaz 2021, 4, 244–254. [Google Scholar] [CrossRef]
- Mogharrab, J.M.; Ayatollahi, S.; Pishvaie, M.R. Experimental study and surface complexation modeling ofnon-monotonic wettability behavior due to change in brine salinity/composition: Insight into anhydrite impurity in carbonates. J. Mol. Liq. 2022, 365, 120117. [Google Scholar] [CrossRef]
- Papadopoulo, M.; Kaddouh, S.; Pacitto, P.; Prieur Vernat, A. Life Cycle Assessment of the European Natural Gas Chain Focused on Three Environmental Impact Indicators; Final Report; Marcogaz: Brussels, Belgium, 2011; pp. 1–189. [Google Scholar]
- Prieur-Vernat, A.; Yoshida, S. Opportunities and Challenges of LCA Applied to the Natural Gas Industry; Program Committee A: Sustainability, 2012–2015 Triennium Work Report; International Gas Union (IGU): Barcelona, Spain, 2015; pp. 1–34. [Google Scholar]
- Rogowska, D. GHG emission in the life cycle of motor fuel. Part I—guidelines for the determination of production mass balance. Nafta-Gaz 2014, 9, 639–646. [Google Scholar]
- Sapkota, K.; Oni, A.O.; Kumar, A. Techno-economic and life cycle assessments of the natural gas supply chain from production sites in Canada to north and southwest. J. Nat. Gas Sci. Eng. 2018, 52, 401–409. [Google Scholar] [CrossRef]
- Niemczewska, J.; Zaleska-Bartosz, J. Introduction to the evaluation of the environmental footprint for the oil and natural gas production sector. Nafta-Gaz 2020, 8, 527–532. [Google Scholar] [CrossRef]








| Analysis | Unit | W-1 | W-2 |
|---|---|---|---|
| pH | 5.9 | 4.8 | |
| Eh | g/cm3 | −108 | −117.8 |
| Density (20 °C) | mV | 0.997 | 1.182 |
| Total dissolved substances | mg/dm3 | 551 | 306,428 |
| Residue of roasted (in 600 °C) | mg/dm3 | 318 | 288,904 |
| Total suspended solids | mg/dm3 | 76 | 159 |
| COD | mg O2/dm3 | 15,023 | 13,589 |
| BOD | mg O2/dm3 | 1875 | 2258 |
| TOC | mg/dm3 | 1004 | 1059 |
| TPH | mg/dm3 | 64 | 284 |
| Organic substances (dichloromethane extract) | mg/dm3 | 91 | 1102 |
| Anionic surfactants | mg/dm3 | 1.23 | 18.9 |
| Nonionic surfactants | mg/dm3 | 247 | 1.73 |
| Chloride Cl− | mg/dm3 | 129 | 176,615 |
| Sulphates SO42− | mg/dm3 | 4.3 | 189 |
| Carbonates CO32− | mg/dm3 | – | – |
| Bicarbonates HCO3− | mg/dm3 | 215 | 169 |
| Nitrates NO3− | mg/dm3 | – | – |
| Ammonium NH4+ | mg/dm3 | – | – |
| Phosphates PO43− | mg/dm3 | – | – |
| Bromides Br− | mg/dm3 | 4.12 | 249.3 |
| Sodium Na+ | mg/dm3 | 61.9 | 68,841 |
| Potassium K+ | mg/dm3 | 28.6 | 588 |
| Calcium Ca2+ | mg/dm3 | 18.6 | 35,258 |
| Magnesium Mg 2+ | mg/dm3 | 12.7 | 4974 |
| Ferrous ion Fe2+ | mg/dm3 | 2.10 | 6.50 |
| Ferric ion Fe3+ | mg/dm3 | 16.00 | 56.40 |
| Manganese Mn2+ | mg/dm3 | 3.91 | 7.05 |
| Copper Cu | mg/dm3 | 0.021 | 0.009 |
| Lead Pb | mg/dm3 | 0.068 | 0.035 |
| Zinc Zn | mg/dm3 | 0.651 | 0.358 |
| Tin Sn | mg/dm3 | 0.023 | 0.51 |
| Nickel Ni | mg/dm3 | 0.067 | 0.129 |
| Cobalt Co | mg/dm3 | 0.009 | 0.028 |
| Cadmium Cd | mg/dm3 | 0.003 | 0.048 |
| Strontium Sr | mg/dm3 | 0.061 | 3012 |
| Barium Ba | mg/dm3 | 0.038 | 81.0 |
| Silicon Si | mg/dm3 | 3.18 | 4.26 |
| Aluminum Al | mg/dm3 | 0.061 | 0.056 |
| No. | Main Issues | Reference |
|---|---|---|
| 1 | Interactions between brine and rock minerals in static and dynamic system. | [40] |
| 2 | Study of hydrochemical simulations of a dual-layer geothermal reservoir to the long-term impact of barite scale formation on well injectivity. | [41] |
| 3 | Description of mechanistic model constructed for low-salinity water injection to consider geochemical reaction issues in low-salinity flooding among surface sites and aqueous solution. | [18] |
| 4 | Integrated open-source simulator to model hydrogeochemical processes at various scales of interest including pore-scale and reservoir-scale. | [42] |
| 5 | Modeling (with PHREEQC software) of mineral precipitation and deposition in the porous media controlled by deep bed filtration model. | [44] |
| 6 | Study of fine particle migration in the rock causing formation damage and permeability impairment. | [43] |
| 7 | Investigation of the carbonate/brine interactions, using geochemical modeling, during low-salinity water injection for enhanced oil recovery (EOR). | [45] |
| 8 | Modeling of different geochemical effects such as multivalent cation exchange and mineral dissolution during flow and transport in low-salinity waterflooding. | [46] |
| 9 | Comparison of thermodynamic data files from PHREEQC software package and influence of TDF choice on modeling results. | [47] |
| 10 | Studies of influence of anhydrite on wettability of calcite rock during low-salinity water flooding. | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowicz, P.; Steliga, T.; Wojtowicz, K. Analysis of Temperature Influence on Precipitation of Secondary Sediments during Water Injection into an Absorptive Well. Energies 2022, 15, 9130. https://doi.org/10.3390/en15239130
Jakubowicz P, Steliga T, Wojtowicz K. Analysis of Temperature Influence on Precipitation of Secondary Sediments during Water Injection into an Absorptive Well. Energies. 2022; 15(23):9130. https://doi.org/10.3390/en15239130
Chicago/Turabian StyleJakubowicz, Piotr, Teresa Steliga, and Katarzyna Wojtowicz. 2022. "Analysis of Temperature Influence on Precipitation of Secondary Sediments during Water Injection into an Absorptive Well" Energies 15, no. 23: 9130. https://doi.org/10.3390/en15239130
APA StyleJakubowicz, P., Steliga, T., & Wojtowicz, K. (2022). Analysis of Temperature Influence on Precipitation of Secondary Sediments during Water Injection into an Absorptive Well. Energies, 15(23), 9130. https://doi.org/10.3390/en15239130






