Coupling Microbial Fuel Cell and Hydroponic System for Electricity Generation, Organic Removal, and Nutrient Recovery via Plant Production from Wastewater
Abstract
1. Introduction
2. Methodology
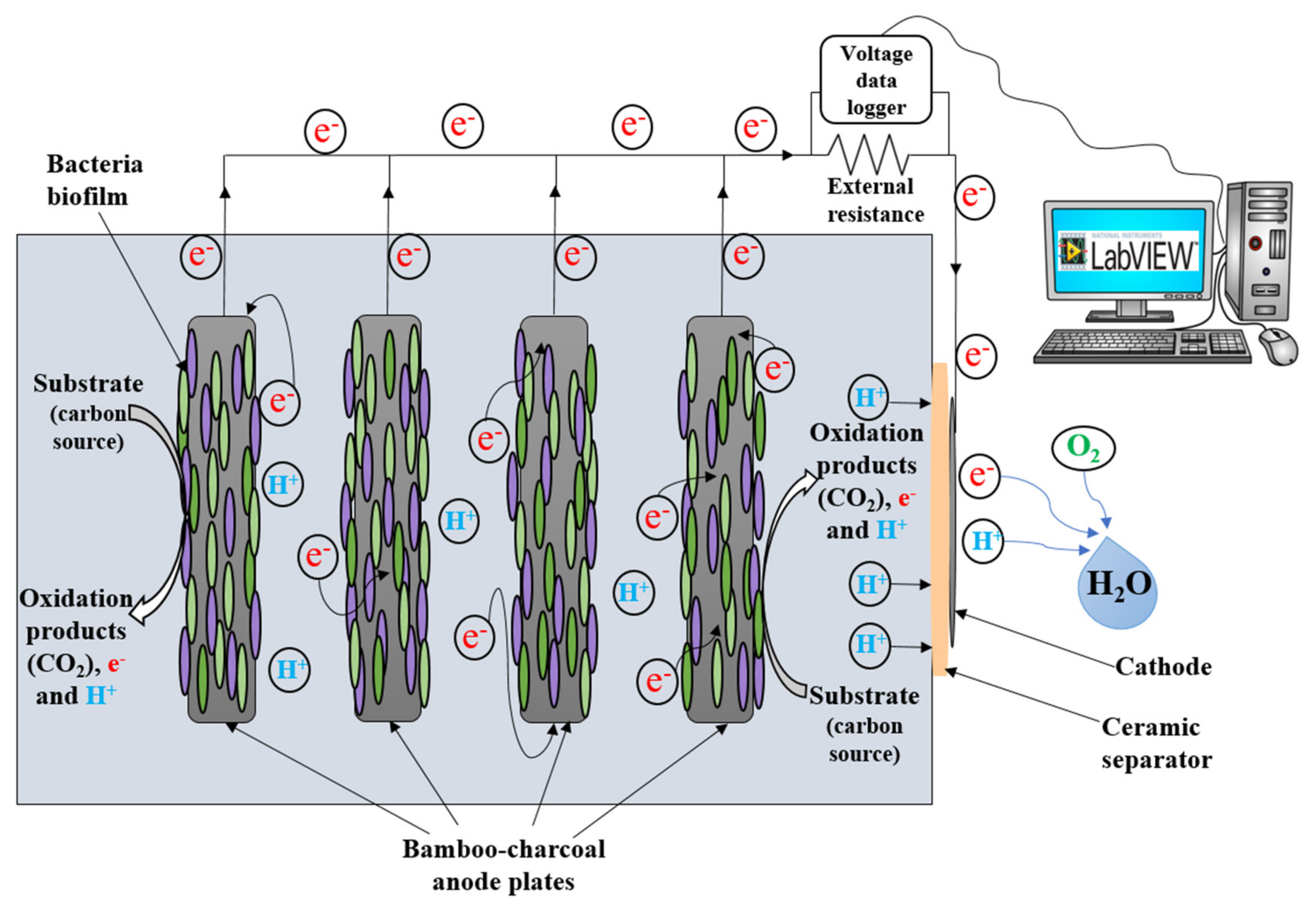
2.1. Microbial Fuel Cell
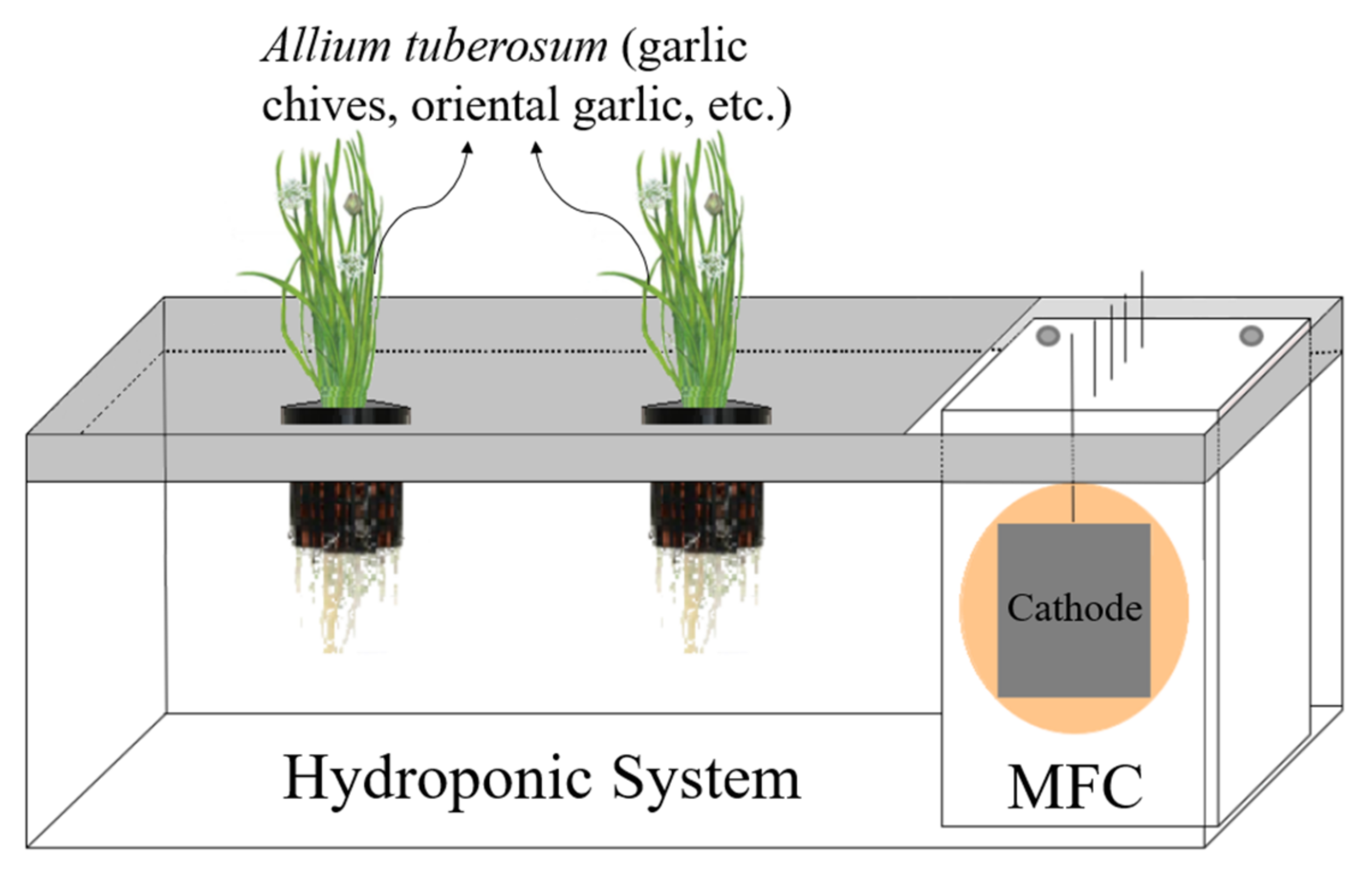
2.2. MFC—Hydroponic System
2.3. Bacterial Culture and Substrate Wastewater
2.4. MFC-Hyp System Operation, Monitoring, and Analyses
3. Results
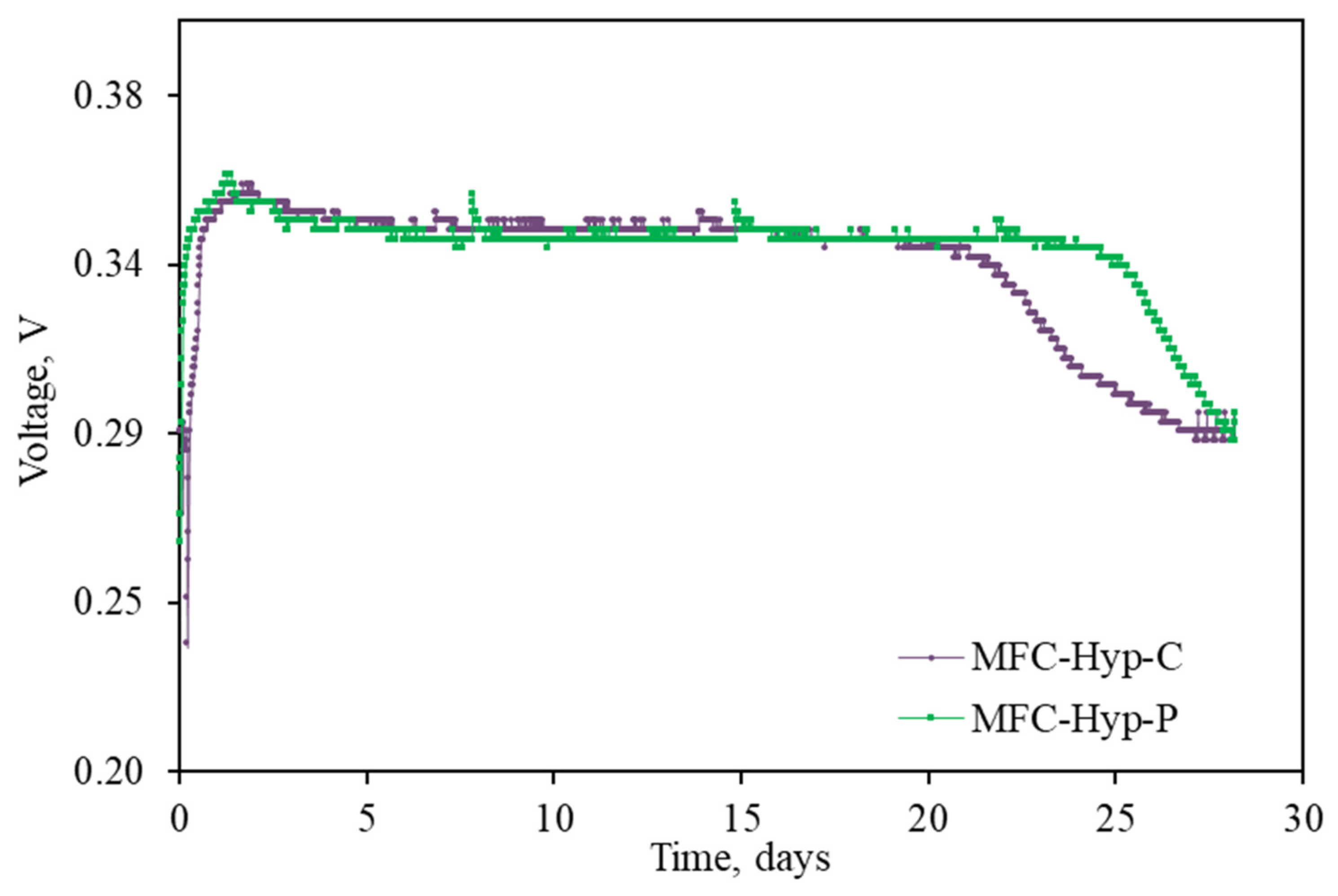
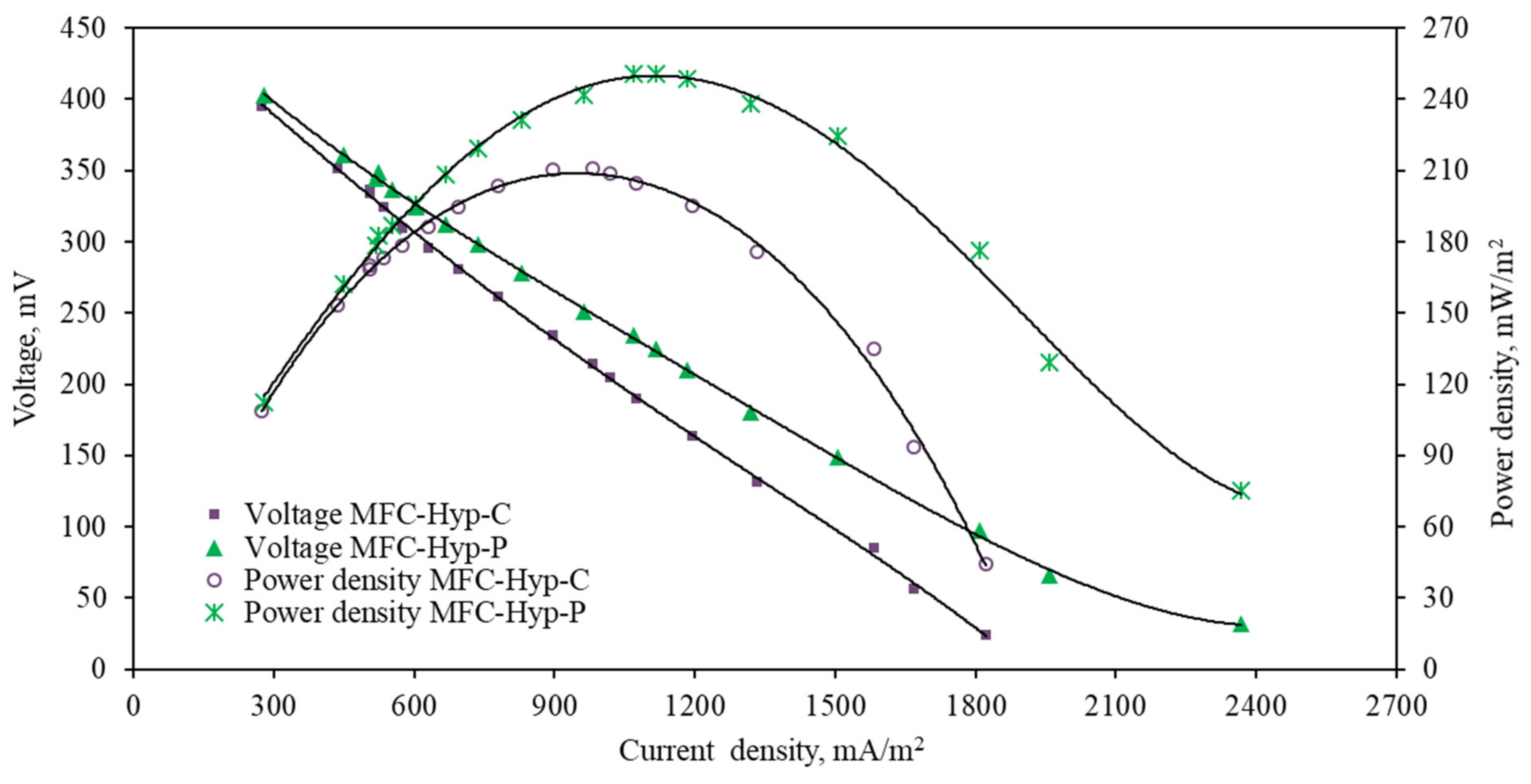
3.1. MFC-Hyp Performance
3.2. Nutrient and COD Removal
3.3. Phosphate Recovery
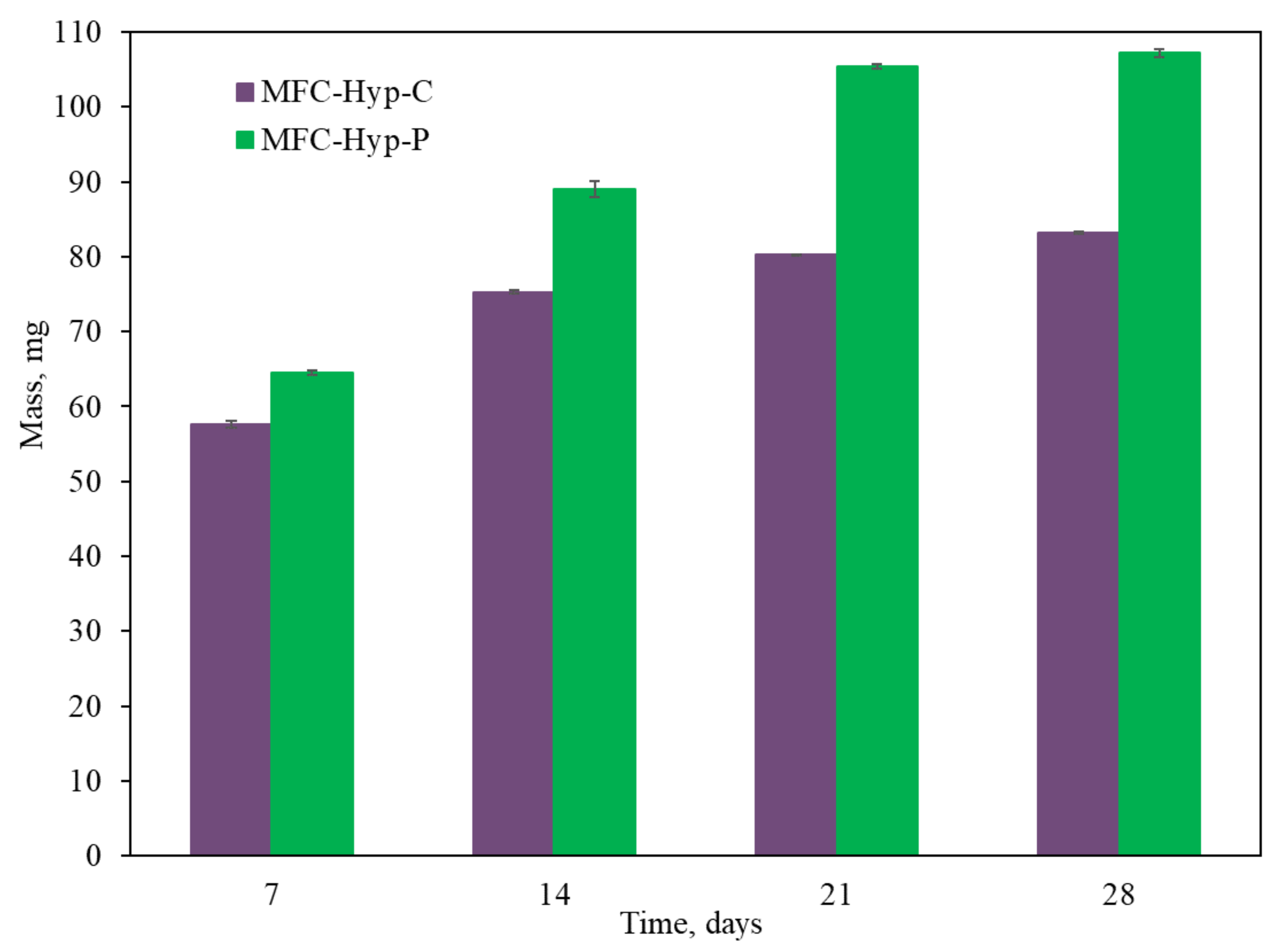
3.4. Plant Biomass
3.5. Coulombic Efficiency
4. Discussion
4.1. MFC-Hyp Performance
4.2. Nutrient and COD Removal
4.3. Plant Biomass
4.4. Coulombic Efficiency (CE)
4.5. Ceramic Separator
5. Conclusions
6. Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jingyu, H.; Miwornunyuie, N.; Ewusi-Mensah, D.; Koomson, D. Assessing the factors influencing the performance of constructed wetland–microbial fuel cell integration. Water Sci. Technol. 2020, 81, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.N.; Nguyen, H.T.; Noori, T.; Min, B. Potential applications of algae in the cathode of microbial fuel cells for enhanced electricity generation with simultaneous nutrient removal and algae biorefinery: Current status and future perspectives. Bioresour. Technol. 2019, 292, 122010. [Google Scholar] [CrossRef] [PubMed]
- UN Environment Annual Report 2017. Available online: https://www.unep.org/annualreport/2017/index.php (accessed on 7 January 2022).
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Electricity generation and struvite recovery from human urine using microbial fuel cells. J. Chem. Technol. Biotechnol. 2016, 91, 647–654. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Zhou, Y.; Mao, X.Z. Eutrophication control strategies for highly anthropogenic influenced coastal waters. Sci. Total Environ. 2020, 705, 135760. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.; Smit, A. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Nghiem, L.D.; Zhang, X.; Wang, J. Effect of organic loading rate on the recovery of nutrients and energy in a dual-chamber microbial fuel cell. Bioresour. Technol. 2019, 281, 367–373. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Geerts, S.; Marchi, A.; Weemaes, M. Full-scale phosphorus recovery from digested wastewater sludge in Belgium—Part II: Economic opportunities and risks. Water Sci. Technol. 2015, 71, 495–502. [Google Scholar] [CrossRef]
- Marchi, A.; Geerts, S.; Weemaes, M.; Wim, S.; Christine, V. Full-scale phosphorus recovery from digested wastewater sludge in Belgium-part I: Technical achievements and challenges. Water Sci. Technol. 2015, 71, 487–494. [Google Scholar] [CrossRef]
- Mihelcic, J.R.; Fry, L.M.; Shaw, R. Global potential of phosphorus recovery from human urine and feces. Chemosphere 2011, 84, 832–839. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Freguia, S.; Logrieco, M.E.; Monetti, J.; Ledezma, P.; Virdis, B.; Tsujimura, S. Self-Powered Bioelectrochemical Nutrient Recovery for Fertilizer Generation from Human Urine. Sustainability 2019, 11, 5490. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Zhang, S.; Luo, G.; Liu, Y. Impacts of hydraulic retention time on a continuous flow mode dual-chamber microbial fuel cell for recovering nutrients from municipal wastewater. Sci. Total Environ. 2020, 734, 139220. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef]
- Chen, X.; Sun, D.; Zhang, X.; Liang, P.; Huang, X. Novel Self-driven Microbial Nutrient Recovery Cell with Simultaneous Wastewater Purification. Sci. Rep. 2015, 5, 15744. [Google Scholar] [CrossRef]
- Gardner-Dale, D.; Bradley, I.; Guest, J. Influence of solids residence time and carbon storage on nitrogen and phosphorus recovery by microalgae across diel cycles. Water Res. 2017, 121, 231–239. [Google Scholar] [CrossRef]
- Electric Power Research Institute (EPRI). Water & Sustainability: U. S. Electricity Consumption for the Water Supply & Treatment; The Next Half Century Topical Report; EPRI: Palo Alto, CA, USA, 2002. [Google Scholar]
- U.S. Department of Energy. The Water-Energy Nexus: Challenges and Opportunities; US Department of Energy: Washington, DC, USA, 2014.
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: Nutrient, organics removal and bioenergy production. Chem. Eng. J. 2018, 332, 277–285. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Paucar, N.; Sato, C. Microbial Fuel Cell for Energy Production, Nutrient Removal and Recovery from Wastewater: A Review. Processes 2021, 9, 1318. [Google Scholar] [CrossRef]
- Paucar, N.E.; Sato, C. An Overview of Microbial Fuel Cells within Constructed Wetland for Simultaneous Nutrient Removal and Power Generation. Energies 2022, 15, 6841. [Google Scholar] [CrossRef]
- Sato, C.; Paucar, N.E.; Chiu, S.; Mahmud, M.Z.I.M.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes 2021, 9, 2194. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Ieropoulos, I.A. Microbial fuel cells directly powering a microcomputer. J. Power Sources 2019, 446, 227328. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Cecconet, D.; Molognoni, D.; Capodaglio, A.G. Sustainable processing of dairy wastewater: Long-term pilot application of a bio-electrochemical system. J. Clean. Prod. 2018, 189, 563–569. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Zhi, W.; Ge, Z.; He, Z.; Zhang, H. Methods for understanding microbial community structures and functions in microbial fuel cells: A review. Bioresour. Technol. 2014, 171, 461–468. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Rossi, R.; Jones, D.; Myung, J.; Zikmund, E.; Yang, W.; Gallego, Y.A.; Pant, D.; Evans, P.J.; Page, M.A.; Cropek, D.M.; et al. Evaluating a multi-panel air cathode through electrochemical and biotic tests. Water Res. 2018, 148, 51–59. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total. Environ. 2020, 698, 134154. [Google Scholar] [CrossRef]
- Ghadge, A.N.; Ghangrekar, M. Development of low cost ceramic separator using mineral cation exchanger to enhance performance of microbial fuel cells. Electrochimica Acta 2015, 166, 320–328. [Google Scholar] [CrossRef]
- Sato, C.; Martinez, R.G.; Shields, M.S.; Gracia, A.P.; Schoen, M.P. Behaviour of Microbial Fuel Cell in a start-up phase. Int. J. Environ. Eng. 2009, 1, 36. [Google Scholar] [CrossRef]
- Li, Z.; Haynes, R.; Sato, E.; Shields, M.S.; Fujita, Y.; Sato, C. Microbial Community Analysis of a Single Chamber Microbial Fuel Cell Using Potato Wastewater. Water Environ. Res. 2014, 86, 324–330. [Google Scholar] [CrossRef]
- Holmes, R.M.; Aminot, A.; Kérouel, R.; Hooker, B.A.; Peterson, B. A simple and precise method for measuring ammonium in marine and freshwater ecosystem. Can. J. Fish. Aquat. Sci. 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar]
- Chen, P.S.; Toribara, T.Y., Jr.; Warner, H. Microdetermination of phosphorus. Anal. Chem. 1956, 28, 1756–1758. [Google Scholar] [CrossRef]
- Rizzoni, G. Principles and Applications of Electrical Engineering, 2nd ed.; IRWIN: Chicago, IL, USA, 1996; pp. 97–98. [Google Scholar]
- Gupta, S.; Nayak, A.; Roy, C.; Yadav, A.K. An algal assisted constructed wetland-microbial fuel cell integrated with sand filter for efficient wastewater treatment and electricity production. Chemosphere 2021, 263, 128132. [Google Scholar] [CrossRef]
- Yang, X.-L.; Li, T.; Xia, Y.-G.; Singh, R.P.; Song, H.-L.; Zhang, H.; Wang, Y.-W. Microbial fuel cell coupled ecological floating bed for enhancing bioelectricity generation and nitrogen removal. Int. J. Hydrogen Energy 2021, 46, 11433–11444. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- USEPA (The United States Environmental Protection Agency). Quality Criteria for Water 1986; U.S. Environmental Protection Agency Report 440/5-86-001; Office of Water: Washington, DC, USA, 1986.
- USEPA (The United States Environmental Protection Agency). Free Water Surface Wetlands for Wastewater Treatment: A Technology Assessment Factsheet; USEPA: Washington, DC, USA, 2000.
- Khuman, C.N.; Bhowmick, G.D.; Ghangrekar, M.M.; Mitra, A. Effect of Using a Ceramic Separator on the Performance of Hydroponic Constructed Wetland-Microbial Fuel Cell. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020005. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chiranjeevi, P.; Sukrampal; Patil, S.A. Integrated drip hydroponics-microbial fuel cell system for wastewater treatment and resource recovery. Bioresour. Technol. Rep. 2020, 9, 100392. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef] [PubMed]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2017, 200, 198–220. [Google Scholar] [CrossRef]
- Colares, G.S.; Dell’Osbel, N.; Barbosa, C.V.; Lutterbeck, C.; Oliveira, G.A.; Rodrigues, L.R.; Bergmann, C.P.; Lopez, D.R.; Rodriguez, A.L.; Vymazal, J.; et al. Floating treatment wetlands integrated with microbial fuel cell for the treatment of urban wastewaters and bioenergy generation. Sci. Total. Environ. 2021, 766, 142474. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Justin, S.; Beckett, P.; Lythe, S. Root adaptation to soil waterlogging. Aquat. Bot. 1991, 39, 57–73. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for wastewater treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Sapkota, S.; Sapkota, S.; Liu, Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. [Google Scholar] [CrossRef]
- Chaney, R.; Coulombe, B. Effect of phosphate on regulation of FE-stress in soybean and peanut. J. Plant Nutr. 1982, 5, 469–487. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275. [Google Scholar] [CrossRef]
- Saz, Ç.; Türe, C.; Türker, O.C.; Yakar, A. Effect of vegetation type on treatment performance and bioelectric production of constructed wetland modules combined with microbial fuel cell (CW-MFC) treating synthetic wastewater. Environ. Sci. Pollut. Res. 2018, 25, 8777–8792. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Tang, A.; Deng, M.; Wu, R. Organic matter and ammonia removal by a novel integrated process of constructed wetland and microbial fuel cells. RSC Adv. 2019, 9, 5384–5393. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Garaniya, V.; Lewis, T.; Yadav, A.K. Performance of pilot-scale horizontal subsurface flow constructed wetland coupled with a microbial fuel cell for treating wastewater. J. Water Process. Eng. 2020, 33, 100994. [Google Scholar] [CrossRef]
- Chaijak, P.; Sato, C.; Lertworapreecha, M.; Sukkasem, C.; Boonsawang, P.; Paucar, N. Potential of Biochar-Anode in a Ceramic-Separator Microbial Fuel Cell (CMFC) with a Laccase-Based Air Cathode. Pol. J. Environ. Stud. 2019, 29, 499–503. [Google Scholar] [CrossRef]
- Tremouli, A.; Greenman, J.; Ieropoulos, I. Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 2018, 123, 19–25. [Google Scholar] [CrossRef]
- Winfield, J.; Gajda, I.; Greenman, J.; Leroulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303. [Google Scholar] [CrossRef]
- Ren, B.; Wang, T.; Zhao, Y. Two-stage hybrid constructed wetland-microbial fuel cells for swine wastewater treatment and bioenergy generation. Chemosphere 2021, 268, 128803. [Google Scholar] [CrossRef]
- Yakar, A.; Türe, C.; Türker, O.C.; Vymazal, J.; Saz, Ç. Impacts of various filtration media on wastewater treatment and bioelectric production in up-flow constructed wetland combined with microbial fuel cell (UCW-MFC). Ecol. Eng. 2018, 117, 120–132. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. The effects of elecdrode spacing and flow direction on the performance of microbial fuel cells-constructed wetland. Ecol. Eng. 2015, 79, 8–14. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Lehl, H.K.; Thung, W.-E. Hybrid system up-flow constructed wetland integrated with microbial fuel cell for simultaneous wastewater treatment and electricity generation. Bioresour. Technol. 2015, 186, 270–275. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary investigation of constructed wetland incorporating microbial fuel cell: Batch and continuous flow trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef]


| Type of System/Characteristics | Type of Wastewater/Electrodes | Plant Type/External Resistance | Average Voltage mV | Max Power Density mW/m2 | Current Density mA/m2 | CE % | COD Removal % | Nitrate Removal % | Phosphate Removal % | Plant Biomass Grow | Phosphate Recovery % | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two upflow hydroponic CW-MFC (with ceramic separator, without ceramic separator) Continuous mode | Synthetic wastewater Anode and cathode: carbon felts | Canna indica 1000 Ω | With ceramic separator: ~900 mV Without ceramic separator: ~800 mV | With ceramic separator: 258.78 mW.m−3 Without ceramic separator: 91.02 mW.m−3 | With ceramic separator: ~560 mA.m−3 Without ceramic separator: ~190 mA.m−3 | NA | With ceramic separator: 86.2 ± 8.1% Without ceramic separator: 91.5 ± 4.9% | NA | NA | NA | NA | [46] |
| Integrated drip hydroponics-MFC Batch recirculation mode | Domestic sewage collected from the sedimentation tank of the primary treatment unit Anode and cathode: non-catalyzed disc-shaped graphite | Cymbopogon citratus 20 kΩ | In series: 1490 ± 91 mV In parallel: 1580 ± 5 mV | 31.9 mW.m−2 in series and parallel | In series: ~36 mA.m−2 In parallel: ~458 mA.m−2 | NA | 72 ± 2.4% at HRT = 3 h 85.7 ± 0.6% at HRT = 12 h | NA | 83.2 ± 1.1% at HRT = 3 h 85.8 ± 0.6% at HRT = 12 h | Per plant: 45 ± 15 cm 0.216 ± 0.039 g | NA | [47] |
| Floating treatment wetlands-MFC Closed system for 3 weeks | Urban wastewater Cathode: graphite rods Anode: PVC hose filled with graphite sticks | Canna generalis Chrysopogon zizanioides Cyperus papyrus Nanus Hymenachne grumosa Equisetum hyemale 1000 Ω | Maximum voltages in: Open circuit: 225 mV (C generalis) 212 mV (H. grumosa) 144 mV (C. zizanioides) 137.4 (C. papyrus Nanus) 89.6 mV (E. hyemale) Closed circuit: 21.0 mV (C generalis) | 0.93 mWm−2 (When max voltage is 108 mV from all plants in parallel) | NA | NA | 71.4% | TN: 8.4% | TP: 11.4% | NA | NA | [51] |
| Ecological floating bed-MFC After 30 days start-up period, operated continuously for 116 days | Synthetic eutrophication influent Anode and cathode: stainless-steel mesh and carbon felt | Cyperus alternifolius Linn. subsp. flabelliformis (Rottb.) Kukenth (EFB-MFC1) Ceratophyllum demersum Linn. (EFB-MFC2) Eichhornia crassipes (Mart.) Solms Pontereia crassipes Mart. (EFB-MFC3) Ipomoea aquatic Forssk (EFB-MFC4) 500 Ω | Control: 99 mV EFB-MFC1: 125 mV EFB-MFC2: 144 mV EFB-MFC3: 157 mV EFB-MFC4: 161 mV | The maximum power density was EFB-MFC4: 6.03 mWm−2 | NA | NA | Control: 73.88% EFB-MFC1: 73% EFB-MFC2: 76.37% EFB-MFC3: 78.23% EFB-MFC4: 82.49% | TN: Control: 38.74% EFB-MFC1: 34.76% EFB-MFC2: 41.65% EFB-MFC3: 51.21% EFB-MFC4: 55.6% | NA | NA | NA | [42] |
| MFC-Hyp-Plants Batch mode | Synthetic potato wastewater Anode: bamboo charcoal plates Cathode: platinum-coated carbon cloth | Allium tuberosum 973 Ω | Max 403 mV | 250.7 | Max 2367.9 | 4.8 | 80.4 ± 1.39 | 31.7 ± 0.17 | 11.4 ± 0.02 | P1: 6.5 cm P2: 7.5 cm PC: 2.2 cm | 7.3 | This study |
| MFC-Hyp-Control Batch mode | Synthetic potato wastewater Anode: bamboo charcoal plates Cathode: platinum-coated carbon cloth | 973 Ω | Max 396 mV | 210.7 | Max 1821.6 | 5.5 | 78.7 ± 1.66 | 28.9 ± 0.10 | 8.9 ± 0.08 | NA | 4.7 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar, N.E.; Sato, C. Coupling Microbial Fuel Cell and Hydroponic System for Electricity Generation, Organic Removal, and Nutrient Recovery via Plant Production from Wastewater. Energies 2022, 15, 9211. https://doi.org/10.3390/en15239211
Paucar NE, Sato C. Coupling Microbial Fuel Cell and Hydroponic System for Electricity Generation, Organic Removal, and Nutrient Recovery via Plant Production from Wastewater. Energies. 2022; 15(23):9211. https://doi.org/10.3390/en15239211
Chicago/Turabian StylePaucar, N. Evelin, and Chikashi Sato. 2022. "Coupling Microbial Fuel Cell and Hydroponic System for Electricity Generation, Organic Removal, and Nutrient Recovery via Plant Production from Wastewater" Energies 15, no. 23: 9211. https://doi.org/10.3390/en15239211
APA StylePaucar, N. E., & Sato, C. (2022). Coupling Microbial Fuel Cell and Hydroponic System for Electricity Generation, Organic Removal, and Nutrient Recovery via Plant Production from Wastewater. Energies, 15(23), 9211. https://doi.org/10.3390/en15239211







