Membrane Electrode Assembly Degradation Modeling of Proton Exchange Membrane Fuel Cells: A Review
Abstract
:1. Introduction
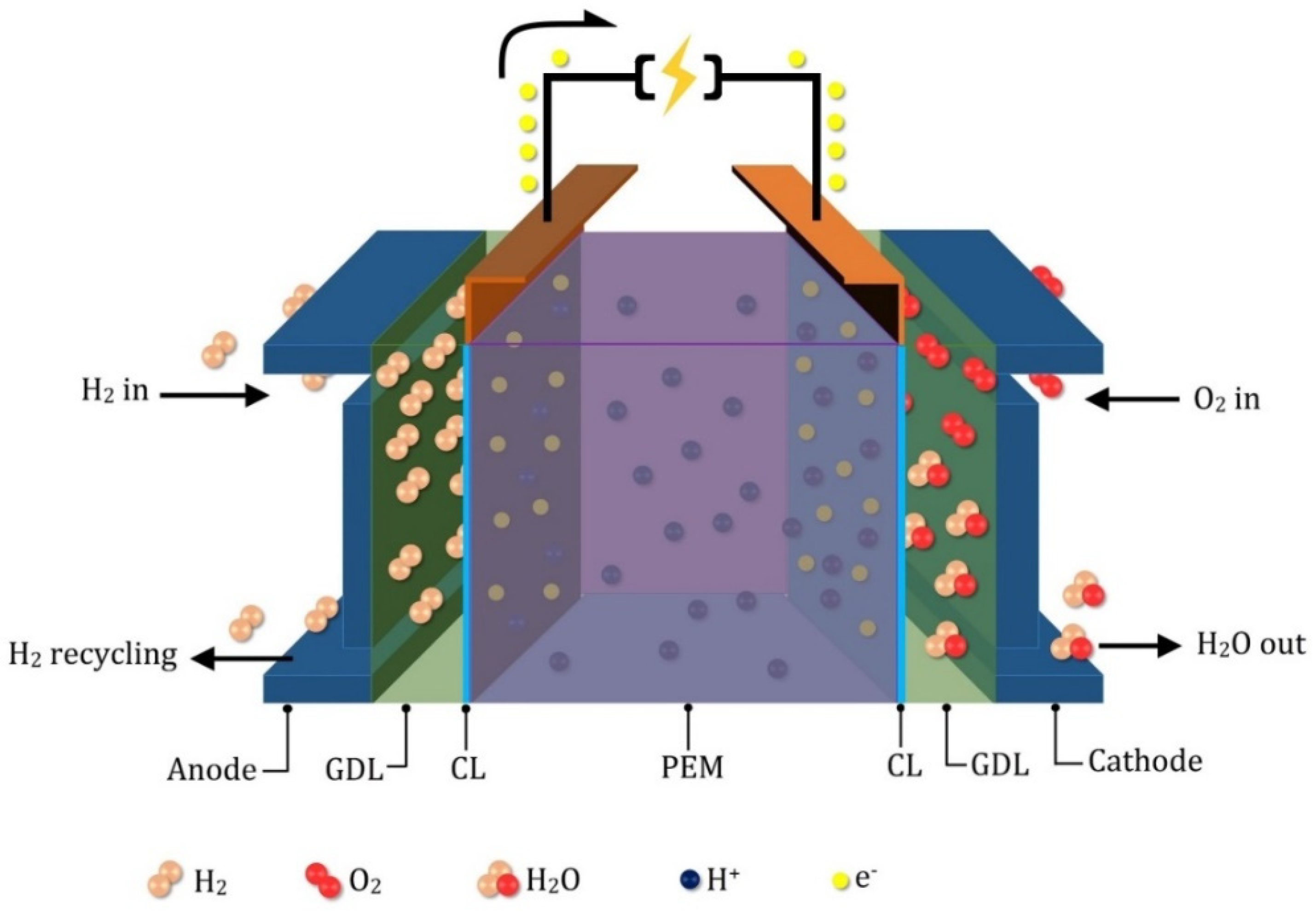
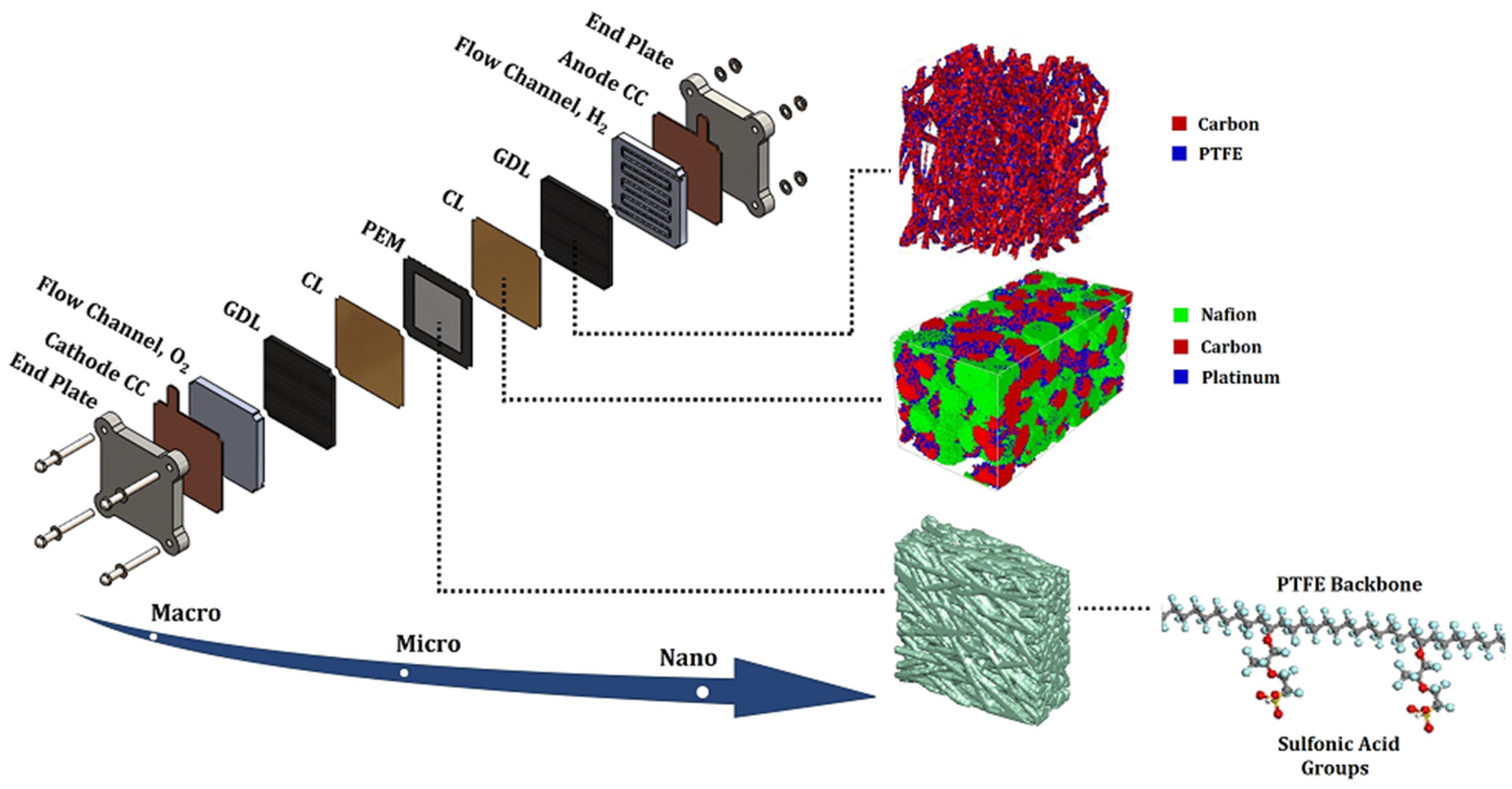
2. MEA Degradation Modeling
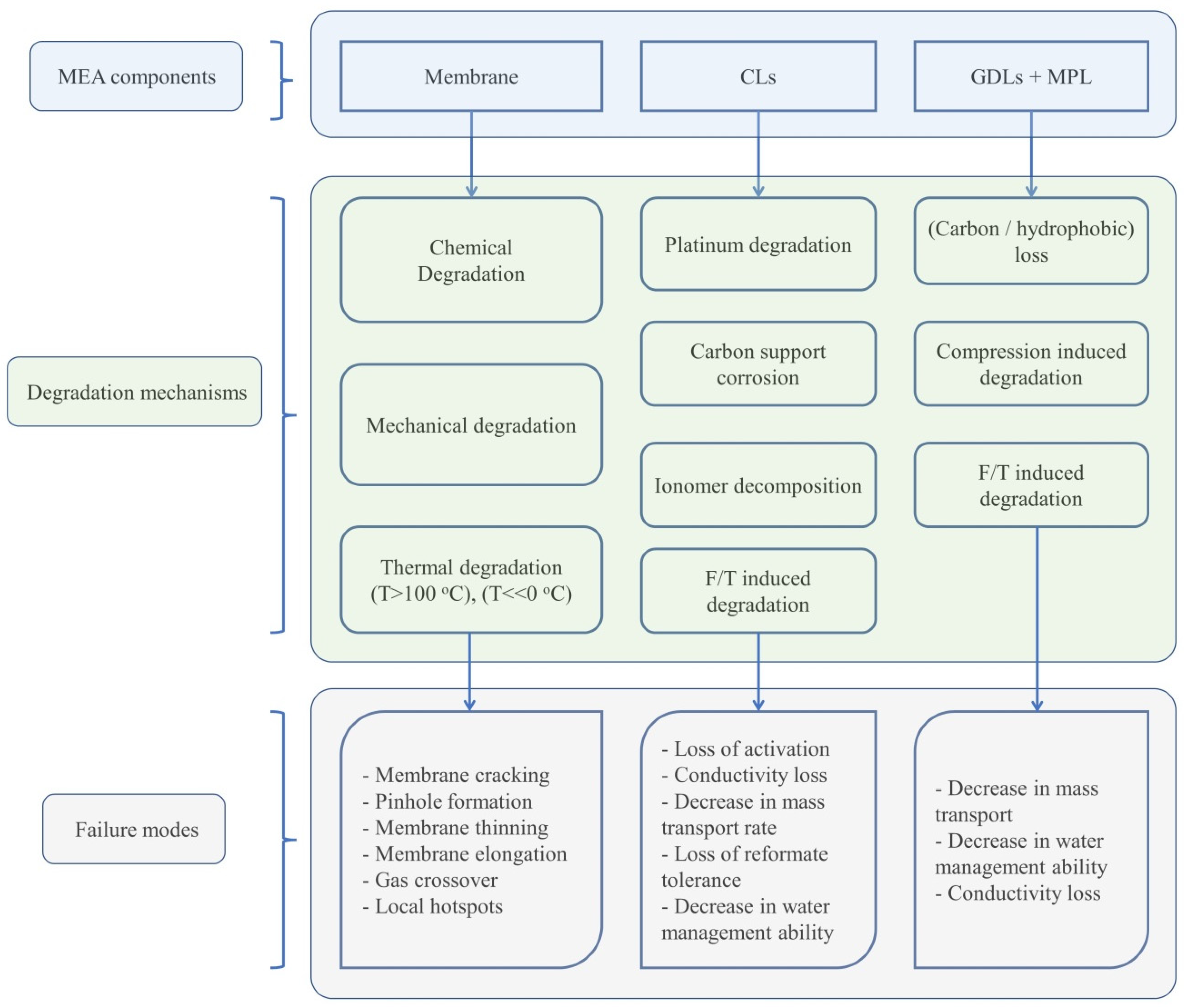
2.1. Proton Exchange Membrane
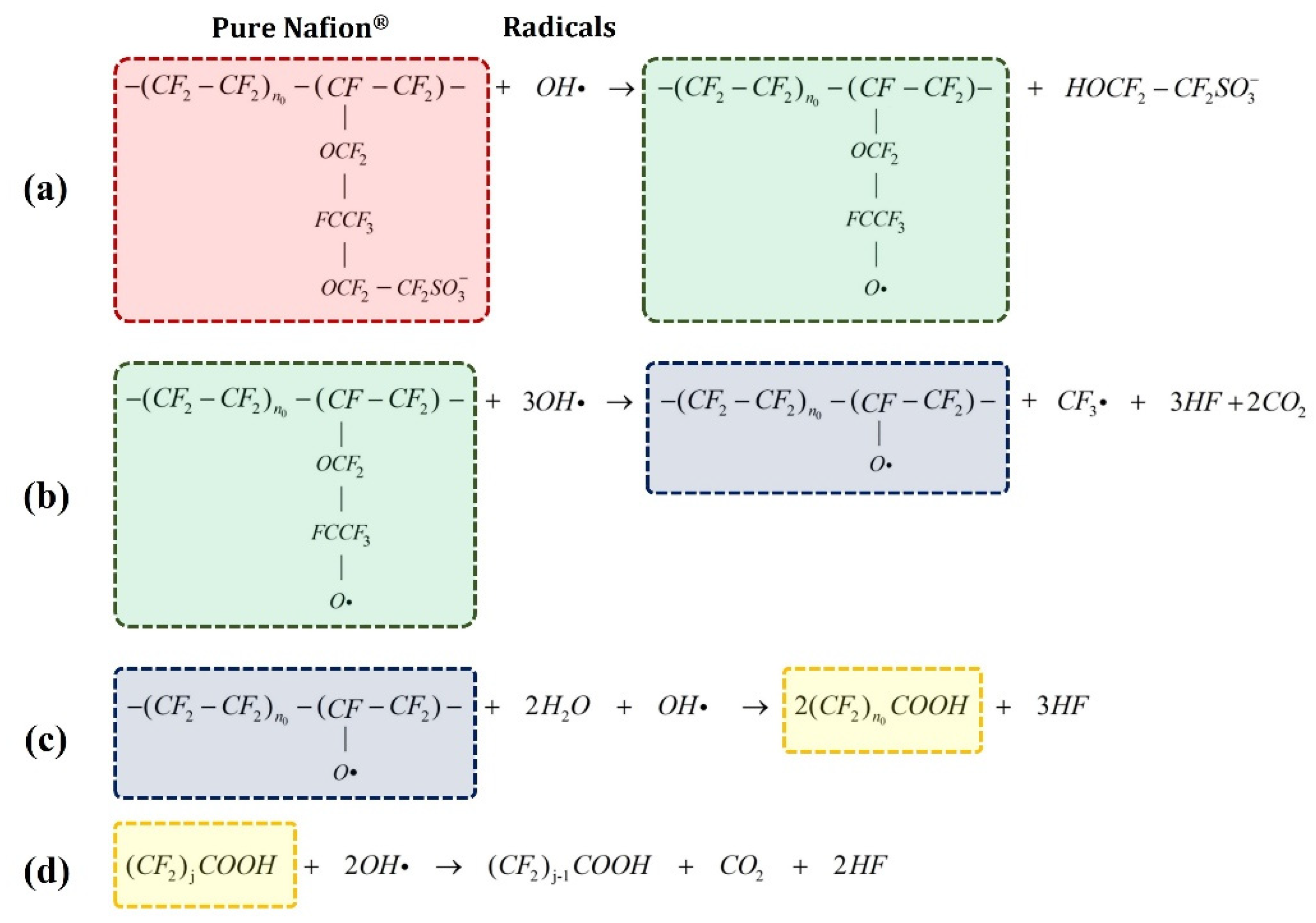
2.1.1. Chemical Degradation
2.1.2. Mechanical Degradation
2.1.3. Thermal Degradation
2.1.4. Brief Summary of Membrane Degradation Models
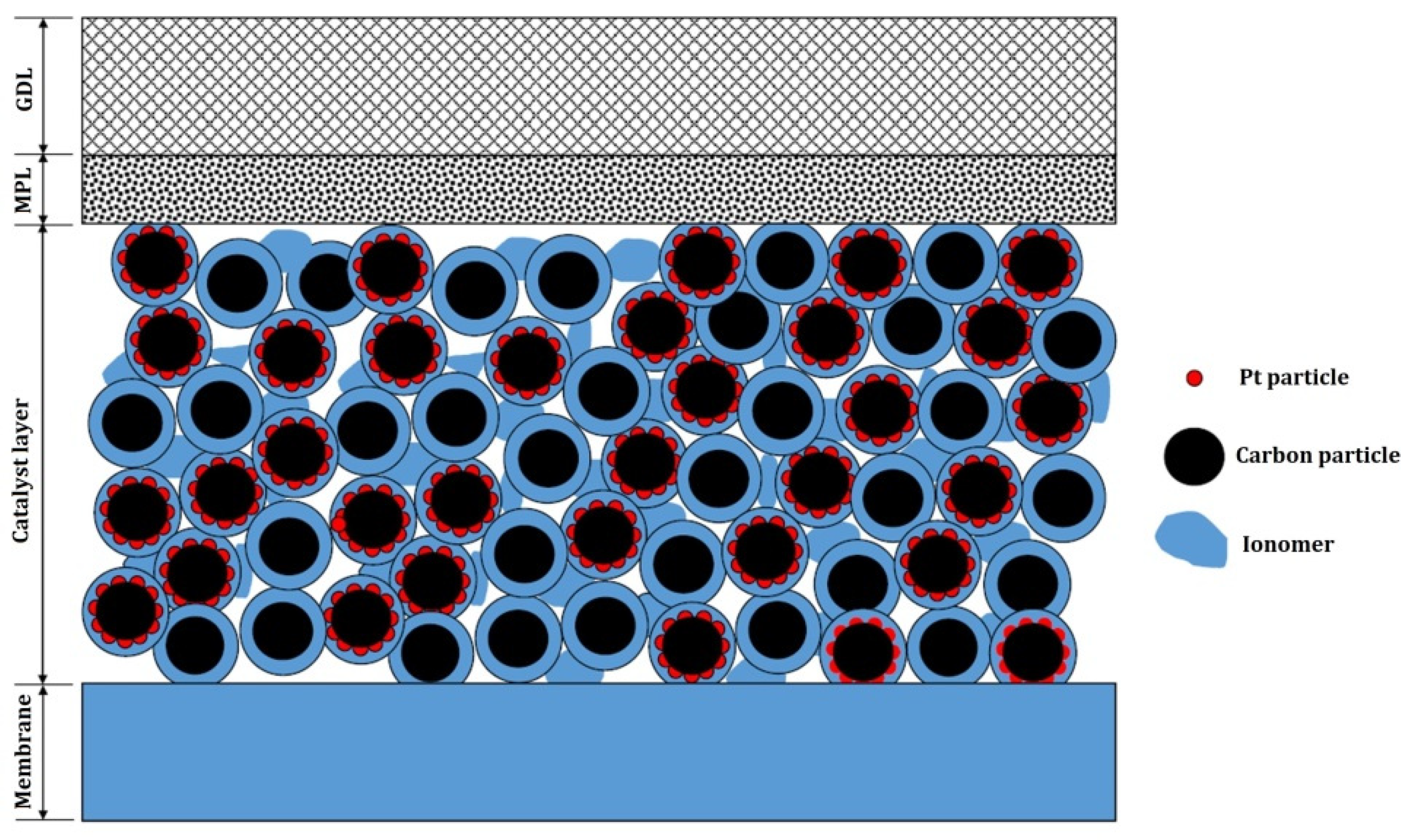
2.2. CL
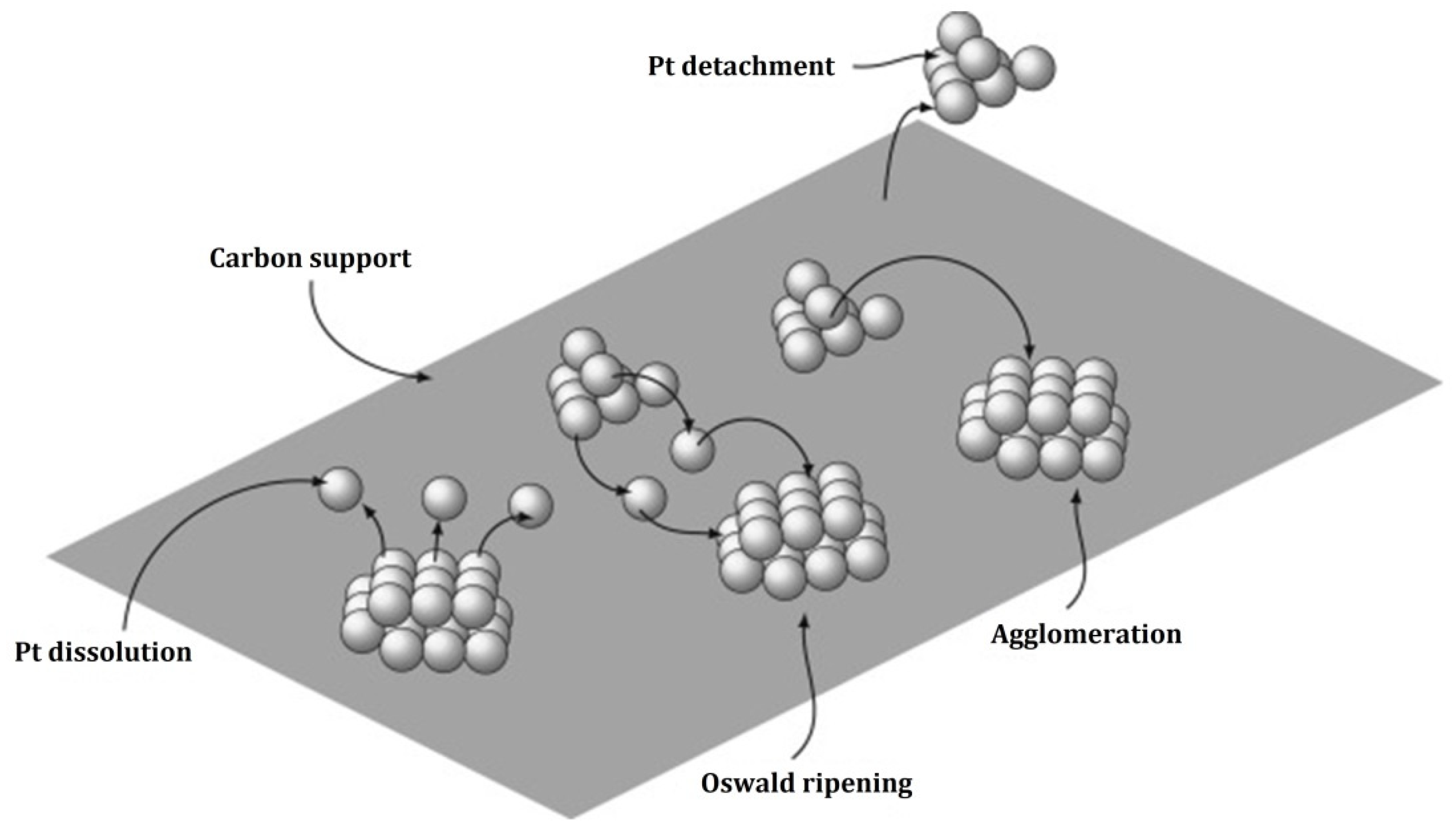
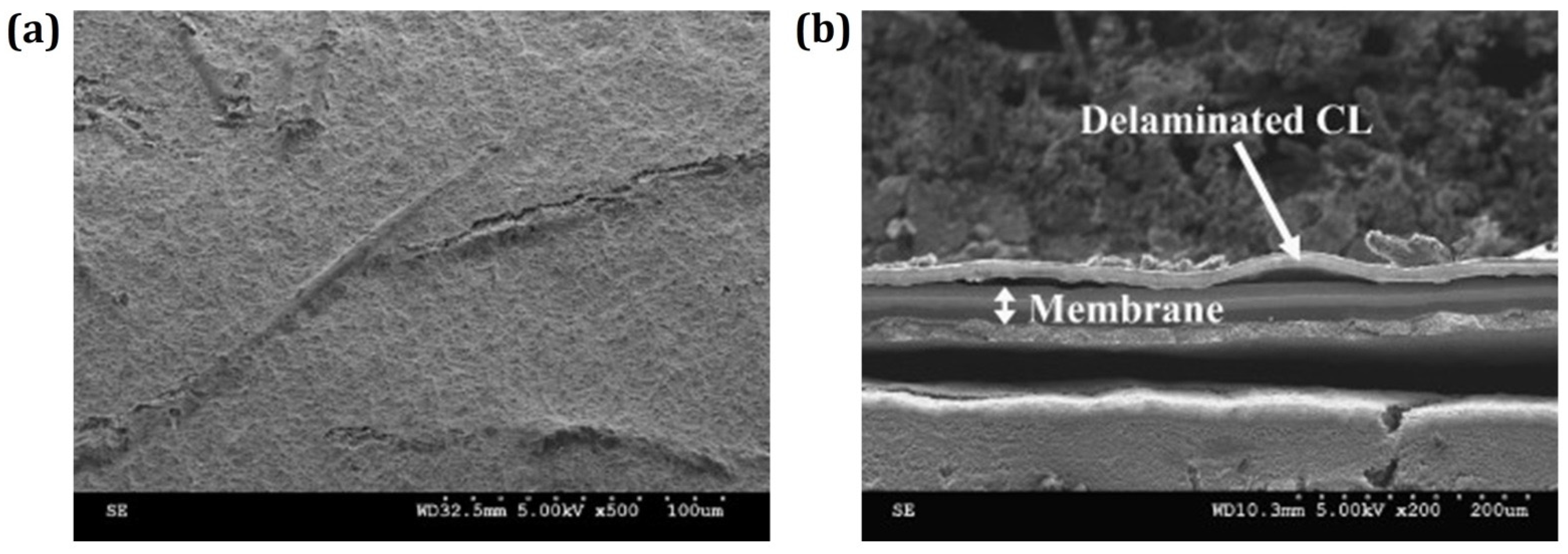
2.2.1. Degradation Mechanism
2.2.2. Degradation Modeling
2.2.3. Brief Summary of CL Degradation Models
2.3. GDL
2.3.1. Degradation Mechanisms
2.3.2. Degradation Modeling
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, X.Y.; Zhakeyev, A.; Wang, H.; Jiao, K.; Zhang, H.; Xuan, J. Accelerating Fuel Cell Development with Additive Manufacturing Technologies: State of the Art, Opportunities and Challenges. Fuel Cells 2019, 19, 636–650. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, P.; Chen, H.; Pei, P. A review of automotive proton exchange membrane fuel cell degradation under start-stop operating condition. Appl. Energy 2018, 223, 249–262. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A. A review of high-temperature proton exchange membrane fuel cell ( HT-PEMFC ) system. Int. J. Hydrog. Energy 2016, 42, 9293–9314. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, G.; Zhang, X.; Sun, C.; Jiao, K.; Wang, Y. Thermal management of polymer electrolyte membrane fuel cells: A review of cooling methods, material properties, and durability. Appl. Energy 2021, 286, 116496. [Google Scholar] [CrossRef]
- Wang, F. −20 °C cold start of fuel cell engine. Shanghai Auto 2017, 8, 3–6. [Google Scholar]
- Dafalla, A.M.; Jiang, F. Stresses and their impacts on proton exchange membrane fuel cells: A review. Int. J. Hydrog. Energy 2018, 43, 2327–2348. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- El-kharouf, A.; Chandan, A.; Hattenberger, M.; Pollet, B.G. Proton exchange membrane fuel cell degradation and testing: Review. J. Energy Inst. 2012, 85, 188–200. [Google Scholar] [CrossRef]
- Ahmadi, P.; Hosein, S.; Afsaneh, H. The effects of driving patterns and PEM fuel cell degradation on the lifecycle assessment of hydrogen fuel cell vehicles. Int. J. Hydrog. Energy 2019, 45, 3595–3608. [Google Scholar] [CrossRef]
- Raeesi, M.; Changizian, S.; Ahmadi, P.; Khoshnevisan, A. Performance analysis of a degraded PEM fuel cell stack for hydrogen passenger vehicles based on machine learning algorithms in real driving conditions. Energy Convers. Manag. 2021, 248, 114793. [Google Scholar] [CrossRef]
- Stropnik, R.; Mlakar, N.; Lotri, A.; Sekav, M.; Mori, M. The influence of degradation effects in proton exchange membrane fuel cells on life cycle assessment modelling and environmental impact indicators. Int. J. Hydrog. Energy 2022, 47, 24223–24241. [Google Scholar] [CrossRef]
- Vichard, L.; Steiner, N.Y.; Zerhouni, N.; Hissel, D. Hybrid fuel cell system degradation modeling methods: A comprehensive review. J. Power Sources 2021, 506, 230071. [Google Scholar] [CrossRef]
- Shahraki, A.F.; Yadav, O.P.; Liao, H. A review on degradation modelling and its engineering applications. Int. J. Perform. Eng. 2017, 13, 299–314. [Google Scholar] [CrossRef]
- Wu, H.-W. A review of recent development: Transport and performance modeling of PEM fuel cells. Appl. Energy 2016, 165, 81–106. [Google Scholar] [CrossRef]
- Tzelepis, S.; Kavadias, K.A.; Marnellos, G.E.; Xydis, G. A review study on proton exchange membrane fuel cell electrochemical performance focusing on anode and cathode catalyst layer modelling at macroscopic level. Renew. Sustain. Energy Rev. 2021, 151, 111543. [Google Scholar] [CrossRef]
- Jiao, K.; Ni, M. Challenges and opportunities in modelling of proton exchange membrane fuel cells (PEMFC). Int. J. Energy Res. 2017, 41, 1793–1797. [Google Scholar] [CrossRef] [Green Version]
- Alaswad, A.; Omran, A.; Sodre, J.R.; Wilberforce, T.; Pignatelli, G.; Dassisti, M.; Baroutaji, A.; Olabi, A.G. Technical and commercial challenges of proton-exchange membrane (Pem) fuel cells. Energies 2021, 14, 144. [Google Scholar] [CrossRef]
- Liu, Q.; Lan, F.; Chen, J.; Zeng, C.; Wang, J. A review of proton exchange membrane fuel cell water management: Membrane electrode assembly. J. Power Sources 2022, 517, 230723. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Araya, S.S.; Li, N.; Liso, V. Degradation and failure modes in proton exchange membrane fuel cells. In PEM Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2022; pp. 199–222. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Prog. Energy Combust. Sci. 2020, 80, 100859. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques DP AGDL: Anode gas diffusion layer ACL: Anode catalyst layer PEM: Polymer electrolyte membrane CGDL: Cathode gas d. Energy Convers. Manag. 2020, 199, 112022. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Hissel, D.; Lu, J.; Hou, M.; Shao, Z. Prognostics methods and degradation indexes of proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2020, 123, 109721. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Han, J.; Nguyen, X.L.; Yu, S.; Goo, Y.M.; Le, D.D. Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols. Energies 2021, 14, 4048. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Li, H.; Zhang, S.; Martin, J.; Wang, H. A review of polymer electrolyte membrane fuel cell durability test protocols. J. Power Sources 2011, 196, 9107–9116. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Zheng, Z.; Pahon, E.; Péra, M.C.; Gao, F. A review on lifetime prediction of proton exchange membrane fuel cells system. J. Power Sources 2022, 529, 231256. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, H.; Brandon, N.P. Gas diffusion layer degradation in proton exchange membrane fuel cells: Mechanisms, characterization techniques and modelling approaches. J. Power Sources 2021, 513, 230560. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ben, I.; Emori, W.; Uzoma, P.C. ScienceDirect Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrog. Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A review of membranes in proton exchange membrane fuel cells: Transport phenomena, performance and durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar] [CrossRef]
- Ramos-s, G.; Dang, N.; Balbuena, P.B. Multi-Scale Simulation Study of Pt-Alloys Degradation for Fuel Cells Applications; Springer: London, UK, 2016; pp. 37–59. [Google Scholar] [CrossRef]
- Jahnke, T.; Futter, G.; Latz, A.; Malkow, T.; Papakonstantinou, G.; Tsotridis, G.; Schott, P.; Gérard, M.; Quinaud, M.; Quiroga, M.; et al. Performance and degradation of Proton Exchange Membrane Fuel Cells: State of the art in modeling from atomistic to system scale. J. Power Sources 2016, 304, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.A. Multi-scale Modeling-based Prediction of PEM Fuel Cells MEA Durability under Automotive Operating Conditions. ECS Trans. 2009, 25, 65. [Google Scholar] [CrossRef]
- Franco, A.A. Physical Multiscale Modeling of Degradation Phenomena in PEM Fuel Cells. In ECS Meeting Abstracts; MA2011-01; IOP Publishing: Bristol, UK, 2011; p. 1905. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Liu, J.; Wang, N.; Abdalla, A.S.; Jiang, F. Numerical Simulation of High Temperature PEM Fuel Cell Performance under Different Key Operating and Design Parameters. J. Adv. Therm. Sci. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Kwan, S.; Vinothkannan, M.; Rhan, A.; Jin, D. Effect of type and stoichiometry of fuels on performance of polybenzimidazole-based proton exchange membrane fuel cells operating at the temperature range of 120 e 160 C. Energy 2022, 238, 121791. [Google Scholar] [CrossRef]
- Wang, X.; Habte, B.T.; Zhang, S.; Yang, H.; Zhao, J.; Jiang, F.; He, Q. Localized Electrochemical Impedance Measurements on Nafion Membranes: Observation and Analysis of Spatially Diverse Proton Transport Using Atomic Force Microscopy. Anal. Chem. 2019, 91, 11678–11686. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N. Fuel cell membranes—Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Li, B.; Yang, D.; Ming, P.; Zhang, C.; Wang, Y. ScienceDirect Review of hydrogen crossover through the polymer electrolyte membrane. Int. J. Hydrog. Energy 2021, 46, 22040–22061. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 35. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Hakim, N.; Mukerjee, S. Degradation mechanism study of perfluorinated proton exchange membrane under fuel cell operating conditions. Electrochim. Acta 2008, 53, 3279–3295. [Google Scholar] [CrossRef]
- Wong, K.H.; Kjeang, E. Macroscopic In-Situ Modeling of Chemical Membrane Degradation in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2014, 161, F823–F832. [Google Scholar] [CrossRef]
- Khattra, N.S.; Hannach, M.; El Wong, K.H.; Lauritzen, M.; Kjeang, E. Estimating the Durability of Polymer Electrolyte Fuel Cell Membranes Using a Fracture Percolation Model. J. Electrochem. Soc. 2020, 167, 013528. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Shi, S.; Fu, Y.; Chen, J.; Lin, Q.; Hu, J.; Li, C.; Li, J.; Chen, X. Embrittlement induced fracture behavior and mechanisms of perfluorosulfonic-acid membranes after chemical degradation. J. Power Sources 2020, 453, 227893. [Google Scholar] [CrossRef]
- Chandesris, M.; Vincent, R.; Guetaz, L.; Roch, J.; Thoby, D.; Quinaud, M. ScienceDirect Membrane degradation in PEM fuel cells: From experimental results to semi-empirical degradation laws. Int. J. Hydrog. Energy 2017, 42, 8139–8149. [Google Scholar] [CrossRef]
- Ehlinger, V.M.; Kusoglu, A.; Weber, A.Z. Modeling Coupled Durability and Performance in Polymer-Electrolyte Fuel Cells: Membrane Effects. J. Electrochem. Soc. 2019, 166, F3255–F3267. [Google Scholar] [CrossRef]
- Ehlinger, V.M.; Crothers, A.R.; Kusoglu, A.; Weber, A.Z. Modeling proton-exchange-membrane fuel cell performance/degradation tradeoffs with chemical scavengers. J. Phys. Energy 2020, 2, 044006. [Google Scholar] [CrossRef]
- Karpenko-Jereb, L.; Sternig, C.; Fink, C.; Tatschl, R. Membrane degradation model for 3D CFD analysis of fuel cell performance as a function of time. Int. J. Hydrog. Energy 2016, 41, 13644–13656. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Guo, X.; Shi, S.W.; Weng, G.J.; Chen, G.; Lu, J. Biaxial fatigue crack growth in proton exchange membrane of fuel cells based on cyclic cohesive finite element method. Int. J. Mech. Sci. 2021, 189, 105946. [Google Scholar] [CrossRef]
- Singh, R.; Sui, P.C.; Wong, K.H.; Kjeang, E.; Knights, S.; Djilali, N. Modeling the Effect of Chemical Membrane Degradation on PEMFC Performance. J. Electrochem. Soc. 2018, 165, F3328–F3336. [Google Scholar] [CrossRef] [Green Version]
- Futter, G.A.; Latz, A.; Jahnke, T. Physical modeling of chemical membrane degradation in polymer electrolyte membrane fuel cells: Influence of pressure, relative humidity and cell voltage. J. Power Sources 2019, 410–411, 78–90. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Zhou, C.; Guerra, M.A.; Qiu, Z.M.; Zawodzinski, T.A.; Schiraldi, D.A. Chemical durability studies of perfluorinated sulfonic acid polymers and model compounds under mimic fuel cell conditions. Macromolecules 2007, 40, 8695–8707. [Google Scholar] [CrossRef]
- Xie, T.; Hayden, C.A. A kinetic model for the chemical degradation of perfluorinated sulfonic acid ionomers: Weak end groups versus side chain cleavage. Polymer 2007, 48, 5497–5506. [Google Scholar] [CrossRef]
- Wong, K.H.; Kjeang, E. In-Situ Modeling of Chemical Membrane Degradation and Mitigation in Ceria-Supported Fuel Cells. J. Electrochem. Soc. 2017, 164, F1179–F1186. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, L.; Hu, Z.; Ding, Y.; Li, J.; Ouyang, M. Dynamic modeling of chemical membrane degradation in polymer electrolyte fuel cells: Effect of pinhole formation. J. Power Sources 2021, 487, 229367. [Google Scholar] [CrossRef]
- Mahmoudi, A.H.; Ramiar, A.; Esmaili, Q. Effect of inhomogeneous compression of gas diffusion layer on the performance of PEMFC with interdigitated flow field. Energy Convers. Manag. 2016, 110, 78–89. [Google Scholar] [CrossRef]
- Kulkarni, N.; Kok, M.D.; Jervis, R.; Iacoviello, F.; Meyer, Q.; Shearing, P.R.; Brett, D.J. The effect of non-uniform compression and flow-field arrangements on membrane electrode assemblies—X-ray computed tomography characterisation and effective parameter determination. J. Power Sources 2019, 426, 97–110. [Google Scholar] [CrossRef]
- Ding, G.; Santare, M.H.; Karlsson, A.M.; Kusoglu, A. Numerical evaluation of crack growth in polymer electrolyte fuel cell membranes based on plastically dissipated energy. J. Power Sources 2016, 316, 114–123. [Google Scholar] [CrossRef]
- Singh, Y.; White, R.T.; Najm, M.; Haddow, T.; Pan, V.; Orfino, F.P.; Dutta, M.; Kjeang, E. Tracking the evolution of mechanical degradation in fuel cell membranes using 4D in situ visualization. J. Power Sources 2019, 412, 224–237. [Google Scholar] [CrossRef]
- Ramani, D.; Singh, Y.; White, R.T.; Wegener, M.; Orfino, F.P.; Dutta, M.; Kjeang, E. 4D in situ visualization of mechanical degradation evolution in reinforced fuel cell membranes. Int. J. Hydrog. Energy 2020, 45, 10089–10103. [Google Scholar] [CrossRef]
- Singh, Y.; Orfino, F.P.; Dutta, M.; Kjeang, E. 3D Failure Analysis of Pure Mechanical and Pure Chemical Degradation in Fuel Cell Membranes. J. Electrochem. Soc. 2017, 164, F1331–F1341. [Google Scholar] [CrossRef]
- Kusoglu, A.; Karlsson, A.M.; Santare, M.H.; Cleghorn, S.; Johnson, W.B. Mechanical behavior of fuel cell membranes under humidity cycles and effect of swelling anisotropy on the fatigue stresses. J. Power Sources 2007, 170, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Kusoglu, A.; Weber, A.Z. A Mechanistic Model for Pinhole Growth in Fuel-Cell Membranes during Cyclic Loads. J. Electrochem. Soc. 2014, 161, E3311–E3322. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Pandian, S.; Dhathathreyan, K.S. Vibration tests on a PEM fuel cell stack usable in transportation application. Int. J. Hydrog. Energy 2009, 34, 3833–3837. [Google Scholar] [CrossRef]
- Çalık, A.; Yıldırım, S.; Tosun, E. Estimation of crack propagation in polymer electrolyte membrane fuel cell under vibration conditions. Int. J. Hydrog. Energy 2017, 42, 23347–23351. [Google Scholar] [CrossRef]
- Ahmed, H.E.U.; Banan, R.; Zu, J.W.; Bazylak, A. Free vibration analysis of a polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 5520–5525. [Google Scholar] [CrossRef]
- Banan, R.; Bazylak, A.; Zu, J. Effect of mechanical vibrations on damage propagation in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2013, 38, 14764–14772. [Google Scholar] [CrossRef]
- Banan, R.; Bazylak, A.; Zu, J. Combined effects of environmental vibrations and hygrothermal fatigue on mechanical damage in PEM fuel cells. Int. J. Hydrog. Energy 2015, 40, 1911–1922. [Google Scholar] [CrossRef]
- Wei, L.; Liao, Z.; Dafalla, A.M.; Jiang, F. Performance Investigation of Proton-Exchange Membrane Fuel Cell with Dean Flow Channels. Energy Technol. 2022, 10, 2100851. [Google Scholar] [CrossRef]
- Liao, Z.; Wei, L.; Mohmed, A.; Guo, J.; Jiang, F. Analysis of the impact of flow field arrangement on the performance of PEMFC with zigzag-shaped channels. Int. J. Heat Mass Transf. 2021, 181, 121900. [Google Scholar] [CrossRef]
- Alentiev, A.; Kostina, J.; Bondarenko, G. Chemical aging of Nafion: FTIR study. Desalination 2006, 200, 32–33. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. Electrochemical/Mechanical Coupling in Ion-Conducting Soft Matter. J. Phys. Chem. Lett. 2015, 6, 4547–4552. [Google Scholar] [CrossRef] [Green Version]
- Kreitmeier, S.; Lerch, P.; Wokaun, A.; Büchi, F.N. Local Degradation at Membrane Defects in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2013, 160, F456–F463. [Google Scholar] [CrossRef]
- Phillips, A.; Ulsh, M.; Neyerlin, K.C.; Porter, J.; Bender, G. Impacts of electrode coating irregularities on polymer electrolyte membrane fuel cell lifetime using quasi in-situ infrared thermography and accelerated stress testing. Int. J. Hydrog. Energy 2018, 43, 6390–6399. [Google Scholar] [CrossRef]
- Huang, Q.; Xiao, C.; Hu, X.; Li, X. Study on the effects and properties of hydrophobic poly(tetrafluoroethylene) membrane. Desalination 2011, 277, 187–192. [Google Scholar] [CrossRef]
- Lim, C.; Wang, C.Y. Effects of hydrophobic polymer content in GDL on power performance of a PEM fuel cell. Electrochim. Acta 2004, 49, 4149–4156. [Google Scholar] [CrossRef]
- Chen, T.; Liu, S.; Zhang, J.; Tang, M. Study on the characteristics of GDL with different PTFE content and its effect on the performance of PEMFC. Int. J. Heat Mass Transf. 2019, 128, 1168–1174. [Google Scholar] [CrossRef]
- Shah, A.A.; Ralph, T.R.; Walsh, F.C. Modeling and Simulation of the Degradation of Perfluorinated Ion-Exchange Membranes in PEM Fuel Cells. J. Electrochem. Soc. 2009, 156, B465. [Google Scholar] [CrossRef]
- Coulon, R.; Bessler, W.; Franco, A. Modeling Chemical Degradation of a Polymer Electrolyte Membrane and its Impact on Fuel Cell Performance. ECS Trans. 2009, 25, 259. [Google Scholar] [CrossRef] [Green Version]
- Won, S.; Oh, K.; Ju, H. Numerical degradation studies of high-temperature proton exchange membrane fuel cells with phosphoric acid-doped PBI membranes. Int. J. Hydrog. Energy 2016, 41, 8296–8306. [Google Scholar] [CrossRef]
- Ding, G.; Karlsson, A.M.; Santare, M.H. Numerical evaluation of fatigue crack growth in polymers based on plastically dissipated energy. Int. J. Fatigue 2017, 94, 89–96. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, D.; Peng, L.; Yi, P.; Lai, X. Mechanical degradation of proton exchange membrane during assembly and running processes in proton exchange membrane fuel cells with metallic bipolar plates. Int. J. Energy Res. 2020, 44, 8622–8634. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, F. Microstructure reconstruction and characterization of PEMFC electrodes. Int. J. Hydrog. Energy 2014, 39, 15894–15906. [Google Scholar] [CrossRef]
- Siegel, N.P.; Ellis, M.W.; Nelson, D.J.; Von Spakovsky, M.R. Single domain PEMFC model based on agglomerate catalyst geometry. J. Power Sources 2003, 115, 81–89. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, P.; Xu, L.; Wang, Y. Modeling nanostructured catalyst layer in PEMFC and catalyst utilization. Front. Chem. Eng. China 2011, 5, 297–302. [Google Scholar] [CrossRef]
- Wu, R.; Liao, Q.; Zhu, X.; Wang, H. Pore network modeling of cathode catalyst layer of proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2012, 37, 11255–11267. [Google Scholar] [CrossRef]
- Zhao, J.; Ozden, A.; Shahgaldi, S.; Alaefour, I.E.; Li, X.; Hamdullahpur, F. Effect of Pt loading and catalyst type on the pore structure of porous electrodes in polymer electrolyte membrane (PEM) fuel cells. Energy 2018, 150, 69–76. [Google Scholar] [CrossRef]
- Hao, L.; Moriyama, K.; Gu, W.; Wang, C.-Y. Modeling and Experimental Validation of Pt Loading and Electrode Composition Effects in PEM Fuel Cells. J. Electrochem. Soc. 2015, 162, F854–F867. [Google Scholar] [CrossRef]
- Kregar, A.; Tavčar, G.; Kravos, A.; Katrašnik, T. Predictive system-level modeling framework for transient operation and cathode platinum degradation of high temperature proton exchange membrane fuel cells. Appl. Energy 2020, 263, 114547. [Google Scholar] [CrossRef]
- Pizzutilo, E.; Geiger, S.; Grote, J.P.; Mingers, A.; Mayrhofer, K.J.; Arenz, M.; Cherevko, S. On the Need of Improved Accelerated Degradation Protocols (ADPs): Examination of Platinum Dissolution and Carbon Corrosion in Half-Cell Tests. J. Electrochem. Soc. 2016, 163, F1510–F1514. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wood, D.L.; Wayne, D.M.; Zawodzinski, T.A.; Atanassov, P.; Borup, R.L. Durability of PEFCs at High Humidity Conditions. J. Electrochem. Soc. 2005, 152, A104. [Google Scholar] [CrossRef]
- Rong, F.; Huang, C.; Liu, Z.S.; Song, D.; Wang, Q. Microstructure changes in the catalyst layers of PEM fuel cells induced by load cycling. Part II. Simulation and understanding. J. Power Sources 2008, 175, 712–723. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Lee, Y.W.; Liang, K.; Causey, H. Effect of sub-freezing temperatures on a PEM fuel cell performance, startup and fuel cell components. J. Power Sources 2006, 160, 1242–1250. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, B.K.; Mench, M.M. Physical degradation of membrane electrode assemblies undergoing freeze/thaw cycling: Diffusion media effects. J. Power Sources 2008, 179, 140–146. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, G.; Xuan, J.; Jiao, K. Three-dimensional multi-phase model of PEM fuel cell coupled with improved agglomerate sub-model of catalyst layer. Energy Convers. Manag. 2019, 199, 112051. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, L.; Hu, Z.; Zhao, Y.; Li, J.; Ouyang, M. Dynamic modeling of Pt degradation and mitigation strategies in polymer electrolyte membrane fuel cells. eTransportation 2022, 12, 100171. [Google Scholar] [CrossRef]
- Malek, K.; Franco, A.A. Microstructure-based modeling of aging mechanisms in catalyst layers of polymer electrolyte fuel cells. J. Phys. Chem. B 2011, 115, 8088–8101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Moriyama, K.; Gu, W.; Arisetty, S.; Wang, C.Y. A One-Dimensional Pt Degradation Model for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2015, 162, F834–F842. [Google Scholar] [CrossRef]
- Franco, A.A.; Tembely, M. Transient Multiscale Modeling of Aging Mechanisms in a PEFC Cathode. J. Electrochem. Soc. 2007, 154, B712. [Google Scholar] [CrossRef]
- Bi, W.; Fuller, T.F. Modeling of PEM fuel cell Pt/C catalyst degradation. J. Power Sources 2008, 178, 188–196. [Google Scholar] [CrossRef]
- Takeshita, T.; Morimoto, Y.; Murata, H.; Hatanaka, T. Analysis of Pt Catalyst Degradation of a PEFC Cathode by TEM Observation and Micro Model Simulation. ECS Trans. 2008, 16, 367. [Google Scholar] [CrossRef]
- Rinaldo, S.G.; Lee, W.; Stumper, J.; Eikerling, M. Nonmonotonic dynamics in Lifshitz-Slyozov-Wagner theory: Ostwald ripening in nanoparticle catalysts. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2012, 86, 041601. [Google Scholar] [CrossRef]
- Holby, E.F.; Morgan, D. Application of Pt Nanoparticle Dissolution and Oxidation Modeling to Understanding Degradation in PEM Fuel Cells. J. Electrochem. Soc. 2012, 159, B578–B591. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Arisetty, S.; Peng, J.K.; Subbaraman, R.; Wang, X.; Kariuki, N.; Myers, D.J.; Mukundan, R.; Borup, R.; Polevaya, O. Dynamics of Particle Growth and Electrochemical Surface Area Loss due to Platinum Dissolution. J. Electrochem. Soc. 2014, 161, F291–F304. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Arisetty, S.; Wang, X.; Wang, X.; Subbaraman, R.; Ball, S.C.; DeCrane, S.; Myers, D.J. Thermodynamics and Kinetics of Platinum Dissolution from Carbon-Supported Electrocatalysts in Aqueous Media under Potentiostatic and Potentiodynamic Conditions. J. Electrochem. Soc. 2013, 160, F447–F455. [Google Scholar] [CrossRef]
- Randrianarizafy, B.; Schott, P.; Gerard, M.; Bultel, Y. Modelling carbon Corrosion during a PEMFC startup: Simulation of mitigation strategies. Energies 2020, 13, 2338. [Google Scholar] [CrossRef]
- Jomori, S.; Nonoyama, N.; Yoshida, T. Analysis and modeling of PEMFC degradation: Effect on oxygen transport. J. Power Sources 2012, 215, 18–27. [Google Scholar] [CrossRef]
- Poornesh, K.K.; Cho, C.D.; Lee, G.B.; Tak, Y.S. Gradation of mechanical properties in gas diffusion electrode. Part 1: Influence of nano-scale heterogeneity in catalyst layer on interfacial strength between catalyst layer and membrane. J. Power Sources 2010, 195, 2709–2717. [Google Scholar] [CrossRef]
- Tavassoli, A.; Lim, C.; Kolodziej, J.; Lauritzen, M.; Knights, S.; Wang, G.G.; Kjeang, E. Effect of catalyst layer defects on local membrane degradation in polymer electrolyte fuel cells. J. Power Sources 2016, 322, 17–25. [Google Scholar] [CrossRef]
- Poornesh, K.K.; Cho, C.D.; Lee, G.B.; Tak, Y.S. Gradation of mechanical properties in gas-diffusion electrode. Part 2: Heterogeneous carbon fiber and damage evolution in cell layers. J. Power Sources 2010, 195, 2718–2730. [Google Scholar] [CrossRef]
- Rong, F.; Huang, C.; Liu, Z.S.; Song, D.; Wang, Q. Microstructure changes in the catalyst layers of PEM fuel cells induced by load cycling. Part I. Mechanical model. J. Power Sources 2008, 175, 699–711. [Google Scholar] [CrossRef]
- Darling, R.M.; Meyers, J.P. Mathematical Model of Platinum Movement in PEM Fuel Cells. J. Electrochem. Soc. 2005, 152, A242. [Google Scholar] [CrossRef]
- Moore, M.; Wardlaw, P.; Dobson, P.; Boisvert, J.J.; Putz, A.; Spiteri, R.J.; Secanell, M. Understanding the Effect of Kinetic and Mass Transport Processes in Cathode Agglomerates. J. Electrochem. Soc. 2014, 161, E3125–E3137. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.Y. Modeling of Transient Platinum Degradation in a Low Pt-Loading PEFC under Current Cycling. J. Electrochem. Soc. 2017, 164, F171–F179. [Google Scholar] [CrossRef] [Green Version]
- Moein-Jahromi, M.; Kermani, M.J.; Movahed, S. Degradation forecast for PEMFC cathode-catalysts under cyclic loads. J. Power Sources 2017, 359, 611–625. [Google Scholar] [CrossRef]
- Moein-Jahromi, M.; Kermani, M.J. Three-dimensional multiphase simulation and multi-objective optimization of PEM fuel cells degradation under automotive cyclic loads. Energy Convers. Manag. 2021, 231, 113837. [Google Scholar] [CrossRef]
- Gwak, G.; Lee, J.; Ghasemi, M.; Choi, J.; Lee, S.W.; Jang, S.S.; Ju, H. Analyzing oxygen transport resistance and Pt particle growth effect in the cathode catalyst layer of polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2020, 45, 13414–13427. [Google Scholar] [CrossRef]
- Ghasemi, M.; Choi, J.; Ju, H. Performance analysis of Pt/TiO2/C catalyst using a multi-scale and two-phase proton exchange membrane fuel cell model. Electrochim. Acta 2021, 366, 137484. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Y.; Zhang, J.; Zhang, T.; Zhang, X.; Chen, H. Mechanical degradation of catalyst layer under accelerated relative humidity cycling in a polymer electrolyte membrane fuel cell. J. Power Sources 2021, 512, 230487. [Google Scholar] [CrossRef]
- Liu, H.; Si, D.; Ding, H.; Wang, S.; Zhang, J.; Liu, Y. Cold start capability and durability of electrospun catalyst layer for proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2021, 46, 11140–11149. [Google Scholar] [CrossRef]
- Ge, S.; Wang, C.-Y. Cyclic Voltammetry Study of Ice Formation in the PEFC Catalyst Layer during Cold Start. J. Electrochem. Soc. 2007, 154, B1399. [Google Scholar] [CrossRef]
- Hwang, G.S.; Kim, H.; Lujan, R.; Mukundan, R.; Spernjak, D.; Borup, R.L.; Kaviany, M.; Kim, M.H.; Weber, A.Z. Phase-change-related degradation of catalyst layers in proton-exchange-membrane fuel cells. Electrochim. Acta 2013, 95, 29–37. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhao, H.; Sui, P.C.; Jiang, P.; Pan, M.; Djilali, N. Numerical analysis of ice-induced stresses in the membrane electrode assembly of a PEM fuel cell under sub-freezing operating conditions. Int. J. Hydrog. Energy 2018, 43, 4563–4582. [Google Scholar] [CrossRef]
- Burlatsky, S.F.; Gummalla, M.; Atrazhev, V.V.; Dmitriev, D.V.; Kuzminyh, N.Y.; Erikhman, N.S. The Dynamics of Platinum Precipitation in an Ion Exchange Membrane. J. Electrochem. Soc. 2011, 158, B322. [Google Scholar] [CrossRef]
- Colombo, E.; Bisello, A.; Casalegno, A.; Baricci, A. Mitigating PEMFC Degradation During Start-Up: Locally Resolved Experimental Analysis and Transient Physical Modelling. J. Electrochem. Soc. 2021, 168, 054508. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, J.; Li, R.; Zhao, J.; Qin, Y.; Zhang, J.; Yin, Y.; Li, X. Effect of humidity and thermal cycling on the catalyst layer structural changes in polymer electrolyte membrane fuel cells. Energy Convers. Manag. 2019, 189, 24–32. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Mu, Y.T.; Tao, W.Q. Lattice Boltzmann method simulation of ice melting process in the gas diffusion layer of fuel cell. Int. J. Heat Mass Transf. 2020, 149, 119121. [Google Scholar] [CrossRef]
- Fishman, Z.; Bazylak, A. Heterogeneous Through-Plane Distributions of Tortuosity, Effective Diffusivity, and Permeability for PEMFC GDLs. J. Electrochem. Soc. 2011, 158, B247. [Google Scholar] [CrossRef]
- Miller, M.; Bazylak, A. A review of polymer electrolyte membrane fuel cell stack testing. J. Power Sources 2011, 196, 601–613. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Lai, X.; Ni, J. A Numerical Model for Predicting Gas Diffusion Layer Failure in Proton Exchange Membrane Fuel Cells. J. Fuel Cell Sci. Technol. 2011, 8, 011011. [Google Scholar] [CrossRef]
- Radhakrishnan, V.; Haridoss, P. Differences in structure and property of carbon paper and carbon cloth diffusion media and their impact on proton exchange membrane fuel cell flow field design. Mater. Des. 2011, 32, 861–868. [Google Scholar] [CrossRef]
- Nitta, I.; Hottinen, T.; Himanen, O.; Mikkola, M. Inhomogeneous compression of PEMFC gas diffusion layer. Part I. Experimental. J. Power Sources 2007, 171, 26–36. [Google Scholar] [CrossRef]
- Oszcipok, M.; Riemann, D.; Kronenwett, U.; Kreideweis, M.; Zedda, M. Statistic analysis of operational influences on the cold start behaviour of PEM fuel cells. J. Power Sources 2005, 145, 407–415. [Google Scholar] [CrossRef]
- Yu, S.; Li, X.; Li, J.; Liu, S.; Lu, W.; Shao, Z.; Yi, B. Study on hydrophobicity degradation of gas diffusion layer in proton exchange membrane fuel cells. Energy Convers. Manag. 2013, 76, 301–306. [Google Scholar] [CrossRef]
- Owejan, J.E.; Yu, P.T. Mitigation of Carbon Corrosion in Non-Active Layers Using Graphitized Carbon Black in PEM Fuel Cells. In ECS Meeting Abstracts; MA2007-02; IOP Publishing: Bristol, UK, 2007; p. 593. [Google Scholar] [CrossRef]
- Millichamp, J.; Mason, T.J.; Neville, T.P.; Rajalakshmi, N.; Jervis, R.; Shearing, P.R.; Brett, D.J. Mechanisms and effects of mechanical compression and dimensional change in polymer electrolyte fuel cells e A review. J. Power Sources 2015, 284, 305–320. [Google Scholar] [CrossRef]
- Yoon, W.; Huang, X. A Multiphysics Model of PEM Fuel Cell Incorporating the Cell Compression Effects. J. Electrochem. Soc. 2010, 157, B680. [Google Scholar] [CrossRef]
- Pauchet, J.; Prat, M.; Schott, P.; Kuttanikkad, S.P. Performance loss of proton exchange membrane fuel cell due to hydrophobicity loss in gas diffusion layer: Analysis by multiscale approach combining pore network and performance modelling. Int. J. Hydrog. Energy 2012, 37, 1628–1641. [Google Scholar] [CrossRef]
- Seidenberger, K.; Wilhelm, F.; Schmitt, T.; Lehnert, W.; Scholta, J. Estimation of water distribution and degradation mechanisms in polymer electrolyte membrane fuel cell gas diffusion layers using a 3D Monte Carlo model. J. Power Sources 2011, 196, 5317–5324. [Google Scholar] [CrossRef]
- Mahdi, E.; Bouziane, K.; Zamel, N.; François, X. Effects of mechanical compression on the performance of polymer electrolyte fuel cells and analysis through in-situ characterisation techniques—A review. J. Power Sources 2019, 424, 8–26. [Google Scholar] [CrossRef] [Green Version]
- Ozden, E.; Tari, I. Proton exchange membrane fuel cell degradation: A parametric analysis using Computational Fluid Dynamics. J. Power Sources 2016, 304, 64–73. [Google Scholar] [CrossRef]
- Espinoza, M.; Andersson, M.; Yuan, J.; Sundén, B. Compress effects on porosity, gas-phase tortuosity, and gas permeability in a simulated PEM gas diffusion layer. Arch. Thermodyn. 2015, 33, 23–40. [Google Scholar] [CrossRef]
- Chi, P.H.; Chan, S.H.; Weng, F.B.; Su, A.; Sui, P.C.; Djilali, N. On the effects of non-uniform property distribution due to compression in the gas diffusion layer of a PEMFC. Int. J. Hydrog. Energy 2010, 35, 2936–2948. [Google Scholar] [CrossRef]
- Chang, W.R.; Hwang, J.J.; Weng, F.B.; Chan, S.H. Effect of clamping pressure on the performance of a PEM fuel cell. J. Power Sources 2007, 166, 149–154. [Google Scholar] [CrossRef]
- Zhang, Z.; He, P.; Dai, Y.J.; Jin, P.H.; Tao, W.Q. Study of the mechanical behavior of paper-type GDL in PEMFC based on microstructure morphology. Int. J. Hydrog. Energy 2020, 45, 29379–29394. [Google Scholar] [CrossRef]
- Hottinen, T.; Himanen, O.; Karvonen, S.; Nitta, I. Inhomogeneous compression of PEMFC gas diffusion layer. Part II. Modeling the effect. J. Power Sources 2007, 171, 113–121. [Google Scholar] [CrossRef]
- Su, Z.Y.; Liu, C.T.; Chang, H.P.; Li, C.H.; Huang, K.J.; Sui, P.C. A numerical investigation of the effects of compression force on PEM fuel cell performance. J. Power Sources 2008, 183, 182–192. [Google Scholar] [CrossRef]
- Nitta, I.; Himanen, O.; Mikkola, M. Thermal conductivity and contact resistance of compressed gas diffusion layer of PEM fuel cell. Fuel Cells 2008, 8, 111–119. [Google Scholar] [CrossRef]
- Yan, X.; Lin, C.; Zheng, Z.; Chen, J.; Wei, G.; Zhang, J. Effect of clamping pressure on liquid-cooled PEMFC stack performance considering inhomogeneous gas diffusion layer compression. Appl. Energy 2020, 258, 114073. [Google Scholar] [CrossRef]
- Atyabi, S.A.; Afshari, E.; Wongwises, S.; Yan, W.M.; Hadjadj, A.; Shadloo, M.S. Effects of assembly pressure on PEM fuel cell performance by taking into accounts electrical and thermal contact resistances. Energy 2019, 179, 490–501. [Google Scholar] [CrossRef]
- Li, W.Z.; Yang, W.W.; Zhang, W.Y.; Qu, Z.G.; He, Y.L. Three-dimensional modeling of a PEMFC with serpentine flow field incorporating the impacts of electrode inhomogeneous compression deformation. Int. J. Hydrog. Energy 2019, 44, 22194–22209. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Hazard, M.W.; Rodgers, J.A.; Stroman, R.O.; Gould, B.D. Influence of Gas Diffusion Media Compression on Open-Cathode Fuel Cells. J. Electrochem. Soc. 2019, 166, F926–F934. [Google Scholar] [CrossRef]
- Taymaz, I.; Benli, M. Numerical study of assembly pressure effect on the performance of proton exchange membrane fuel cell. Energy 2010, 35, 2134–2140. [Google Scholar] [CrossRef]
- Wei, L.; Liao, Z.; Suo, Z.; Chen, X.; Jiang, F. Numerical study of cold start performance of proton exchange membrane fuel cell with coolant circulation. Int. J. Hydrog. Energy 2019, 44, 22160–22172. [Google Scholar] [CrossRef]
- Wei, L.; Dafalla, A.M.; Jiang, F. Effects of reactants/coolant non-uniform inflow on the cold start performance of PEMFC stack. Int. J. Hydrog. Energy 2020, 45, 13469–13482. [Google Scholar] [CrossRef]
- Wei, L.; Liao, Z.; Mohmed Dafalla, A.; Suo, Z.; Jiang, F. Effects of Endplate Assembly on Cold Start Performance of Proton Exchange Membrane Fuel Cell Stacks. Energy Technol. 2021, 9, 2100092. [Google Scholar] [CrossRef]
- Wan, Z.; Chang, H.; Shu, S.; Wang, Y.; Tang, H. A review on cold start of proton exchange membrane fuel cells. Energies 2014, 7, 3179–3203. [Google Scholar] [CrossRef] [Green Version]
- Amamou, A.A.; Kelouwani, S.; Boulon, L.; Agbossou, K. A Comprehensive Review of Solutions and Strategies for Cold Start of Automotive Proton Exchange Membrane Fuel Cells. IEEE Access 2016, 4, 4989–5002. [Google Scholar] [CrossRef]
- Jiang, F.; Fang, W.; Wang, C.Y. Non-isothermal cold start of polymer electrolyte fuel cells. Electrochim. Acta 2007, 53, 610–621. [Google Scholar] [CrossRef]
- Huo, S.; Jiao, K.; Wan, J. On the water transport behavior and phase transition mechanisms in cold start operation of PEM fuel cell. Appl. Energy 2019, 233–234, 776–788. [Google Scholar] [CrossRef]
- He, P.; Mu, Y.T.; Park, J.W.; Tao, W.Q. Modeling of the effects of cathode catalyst layer design parameters on performance of polymer electrolyte membrane fuel cell. Appl. Energy 2020, 277, 115555. [Google Scholar] [CrossRef]
- Liao, Z.; Wei, L.; Dafalla, A.M.; Suo, Z.; Jiang, F. Numerical study of subfreezing temperature cold start of proton exchange membrane fuel cells with zigzag-channeled flow field. Int. J. Heat Mass Transf. 2021, 165, 120733. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Jiang, F. Effect of catalyst layer mesoscopic pore-morphology on cold start process of PEM fuel cells. Front. Energy 2021, 15, 460–472. [Google Scholar] [CrossRef]
- Yang, X.G.; Tabuchi, Y.; Kagami, F.; Wang, C.-Y. Durability of Membrane Electrode Assemblies under Polymer Electrolyte Fuel Cell Cold-Start Cycling. J. Electrochem. Soc. 2008, 155, B752. [Google Scholar] [CrossRef] [Green Version]
- Ozden, A.; Shahgaldi, S.; Zhao, J.; Li, X.; Hamdullahpur, F. Degradations in porous components of a proton exchange membrane fuel cell under freeze-thaw cycles: Morphology and microstructure effects. Int. J. Hydrog. Energy 2020, 45, 3618–3631. [Google Scholar] [CrossRef]
- Shimpalee, S.; Electrochem, J.; Soc, F.; Shimpalee, S.; Satjaritanun, P. Multiscale Modeling of PEMFC Using Co-Simulation Approach. J. Electrochem. Soc. 2019, 166, F534. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Xiao, L.; Bazylak, A.; Gao, X.; Sui, P. Pore-scale modeling of gas diffusion layers: Effects of compression on transport properties. J. Power Sources 2021, 496, 229822. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, Z.; Wu, X.; Xu, P. The impact of sample size on transport properties of carbon-paper and carbon-cloth GDLs: Direct simulation using the lattice Boltzmann model. Int. J. Heat Mass Transf. 2018, 118, 1325–1339. [Google Scholar] [CrossRef]
- Bosomoiu, M.; Tsotridis, G.; Bednarek, T. Study of effective transport properties of fresh and aged gas diffusion layers. J. Power Sources 2015, 285, 568–579. [Google Scholar] [CrossRef]

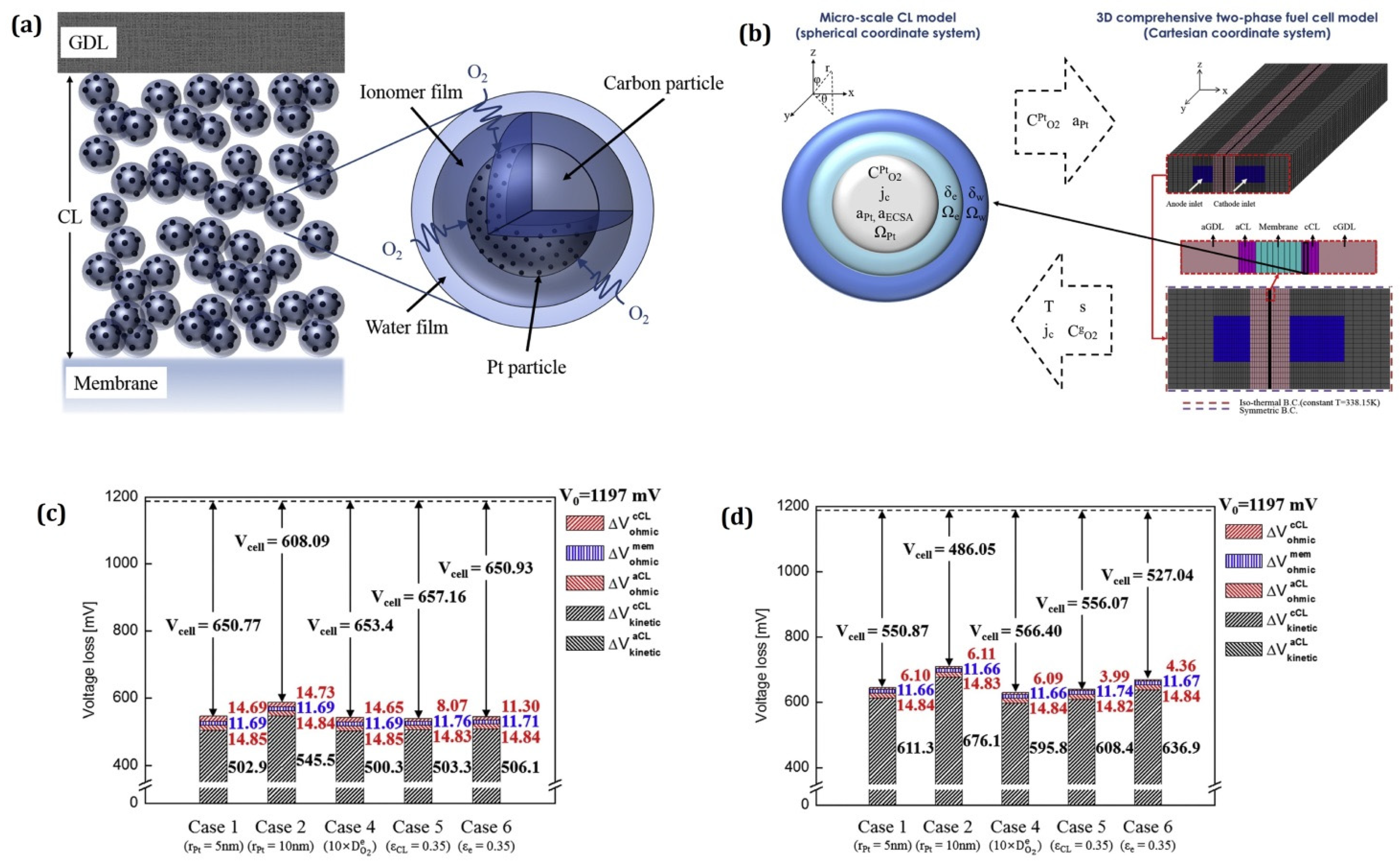

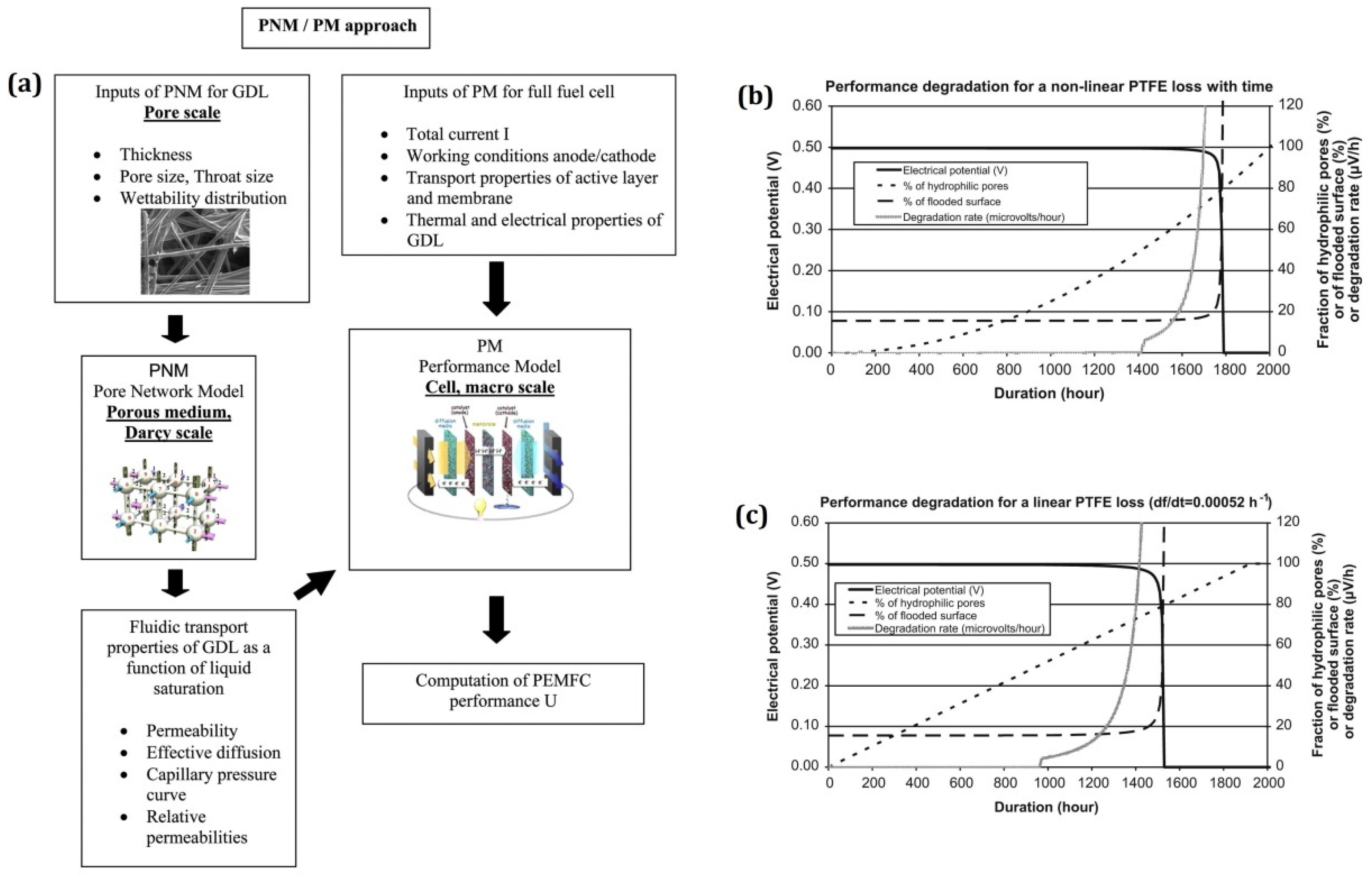
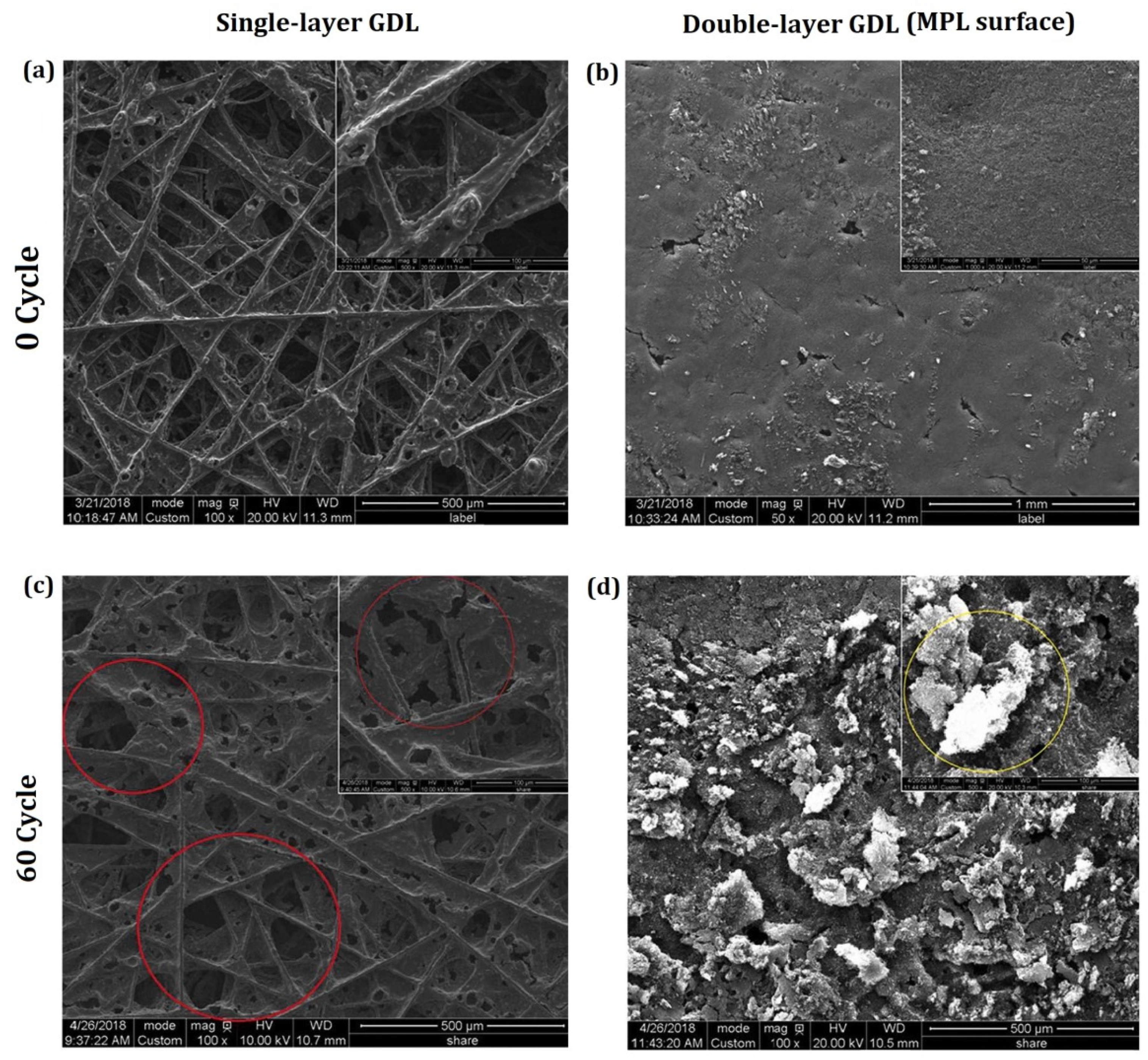
| Authors | Year | Model Description |
|---|---|---|
| Tao Xie et al. [56] | 2007 | Chemical degradation: Modeling main chain carboxylic acid unzipping and side chain cleaving |
| Ahmet Kusoglu et al. [65] | 2007 | Mechanical degradation: Modeling the physical response of membrane under humidity cycling |
| A. A. Shah et al. [81] | 2009 | Chemical degradation: Modeling formation of hydroxyl radicals via Fenton reactions |
| Romain Coulon et al. [82] | 2010 | Chemical degradation: Modeling hydroxyl radical formation via Fenton reaction and a radical mechanism of side chain decomposition. |
| H. E. U. Ahmed et al. [69,71] | 2011 | Mechanical degradation: Modeling natural frequency and mode shapes under exited vibration |
| Roshanak Banan et al. [70,71] | 2013, 2015 | Mechanical degradation: Modeling mechanical vibration |
| Kusoglu and Weber et al. [66] | 2014 | Mechanical degradation: Modeling of pinhole growth under environmental cycling loads |
| Seongyeon Won et al. [83] | 2016 | Thermo-chemical degradation: Modeling the degradation of long-run cell voltage |
| Guoliang Ding et al. [61,84] | 2016, 2017 | Mechanical degradation: Modeling crack growth and fatigue due to clamping pressure under humidity cycling |
| L. Karpenko-Jereb et al. [50] | 2016 | Physio-chemical degradation: Temperature, relative humidity, and cell voltage |
| Ka Hung Wong et al. [57] | 2017 | Chemical degradation: Modeling low-voltage degradation of Ceria-supported membrane |
| R. Singh et al. [52] | 2018 | Chemical degradation: Modeling the sequential degradation of PFSA |
| Georg A. Futter et al. [53] | 2019 | Chemical degradation: Modeling of hydrogen peroxide formation |
| Victoria M Ehlinger et al. [48,49] | 2019, 2020 | Physio-chemical degradation: Modeling of pinhole growth under couples mechanical and chemical effect |
| Wenqing Liu et al. [85] | 2020 | Mechanical degradation: Modeling of stress and strain evolution during assembly and operation |
| Weibo Zheng et al. [58] | 2021 | Chemical degradation: Modeling spatial distribution of hydrogen peroxide |
| Y.X. Wang et al. [51] | 2021 | Mechanical degradation: Modeling of crack growth under humidity cycling |
| Author/s | Year of Publication | Degradation Mechanism/Model Description |
|---|---|---|
| Franco and M. Tembely [102] | 2007 | Pt degradation: Modeling of aging mechanisms in a PEMFC cathode |
| Rong F. et al. [95,114] | 2008 | Structural changes in CL: Modeling of CL microstructure changes induced by load cycling |
| Bi and T. F. Fuller [103] | 2008 | Pt/C catalyst degradation: Modeling of Pt/C catalyst degradation processes using physics-based model |
| T. Takeshita et al. [104] | 2008 | Pt Catalyst Degradation: Modeling of Pt Catalyst degradation using 1D macro model |
| Poornesh K.K. et al. [111,113] | 2010 | Pt/C catalyst degradation: Effect of gradation in catalyst layer on interfacial strength between membrane and catalyst layer |
| Burlatsky S.F. et al. [127] | 2011 | Ionomer decomposition: Modeling of platinum diffusion, precipitation, and band formation in the membrane |
| Colombo E. et al. [128] | 2011 | Pt/C degradation: Modeling the key role of platinum oxides using transient and physical 2D model |
| S. G. Rinaldo et al. [105] | 2012 | Pt degradation: Modeling framework for surface area loss and mass balance phenomena in supported Pt nanoparticle catalysts |
| E. F. Holby and D. Morgan [106] | 2012 | Pt degradation: Modeling of Pt nanoparticle dissolution and oxidation in PEMFCs |
| R. K. Ahluwalia et al. [107,108] | 2014, 2013 | Pt degradation: Modeling the effects of coalescence/sintering of Pt particles on particle growth and ECSA loss |
| Y. Li, K. Moriyama et al. [101] | 2015 | Pt degradation: Modeling of Pt degradation, Ostwald ripening on carbon support and Pt dissolution-re-precipitation through the ionomer phase |
| Li Y. and C.Y. Wang [117] | 2017 | Pt degradation: Modeling of transient platinum degradation under current cycling |
| Moein-Jahromi et al. [118,119] | 2017, 2021 | Pt/C catalyst degradation: Modeling of ECSA degradation, Pt particle growth, the agglomerates via Ostwald ripening, and Pt mass loading loss under cyclic load |
| Randrianarizafy et al. [109] | 2020 | Carbon corrosion: Modeling of the carbon support corrosion and mitigation strategies through the use of a pseudo-3D model |
| Gwak G. et al. [120] | 2020 | Pt degradation: Modeling the oxygen transport resistance and Pt particle growth effect in CL |
| Ghasemi M. et al. [121] | 2021 | Pt degradation: Investigating the usage of TiO2 as Pt catalyst support under different degrees of CL aging using multi-scale two-phase model |
| Chang Y. et al. [129] | 2021 | Structural changes in CL: Modeling the structural changes in CL under humidity and thermal cycling |
| Liu et al. [122] | 2021 | Mechanical degradation: Microstructure changes under accelerated relative humidity cycling |
| Weibo Zheng et al. [99] | 2022 | Pt degradation: Modeling of Pt degradation in the membrane electrode assembly considering Pt mass loss and particle growth mechanisms |
| Authors | Year of Publication | Model Description |
|---|---|---|
| Pauchet J. et al. [141] | 2012 | Modeling of the effect of hydrophobicity loss of GDL on performance of a PEMFC |
| Seidenberger K. et al. [142] | 2012 | PTFE degradation: Modeling of water distribution and PTFE degradation mechanisms in PEMFCs |
| Bosomoiu M. et al. [172] | 2015 | Modeling the effective transport properties for fresh GDL vs. aged GDL. |
| Zhang Z. et al. [148] | 2020 | Modeling the microstructure morphology of carbon paper-type GDL using FEM model |
| Zhu L. et al. [170] | 2021 | Simulation approach combining a pore-scale model and lattice Boltzmann method for GDL to study the compression effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dafalla, A.M.; Wei, L.; Habte, B.T.; Guo, J.; Jiang, F. Membrane Electrode Assembly Degradation Modeling of Proton Exchange Membrane Fuel Cells: A Review. Energies 2022, 15, 9247. https://doi.org/10.3390/en15239247
Dafalla AM, Wei L, Habte BT, Guo J, Jiang F. Membrane Electrode Assembly Degradation Modeling of Proton Exchange Membrane Fuel Cells: A Review. Energies. 2022; 15(23):9247. https://doi.org/10.3390/en15239247
Chicago/Turabian StyleDafalla, Ahmed Mohmed, Lin Wei, Bereket Tsegai Habte, Jian Guo, and Fangming Jiang. 2022. "Membrane Electrode Assembly Degradation Modeling of Proton Exchange Membrane Fuel Cells: A Review" Energies 15, no. 23: 9247. https://doi.org/10.3390/en15239247
APA StyleDafalla, A. M., Wei, L., Habte, B. T., Guo, J., & Jiang, F. (2022). Membrane Electrode Assembly Degradation Modeling of Proton Exchange Membrane Fuel Cells: A Review. Energies, 15(23), 9247. https://doi.org/10.3390/en15239247








