A Novel High-Performance Anode Material with an Enlarged Potential Window for a Hybrid Energy Storage System
Abstract
:1. Introduction
2. Experimental Method
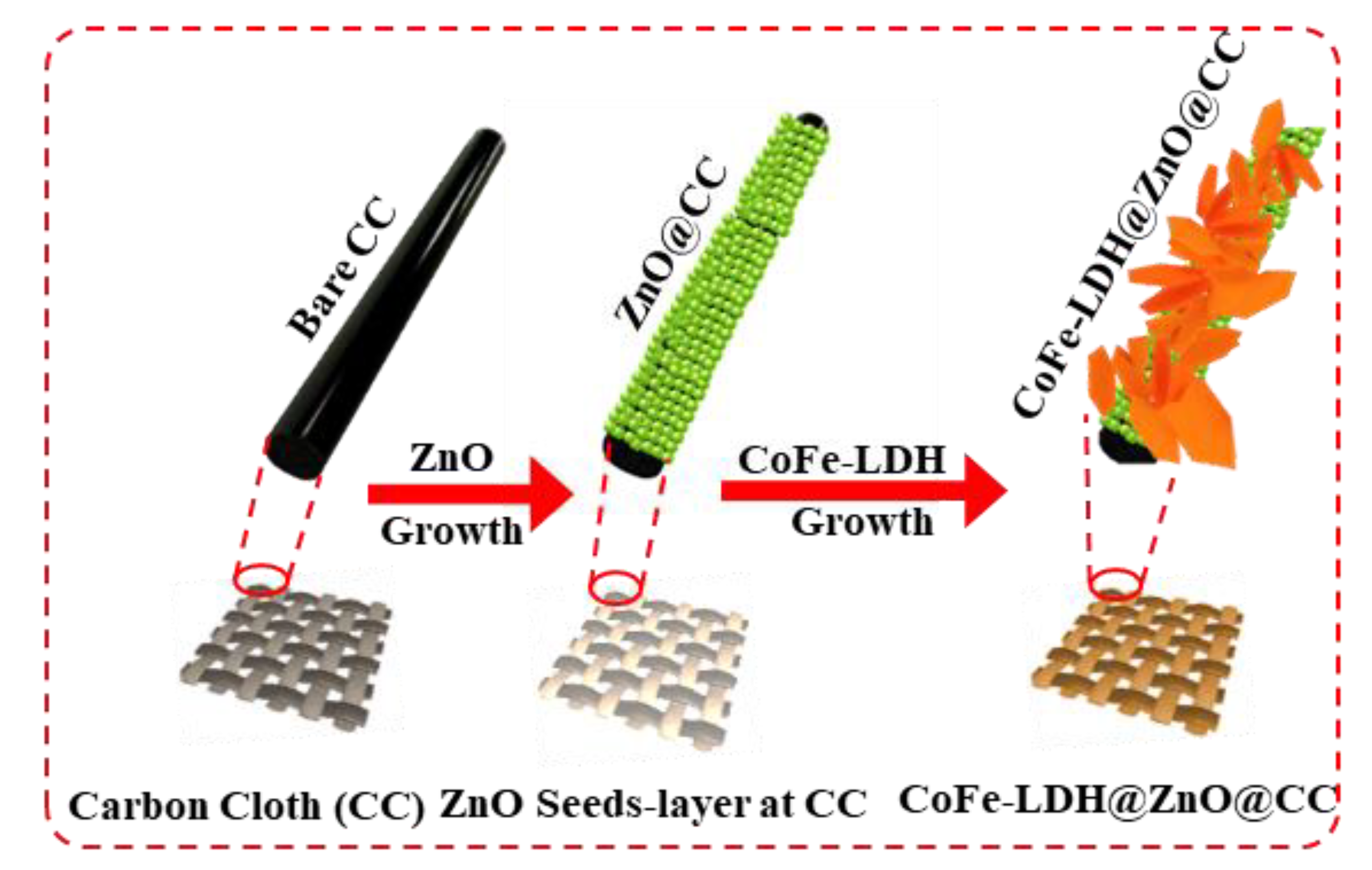
2.1. Synthesis of ZnO@CC
2.2. Synthesis of CoFe-LDH@ZnO@CC
2.3. Physical Characterization
2.4. Electrochemical Measurements
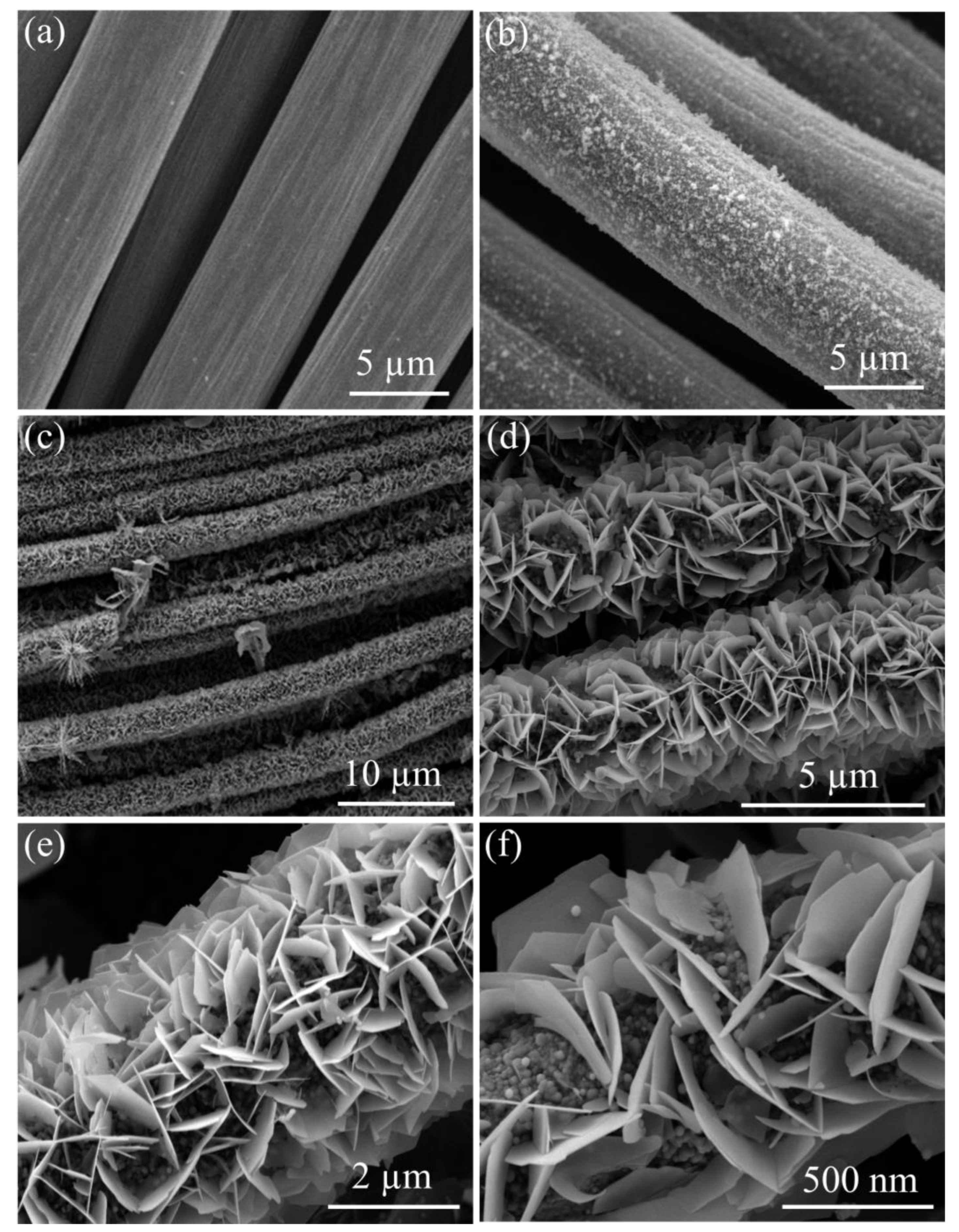
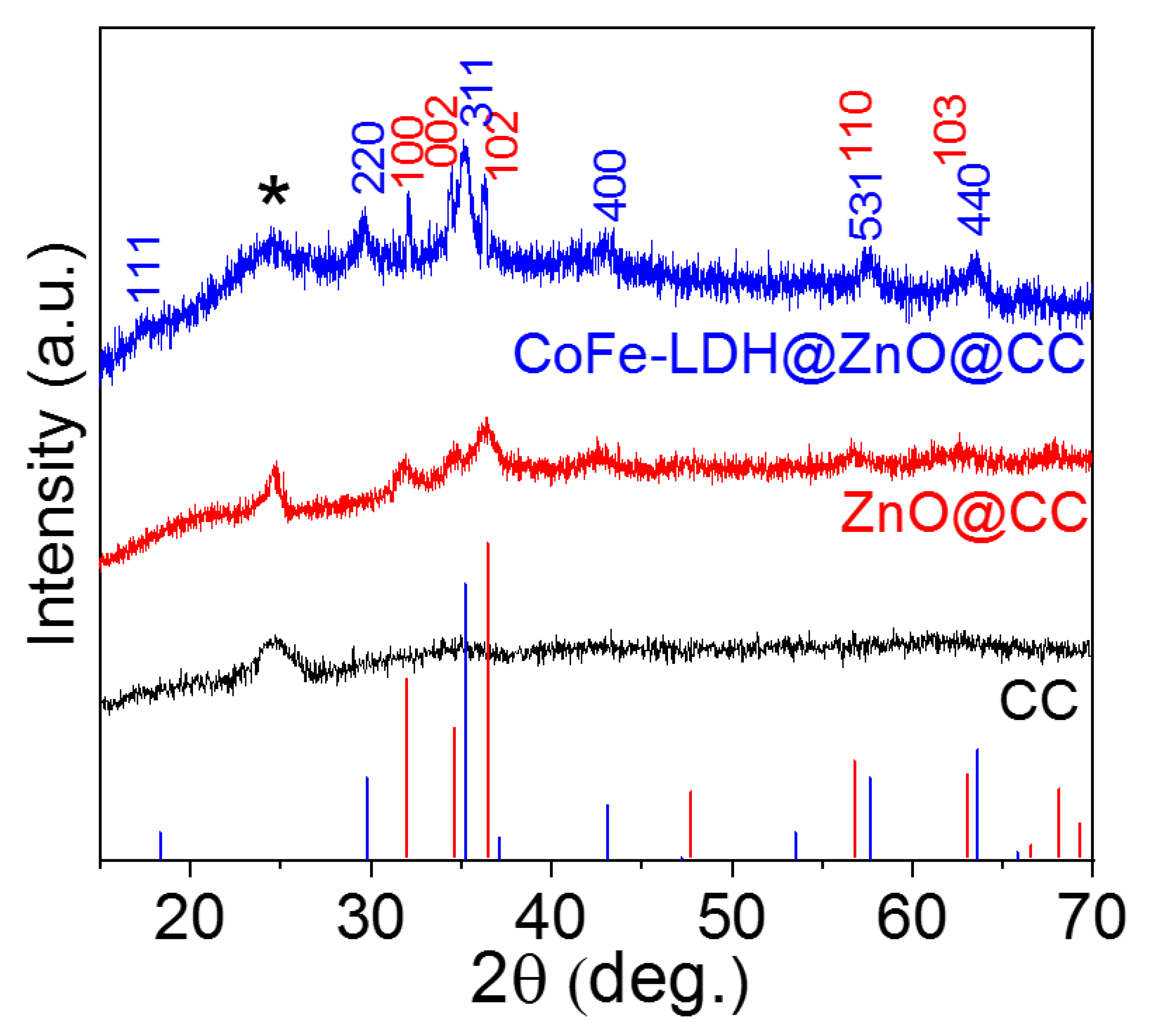
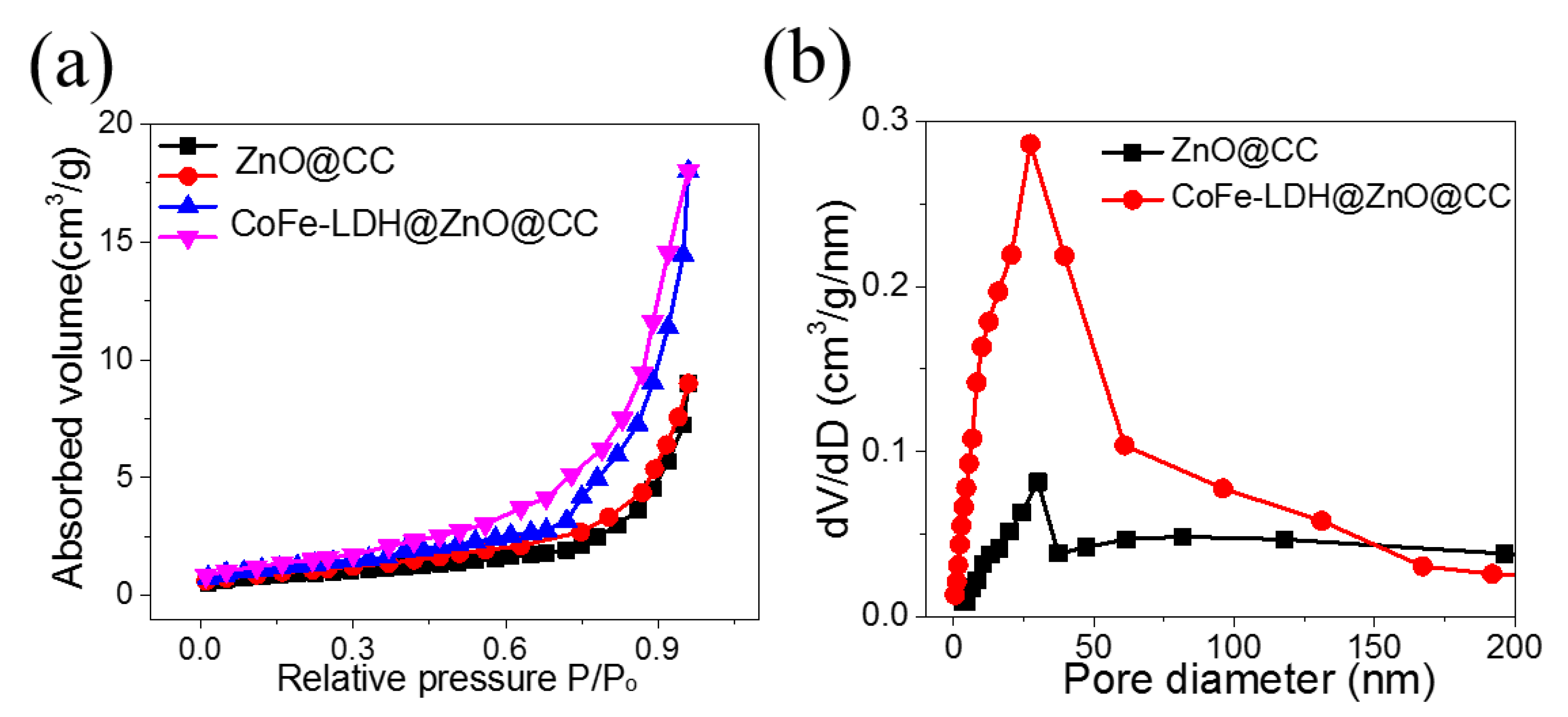
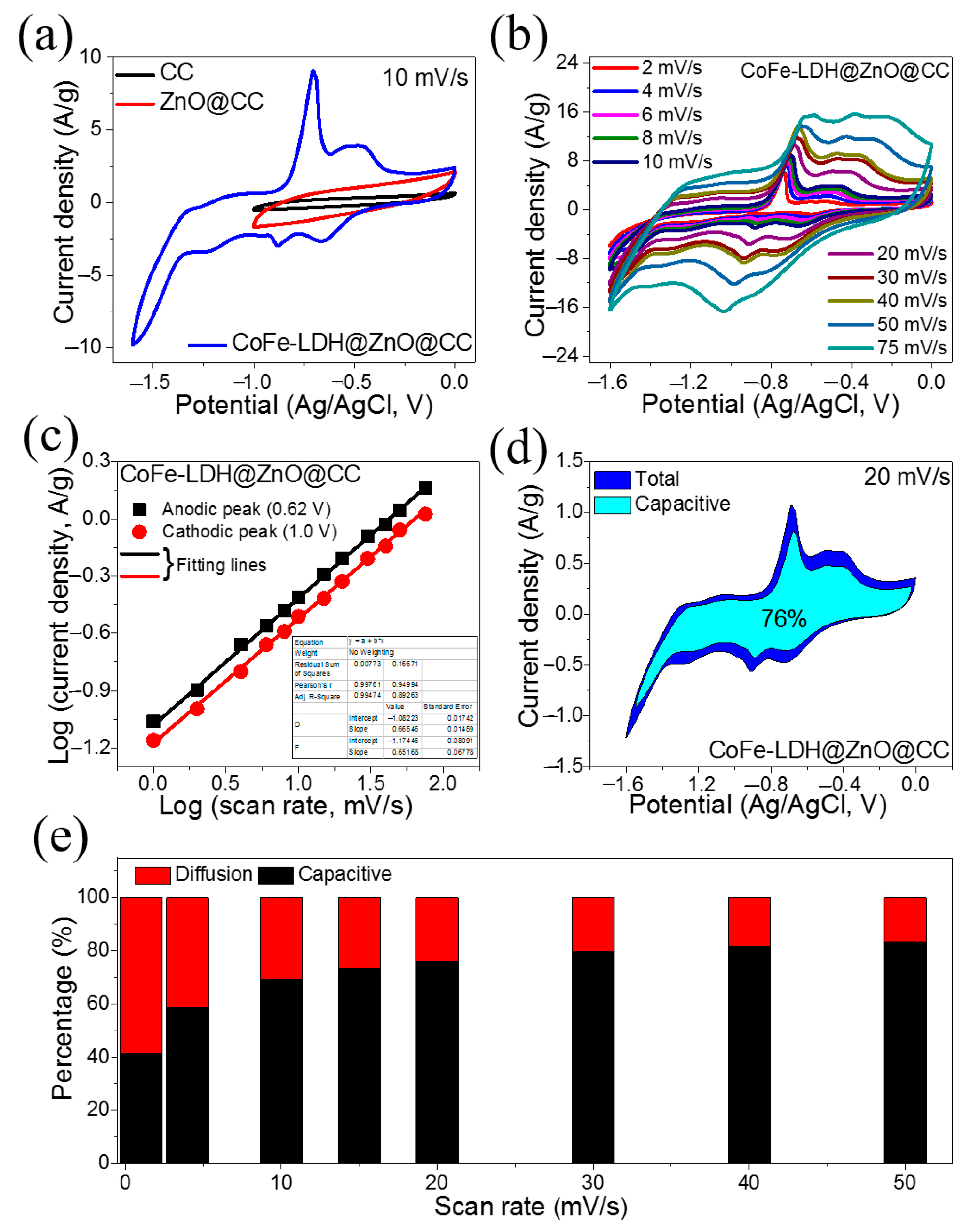
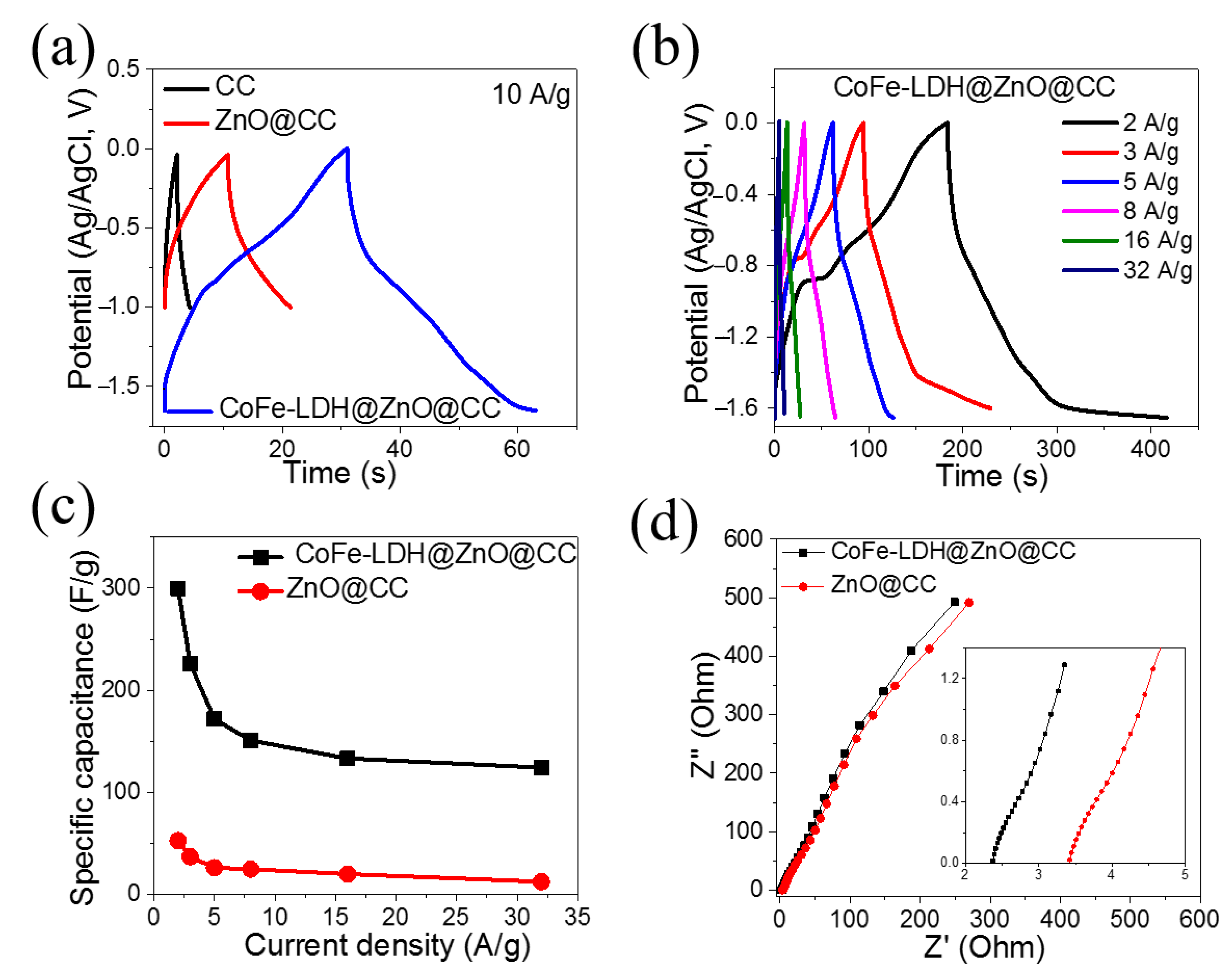
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ullah, N.; Zhang, Y.; Shaheen, A.; Javed, M.S.; Lin, L.; Shah, S.B.; Liu, G.; Qiao, G. One-step synthesis of unique catalyst Ni9S8@ C for excellent MOR performances. Int. J. Hydrogen Energy 2019, 44, 24525–24533. [Google Scholar] [CrossRef]
- Javed, M.S.; Zhang, X.; Ali, S.; Mateen, A.; Idrees, M.; Sajjad, M.; Batool, S.; Ahmad, A.; Imran, M.; Najam, T.; et al. Heterostructured bimetallic–sulfide@layered Ti3C2Tx–MXene as a synergistic electrode to realize high-energy-density aqueous hybrid-supercapacitor. Nano Energy 2022, 101, 107624. [Google Scholar] [CrossRef]
- Mateen, A.; Javed, M.S.; Khan, S.; Saleem, A.; Majeed, M.K.; Khan, A.J.; Tahir, M.F.; Ahmad, M.A.; Assiri, M.A.; Peng, K.-Q. Metal-organic framework-derived walnut-like hierarchical Co-O-nanosheets as an advanced binder-free electrode material for flexible supercapacitor. J. Energy Storage 2022, 49, 104150. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Ali, S.; Zhang, X.; Hussain, I.; Imran, M.; Shah, S.S.A.; Han, W. The Emergence of 2D MXenes Based Zn-Ion Batteries: Recent Development and Prospects. Small 2022, 18, 2201989. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, Z.; Chen, L.; Wang, W.; Ai, Q.; Hou, G.; Li, Y.; Lou, J.; Ci, L. Nitrogen and sulfur co-doped porous carbon fibers film for flexible symmetric all-solid-state supercapacitors. Carbon 2020, 158, 456–464. [Google Scholar] [CrossRef]
- Dai, J.; Wang, L.; Xie, A.; He, J.; Yan, Y. Reactive template and confined self-activation strategy: Three-dimensional interconnected hierarchically porous N/O-doped carbon foam for enhanced supercapacitors. ACS Sustain. Chem. Eng. 2019, 8, 739–748. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Hussain, I.; Ahmad, A.; Mubashir, M.; Khan, S.; Assiri, M.A.; Eldin, S.M.; Shah, S.S.A.; Han, W. Recent Progress in the Design of Advanced MXene/Metal Oxides-Hybrid Materials for Energy Storage Devices. Energy Storage Mater. 2022, 53, 827–872. [Google Scholar] [CrossRef]
- Javed, M.S.; Mateen, A.; Hussain, I.; Ali, S.; Asim, S.; Ahmad, A.; Eldin, E.t.; Bajaber, M.A.; Najam, T.; Han, W. The Quest for Negative Electrode Materials for Supercapacitors: 2D Materials as a Promising Family. Chem. Eng. J. 2022, 452, 139455. [Google Scholar] [CrossRef]
- Senokos, E.; Reguero, V.; Palma, J.; Vilatela, J.; Marcilla, R. Macroscopic fibres of CNTs as electrodes for multifunctional electric double layer capacitors: From quantum capacitance to device performance. Nanoscale 2016, 8, 3620–3628. [Google Scholar] [CrossRef]
- Lan, Y.; Li, M.; Fan, W.; Deng, Q.; Zeng, Z.; Wang, J.; Deng, S. Functional molecules regulated and intercalated nickel-cobalt LDH nano-sheets on carbon fiber cloths as an advanced free-standing electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 321, 134708. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, Y.; Zhu, X.; Fan, M.; Wang, Y. Compounds. CNT/Co3S4@ NiCo LDH ternary nanocomposites as battery-type electrode materials for hybrid supercapacitors. J. Alloy. Compd. 2020, 824, 153937. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lee, J.-M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Zhang, C.; Xu, S.; Wei, M.; Duan, X. Co3O4@ layered double hydroxide core/shell hierarchical nanowire arrays for enhanced supercapacitance performance. Nano Energy 2014, 7, 134–142. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Li, Z.; Yang, W.; Wang, B.; Hou, M.; He, Y.; Liu, Q.; Mann, T.; Yang, P. Graphene nanosheet/Ni2+/Al3+ layered double-hydroxide composite as a novel electrode for a supercapacitor. Chem. Mater. 2011, 23, 3509–3516. [Google Scholar] [CrossRef]
- Padmini, M.; Kiran, S.K.; Lakshminarasimhan, N.; Sathish, M.; Elumalai, P. High-performance solid-state hybrid energy-storage device consisting of reduced graphene-oxide anchored with NiMn-layered double hydroxide. Electrochim. Acta 2017, 236, 359–370. [Google Scholar] [CrossRef]
- Ma, H.; He, J.; Xiong, D.-B.; Wu, J.; Li, Q.; Dravid, V.; Zhao, Y. Nickel cobalt hydroxide@ reduced graphene oxide hybrid nanolayers for high performance asymmetric supercapacitors with remarkable cycling stability. ACS Appl. Mater. Interfaces 2016, 8, 1992–2000. [Google Scholar] [CrossRef]
- Zhan, T.; Zhang, Y.; Liu, X.; Lu, S.; Hou, W. NiFe layered double hydroxide/reduced graphene oxide nanohybrid as an efficient bifunctional electrocatalyst for oxygen evolution and reduction reactions. J. Power Sources 2016, 333, 53–60. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, C.; Fang, J.; Cheng, J.; Zhang, X.; Dong, S.; Zhang, L. Asymmetric electrochemical capacitors with high energy and power density based on graphene/CoAl-LDH and activated carbon electrodes. RSC Adv. 2013, 3, 2483–2490. [Google Scholar] [CrossRef]
- Zheng, C.-H.; Yao, T.; Xu, T.-R.; Wang, H.-A.; Huang, P.-F.; Yan, Y.; Fang, D.-L. Growth of ultrathin NiCoAl layered double hydroxide on reduced graphene oxide and superb supercapacitive performance of the resulting composite. J. Alloy. Compd. 2016, 678, 93–101. [Google Scholar] [CrossRef]
- Ma, K.; Cheng, J.; Zhang, J.; Li, M.; Liu, F.; Zhang, X. Dependence of Co/Fe ratios in Co-Fe layered double hydroxides on the structure and capacitive properties. Electrochim. Acta 2016, 198, 231–240. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; Zhou, L.; Zhang, R.; Zhang, C.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@ layered double hydroxide core–shell nanoarrays. Nano Energy 2016, 20, 294–304. [Google Scholar] [CrossRef]
- Ma, K.; Liu, F.; Zhang, M.; Zhang, X.; Cheng, J. Core/shell microrod arrays of NiO/Co-Fe layered double hydroxides deposited on nickel foam for energy storage and conversion. Electrochim. Acta 2017, 225, 425–434. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, V.; Khosla, A.; Kumar, S.; Arya, S. High performance asymmetric supercapacitor based on vertical nanowire arrays of a novel Ni@Co–Fe LDH core@shell as negative and Ni(OH)2 as positive electrode. Nanotechnology 2020, 31, 245401. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Park, C.; Aldalbahi, A.; El-Newehy, M.; Lee, H.S.; Yoon, S.S. Wearable fabric supercapacitors based on CNTs and polyhedral ZnO with a wide potential window. Int. J. Energy Res. 2022, 46, 8186–8200. [Google Scholar] [CrossRef]
- Kasap, S.; Kaya, I.I.; Repp, S.; Erdem, E. Superbat: Battery-like supercapacitor utilized by graphene foam and zinc oxide (ZnO) electrodes induced by structural defects. Nanoscale Adv. 2019, 1, 2586–2597. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wei, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Wang, G.; Bakenov, Z. Synthesis and electrochemical investigation of highly dispersed ZnO nanoparticles as anode material for lithium-ion batteries. Ionics 2016, 22, 1387–1393. [Google Scholar] [CrossRef]
- Sun, H.; Yang, X.; Zhao, L.; Xu, T.; Lian, J. One-pot hydrothermal synthesis of octahedral CoFe/CoFe2O4 submicron composite as heterogeneous catalysts with enhanced peroxymonosulfate activity. J. Mater. Chem. A 2016, 4, 9455–9465. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn layered double hydroxide/carbon nanotubes architecture with superb energy density for flexible supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Yin, M.; Shi, J.; Zhang, W.; Wang, X.; Wu, Y.; Ma, J.; Yuan, D.; Jia, C. Co3O4@ Co3S4 core-shell neuroid network for high cycle-stability hybrid-supercapacitors. J. Power Sources 2021, 485, 229315. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xie, Z.; Wang, D.; Yuan, Y.; Kang, H.; Su, B.; Cheng, Z.; Liu, Y. Modified MXene/holey graphene films for advanced supercapacitor electrodes with superior energy storage. Adv. Sci. 2018, 5, 1800750. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Khan, A.J.; Ahmad, A.; Siyal, S.H.; Akram, S.; Zhao, G.; Bahajjaj, A.A.A.; Ouladsmane, M.; Alfakeer, M. Design and fabrication of bimetallic oxide nanonest-like structure/carbon cloth composite electrode for supercapacitors. Ceram. Int. 2021, 47, 30747–30755. [Google Scholar] [CrossRef]
- Mohd, A.; Zainal, N.; Tan, K.-K.; AbuBakar, S. Resveratrol affects Zika virus replication in vitro. Sci. Rep. 2019, 9, 14336. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Pang, H.; Lou, X.W.D. Facile synthesis of mesoporous Ni 0.3 Co 2.7 O4 hierarchical structures for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 3619–3626. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Li, F.; Tan, L.; Ma, H.; Zhang, L.; Zhao, Y. Oriented synthesis of Co3O4 core-shell microspheres for high-performance asymmetric supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 1–8. [Google Scholar] [CrossRef]
- Tummala, R.; Guduru, R.K.; Mohanty, P.S. Nanostructured Co3O4 electrodes for supercapacitor applications from plasma spray technique. J. Power Sources 2012, 209, 44–51. [Google Scholar] [CrossRef]
- Xie, L.; Li, K.; Sun, G.; Hu, Z.; Lv, C.; Wang, J.; Zhang, C. Preparation and electrochemical performance of the layered cobalt oxide (Co3O4) as supercapacitor electrode material. J. Solid State Electrochem. 2013, 17, 55–61. [Google Scholar] [CrossRef]
- Racik, M.; Manikandan, A.; Mahendiran, M.; Madhavan, J.; Raj, M.V.A.; Mohamed, M.G.; Maiyalagan, T. Hydrothermal synthesis and characterization studies of α-Fe2O3/MnO2 nanocomposites for energy storage supercapacitor application. Ceram. Int. 2020, 46, 6222–6233. [Google Scholar] [CrossRef]
- Hou, X.-Y.; Yan, X.-L.; Wang, X.; Zhai, Q.-G. Tuning the porosity of mesoporous NiO through calcining isostructural Ni-MOFs toward supercapacitor applications. J. Solid State Chem. 2018, 263, 72–78. [Google Scholar] [CrossRef]
- Ramadevi, P.; Sangeetha, A.; Kousi, F.; Shanmugavadivu, R. Structural and electrochemical investigation on pure and aluminium doped nickel ferrite nanoparticles for supercapacitor application. Mater. Today: Proc. 2020, 33, 2116–2121. [Google Scholar] [CrossRef]
- Yang, S.; Han, Z.; Sun, J.; Yang, X.; Hu, X.; Li, C.; Cao, B. Controllable ZnFe2O4/reduced graphene oxide hybrid for high-performance supercapacitor electrode. Electrochim. Acta 2018, 268, 20–26. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Hao, X.D.; Diao, Z.P.; Li, J.; Guan, Y.M. One-pot controllable synthesis of flower-like CoFe2O4/FeOOH nanocomposites for high-performance supercapacitors. Mater. Lett. 2014, 123, 229–234. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K.; Meyrick, D. Electrochemical performances of CoFe2O4 nanoparticles and a rGO based asymmetric supercapacitor. RSC Adv. 2015, 5, 99959–99967. [Google Scholar] [CrossRef]

| Sr. No. | Electrode Materials | Electrolyte | Specific Capacitance (F/g) | Current Density (A/g) | No. of Cycles (n) | Retention Rate (%) | References |
|---|---|---|---|---|---|---|---|
| 1 | CoFe-LDH@ZnO@CC | Na2SO4 | 299.8 | 2 | 5000 | 97.7 | This work |
| 2 | Co3O4 microspheres | KOH | 261.1 | 0.5 | 2000 | 90.2 | [38] |
| 3 | Co3O4 particulates | KOH | 224.38 | 2.75 | 1000 | 72.2 | [39] |
| 4 | Co3O4 flakes | KOH | 263 | 20 | 1000 | 89.4 | [40] |
| 5 | α-Fe2O3 | Na2SO4 | 216 | 1 | 1000 | 89.2 | [41] |
| 6 | NiO | KOH | 116 | 1 | 2000 | 84 | [42] |
| 7 | Al doped NiFe2O4 | Na2SO4 | 250.9 | 0.5 | 1000 | 96 | [43] |
| 8 | NiFe2O4 | Na2SO4 | 240.9 | 1 | 2000 | 92.9 | [44] |
| 9 | CoFe2O4/FeOOH | KOH | 232 | 0.5 | 1000 | 91.3 | [45] |
| 10 | CoFe2O4/rGO | KOH | 194 | 1 | 2500 | 71.9 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateen, A.; Zubair, M.; Saleem, M.; Golubenkova, A.; Voskressensky, L.; Alothman, A.A.; Ouladsmane, M.; Ahmad, A.; Javed, M.S. A Novel High-Performance Anode Material with an Enlarged Potential Window for a Hybrid Energy Storage System. Energies 2022, 15, 9577. https://doi.org/10.3390/en15249577
Mateen A, Zubair M, Saleem M, Golubenkova A, Voskressensky L, Alothman AA, Ouladsmane M, Ahmad A, Javed MS. A Novel High-Performance Anode Material with an Enlarged Potential Window for a Hybrid Energy Storage System. Energies. 2022; 15(24):9577. https://doi.org/10.3390/en15249577
Chicago/Turabian StyleMateen, Abdul, Muhammad Zubair, Muhammad Saleem, Alexandra Golubenkova, Leonid Voskressensky, Asma A. Alothman, Mohamed Ouladsmane, Awais Ahmad, and Muhammad Sufyan Javed. 2022. "A Novel High-Performance Anode Material with an Enlarged Potential Window for a Hybrid Energy Storage System" Energies 15, no. 24: 9577. https://doi.org/10.3390/en15249577






