Local Charge Carrier Dynamics for Photocatalytic Materials Using Pattern-Illumination Time-Resolved Phase Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2 and α-Fe2O3 Films
2.2. PI-PM Method
3. Results
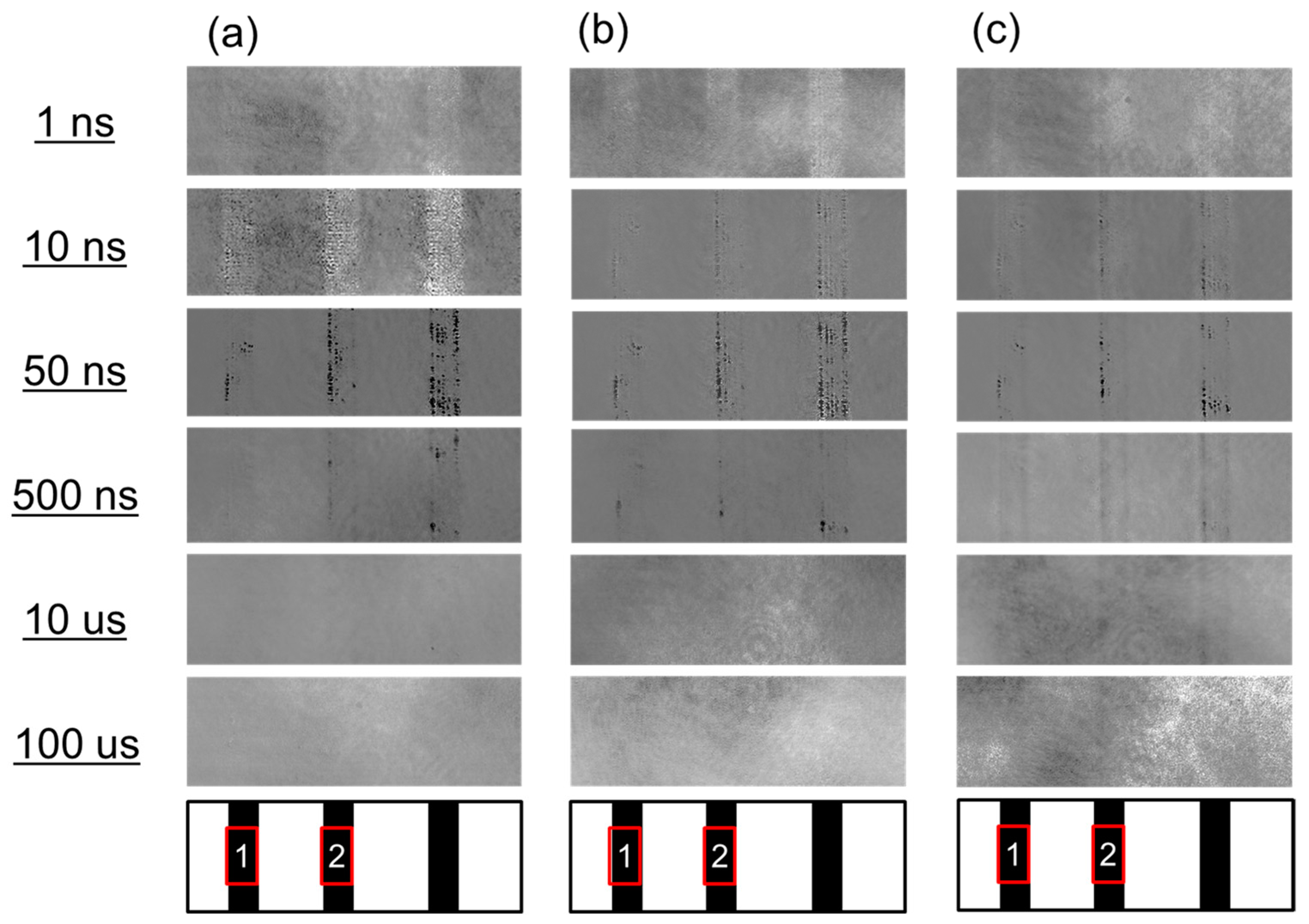
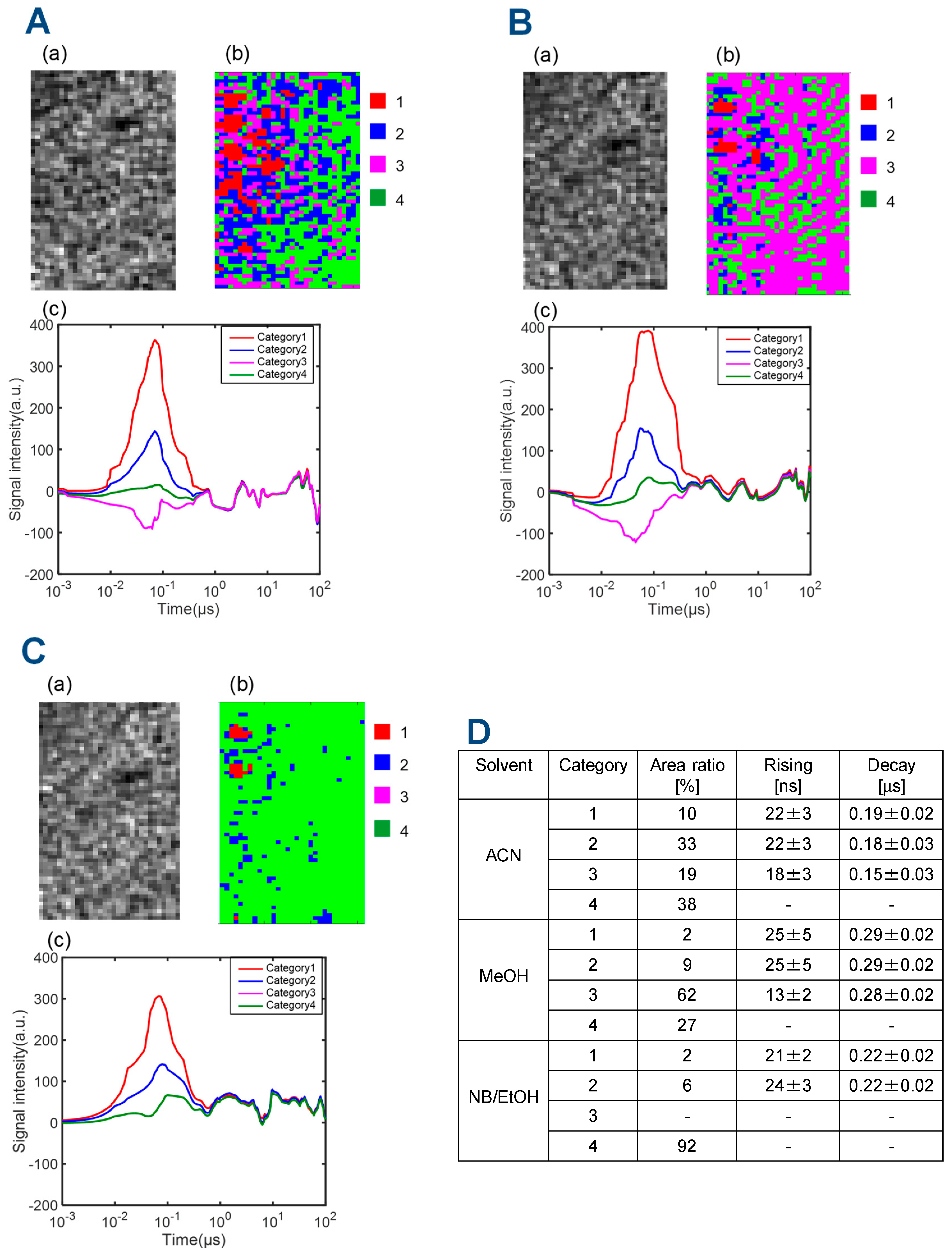
3.1. Charge Carrier Dynamics of a TiO2 Particulate Film
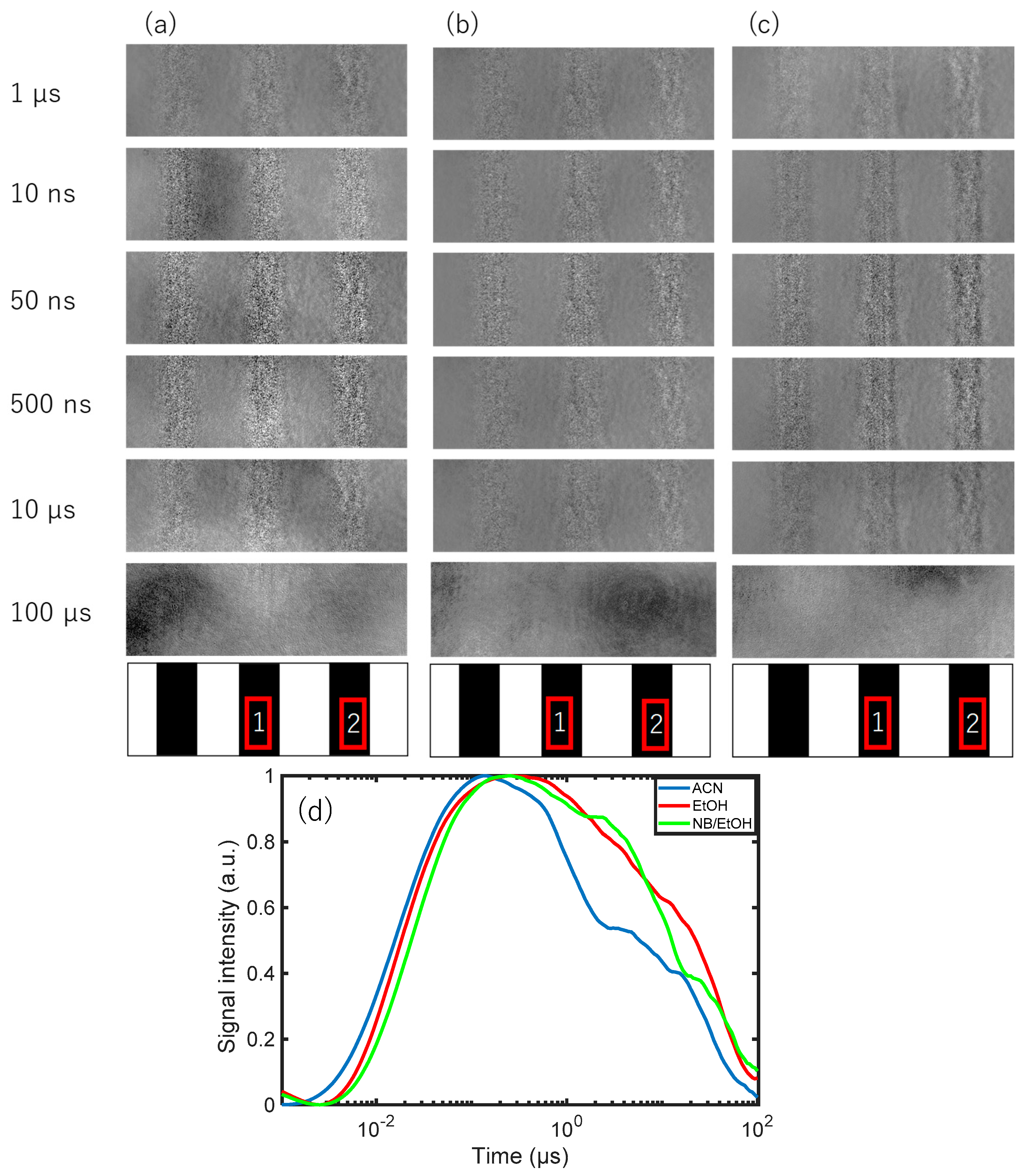
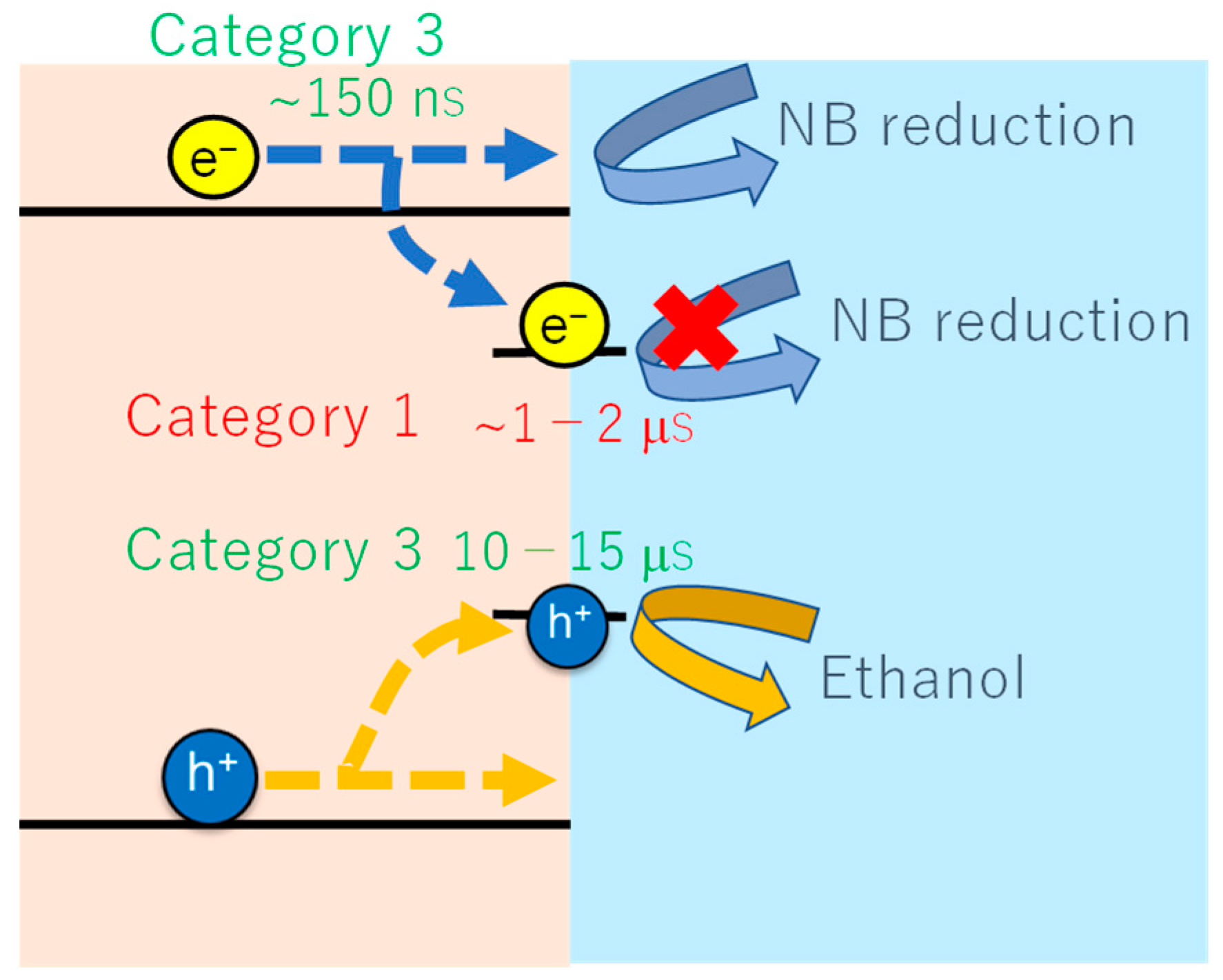
3.2. Charge Carrier Dynamics of an Fe2O3 Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Domen, K. Reaction Systems for Solar Hydrogen Production via Water Splitting with Particulate Semiconductor Photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.-J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.-P. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-Light Photocatalysts: Prospects and Challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent Advances and Perspectives for Solar-Driven Water Splitting Using Particulate Photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef]
- Maeda, K.; Higashi, M.; Lu, D.; Abe, R.; Domen, K. Efficient Nonsacrificial Water Splitting through Two-Step Photoexcitation by Visible Light Using a Modified Oxynitride as a Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2010, 132, 5858–5868. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable Water Splitting on Particulate Photocatalyst Sheets with a Solar-to-Hydrogen Energy Conversion Efficiency Exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. Stoichiometric Water Splitting into H2 and O2 Using a Mixture of Two Different Photocatalysts and an IO3−/I− Shuttle Redox Mediator under Visible Light Irradiation. Chem. Commun. 2001, 23, 2416–2417. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. A New Photocatalytic Water Splitting System under Visible Light Irradiation Mimicking a Z-Scheme Mechanism in Photosynthesis. J. Photochem. Photobiol. A Chem. 2002, 148, 71–77. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly Efficient Water Splitting into H2 and O2 over Lanthanum-Doped NaTaO3 Photocatalysts with High Crystallinity and Surface Nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic Water Splitting with a Quantum Efficiency of Almost Unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Li, C.; Fan, W.; Chen, S.; Zhang, F. Effective Charge Carrier Utilization of BiVO4 for Solar Overall Water Splitting. Chem.-Eur. J. 2022, 28, e202201812. [Google Scholar] [CrossRef]
- Choi, M.-J.; Kim, T.L.; Choi, K.S.; Sohn, W.; Lee, T.H.; Lee, S.A.; Park, H.; Jeong, S.Y.; Yang, J.W.; Lee, S.; et al. Controlled Band Offsets in Ultrathin Hematite for Enhancing the Photoelectrochemical Water Splitting Performance of Heterostructured Photoanodes. ACS Appl. Mater. Interfaces 2022, 14, 7788–7795. [Google Scholar] [CrossRef]
- Fu, J.; Fan, Z.; Nakabayashi, M.; Ju, H.; Pastukhova, N.; Xiao, Y.; Feng, C.; Shibata, N.; Domen, K.; Li, Y. Interface Engineering of Ta3N5 Thin Film Photoanode for Highly Efficient Photoelectrochemical Water Splitting. Nat. Commun. 2022, 13, 729. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, W.; Dang, L.; Mao, Y.; Wu, J.; Xu, K. Recent Progress in Doped G-C3N4 Photocatalyst for Solar Water Splitting: A Review. Front. Chem. 2022, 10, 955065. [Google Scholar] [CrossRef]
- Jiménez, J.M.; Perdolt, D.; Berger, T. Reactivity of Hydrogen-Related Electron Centers in Powders, Layers, and Electrodes Consisting of Anatase TiO2 Nanocrystal Aggregates. J. Phys. Chem. C 2021, 125, 13809–13818. [Google Scholar] [CrossRef]
- Yang, G.; Xiong, J.; Lu, M.; Wang, W.; Li, W.; Wen, Z.; Li, S.; Li, W.; Chen, R.; Cheng, G. Co-Embedding Oxygen Vacancy and Copper Particles into Titanium-Based Oxides (TiO2, BaTiO3, and SrTiO3) Nanoassembly for Enhanced CO2 Photoreduction through Surface/Interface Synergy. J. Colloid Interface Sci. 2022, 624, 348–361. [Google Scholar] [CrossRef]
- Qin, S.; Denisov, N.; Zhou, X.; Zdražil, L.; Fehn, D.; Hwang, I.; Bruns, M.; Kim, H.; Meyer, K.; Schmuki, P. Critical Factors for Photoelectrochemical and Photocatalytic H2 Evolution from Grey Anatase (001) Nanosheets. J. Phys. Energy 2022, 4, 044004. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Jia, X.; Du, B.; Li, R.; Wang, B. Enabling High Low-Bias Performance of Fe2O3 Photoanode for Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2022, 633, 555–565. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, C.; Zhang, X.; Fan, L.; Zhou, L.; Chu, Y.; Huang, W.; Wu, C.; Li, J.; Yang, X.; et al. Uniformly Citrate-Assisted Deposition of Small-Sized FeOOH on BiVO4 Photoanode for Efficient Solar Water Oxidation. Electrochim. Acta 2021, 389, 138795. [Google Scholar] [CrossRef]
- Su, Z.; Fang, F.; Li, X.; Han, W.; Liu, X.; Chang, K. Synergistic Surface Oxygen Defect and Bulk Ti3+ Defect Engineering on SrTiO3 for Enhancing Photocatalytic Overall Water Splitting. J. Colloid Interface Sci. 2022, 626, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, J.; Kong, Y.; Zhao, Y.; Chen, S.; Li, D.; Liu, W.; Chen, Y.; Xie, T.; Cui, J.; et al. Unraveling of Cocatalysts Photodeposited Selectively on Facets of BiVO4 to Boost Solar Water Splitting. Nat. Commun. 2022, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Lu, X.; Yang, J.; Tong, Y. Enhanced BiVO4 Photoanode Photoelectrochemical Performance via Borate Treatment and a NiFeOx Cocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 8306–8314. [Google Scholar] [CrossRef]
- Yamakata, A.; Kawaguchi, M.; Murachi, R.; Okawa, M.; Kamiya, I. Dynamics of Photogenerated Charge Carriers on Ni- and Ta-Doped SrTiO3 Photocatalysts Studied by Time-Resolved Absorption and Emission Spectroscopy. J. Phys. Chem. C 2016, 120, 7997–8004. [Google Scholar] [CrossRef]
- Brüninghoff, R.; Wenderich, K.; Korterik, J.P.; Mei, B.T.; Mul, G.; Huijser, A. Time-Dependent Photoluminescence of Nanostructured Anatase TiO2 and the Role of Bulk and Surface Processes. J. Phys. Chem. C 2019, 123, 26653–26661. [Google Scholar] [CrossRef]
- Gallart, M.; Cottineau, T.; Hönerlage, B.; Keller, V.; Keller, N.; Gilliot, P. Temperature Dependent Photoluminescence of Anatase and Rutile TiO2 Single Crystals: Polaron and Self-Trapped Exciton Formation. J. Appl. Phys. 2018, 124, 133104. [Google Scholar] [CrossRef]
- Saini, C.P.; Barman, A.; Banerjee, D.; Grynko, O.; Prucnal, S.; Gupta, M.; Phase, D.M.; Sinha, A.K.; Kanjilal, D.; Skorupa, W.; et al. Impact of Self-Trapped Excitons on Blue Photoluminescence in TiO2 Nanorods on Chemically Etched Si Pyramids. J. Phys. Chem. C 2017, 121, 11448–11454. [Google Scholar] [CrossRef]
- Singh, M.K.; Mehata, M.S. Temperature-Dependent Photoluminescence and Decay Times of Different Phases of Grown TiO2 Nanoparticles: Carrier Dynamics and Trap States. Ceram. Int. 2021, 47, 32534–32544. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Y.; Bai, J.; Seo, H.J. Manipulating Luminescence and Photocatalytic Activities of BiVO4 by Eu3+ Ions Incorporation. J. Phys. Chem. C 2020, 124, 11767–11779. [Google Scholar] [CrossRef]
- Wang, X.; Shen, S.; Feng, Z.; Li, C. Time-Resolved Photoluminescence of Anatase/Rutile TiO2 Phase Junction Revealing Charge Separation Dynamics. Chin. J. Catal. 2016, 37, 2059–2068. [Google Scholar] [CrossRef]
- Ichihara, F.; Sieland, F.; Pang, H.; Philo, D.; Duong, A.-T.; Chang, K.; Kako, T.; Bahnemann, D.W.; Ye, J. Photogenerated Charge Carriers Dynamics on La- and/or Cr-Doped SrTiO3 Nanoparticles Studied by Transient Absorption Spectroscopy. J. Phys. Chem. C 2020, 124, 1292–1302. [Google Scholar] [CrossRef]
- Selim, S.; Francàs, L.; García-Tecedor, M.; Corby, S.; Blackman, C.; Gimenez, S.; Durrant, J.R.; Kafizas, A. WO3/BiVO4: Impact of Charge Separation at the Timescale of Water Oxidation. Chem. Sci. 2019, 10, 2643–2652. [Google Scholar] [CrossRef]
- Grigioni, I.; Ganzer, L.; Camargo, F.V.A.; Bozzini, B.; Cerullo, G.; Selli, E. In Operando Photoelectrochemical Femtosecond Transient Absorption Spectroscopy of WO3/BiVO4 Heterojunctions. ACS Energy Lett. 2019, 4, 2213–2219. [Google Scholar] [CrossRef]
- Ravensbergen, J.; Abdi, F.F.; van Santen, J.H.; Frese, R.N.; Dam, B.; van de Krol, R.; Kennis, J.T.M. Unraveling the Carrier Dynamics of BiVO4: A Femtosecond to Microsecond Transient Absorption Study. J. Phys. Chem. C 2014, 118, 27793–27800. [Google Scholar] [CrossRef]
- Hillman, S.A.J.; Sprick, R.S.; Pearce, D.; Woods, D.J.; Sit, W.-Y.; Shi, X.; Cooper, A.I.; Durrant, J.R.; Nelson, J. Why Do Sulfone-Containing Polymer Photocatalysts Work So Well for Sacrificial Hydrogen Evolution from Water? J. Am. Chem. Soc. 2022, 144, 19382–19395. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, S.; Tu, W.; Yan, S.; He, T.; Zhu, X.; Yao, Y.; Zhou, Y.; Zou, Z. In Situ Determination of Polaron-Mediated Ultrafast Electron Trapping in Rutile TiO2 Nanorod Photoanodes. J. Phys. Chem. Lett. 2021, 12, 10815–10822. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Chen, Y.; Zhang, R.; Li, C.; Zhang, K.; Li, M.; Lin, Y.; Wang, D.; Zou, X.; et al. Interface Engineering Z-Scheme Ti-Fe2O3/In2O3 Photoanode for Highly Efficient Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2021, 290, 120058. [Google Scholar] [CrossRef]
- Fravventura, M.C.; Siebbeles, L.D.A.; Savenije, T.J. Mechanisms of Photogeneration and Relaxation of Excitons and Mobile Carriers in Anatase TiO2. J. Phys. Chem. C 2014, 118, 7337–7343. [Google Scholar] [CrossRef]
- Ziwritsch, M.; Müller, S.; Hempel, H.; Unold, T.; Abdi, F.F.; van de Krol, R.; Friedrich, D.; Eichberger, R. Direct Time-Resolved Observation of Carrier Trapping and Polaron Conductivity in BiVO4. ACS Energy Lett. 2016, 1, 888–894. [Google Scholar] [CrossRef]
- Yamada, Y.; Kanemitsu, Y. Determination of Electron and Hole Lifetimes of Rutile and Anatase TiO2 Single Crystals. Appl. Phys. Lett. 2012, 101, 133907. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.; Onishi, H. Time-Resolved Infrared Absorption Spectroscopy of Photogenerated Electrons in Platinized TiO2 Particles. Chem. Phys. Lett. 2001, 333, 271–277. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.; Onishi, H. Water- and Oxygen-Induced Decay Kinetics of Photogenerated Electrons in TiO2 and Pt/TiO2: A Time-Resolved Infrared Absorption Study. J. Phys. Chem. B 2001, 105, 7258–7262. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.; Onishi, H. Electron- and Hole-Capture Reactions on Pt/TiO2 Photocatalyst Exposed to Methanol Vapor Studied with Time-Resolved Infrared Absorption Spectroscopy. J. Phys. Chem. B 2002, 106, 9122–9125. [Google Scholar] [CrossRef]
- De Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of Microstructure on Local Carrier Lifetime in Perovskite Solar Cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, J.-X. Transient Absorption Microscopy: Technological Innovations and Applications in Materials Science and Life Science. J. Chem. Phys. 2020, 152, 020901. [Google Scholar] [CrossRef]
- Ebihara, M.; Ikeda, T.; Okunaka, S.; Tokudome, H.; Domen, K.; Katayama, K. Charge Carrier Mapping for Z-Scheme Photocatalytic Water-Splitting Sheet via Categorization of Microscopic Time-Resolved Image Sequences. Nat. Commun. 2021, 12, 3716. [Google Scholar] [CrossRef]
- Kuwahara, S.; Katayama, K. Distinction of Electron Pathways at Titanium Oxide/Liquid Interfaces in Photocatalytic Processes and Co-Catalyst Effects. Phys. Chem. Chem. Phys. 2016, 18, 25271–25276. [Google Scholar] [CrossRef]
- Katayama, K.; Chugenji, T.; Kawaguchi, K. Charge Carrier Trapping during Diffusion Generally Observed for Particulate Photocatalytic Films. Energies 2021, 14, 7011. [Google Scholar] [CrossRef]
- Huang, X.; Fan, J.; Li, L.; Liu, H.; Wu, R.; Wu, Y.; Wei, L.; Mao, H.; Lal, A.; Xi, P.; et al. Fast, Long-Term, Super-Resolution Imaging with Hessian Structured Illumination Microscopy. Nat. Biotechnol. 2018, 36, 451–459. [Google Scholar] [CrossRef]
- Katayama, K.; Takeda, Y.; Shimaoka, K.; Yoshida, K.; Shimizu, R.; Ishiwata, T.; Nakamura, A.; Kuwahara, S.; Mase, A.; Sugita, T.; et al. Novel Method of Screening the Oxidation and Reduction Abilities of Photocatalytic Materials. Analyst 2014, 139, 1953–1959. [Google Scholar] [CrossRef]
- Katayama, K.; Chugenji, T.; Kawaguchi, K. Defocus-Induced Phase Contrast Enhancement in Pattern Illumination Time-Resolved Phase Microscopy. AIP Adv. 2021, 11, 115215. [Google Scholar] [CrossRef]
- Katayama, K. Photo-Excited Charge Carrier Imaging by Time-Resolved Pattern Illumination Phase Microscopy. J. Chem. Phys. 2020, 153, 054201. [Google Scholar] [CrossRef]
- Candès, E.J.; Li, X.; Ma, Y.; Wright, J. Robust Principal Component Analysis? J. ACM 2011, 58, 11:1–11:37. [Google Scholar] [CrossRef]
- Miyama, M.J.; Hukushima, K. Real-Space Analysis of Scanning Tunneling Microscopy Topography Datasets Using Sparse Modeling Approach. J. Phys. Soc. Jpn. 2018, 87, 044801. [Google Scholar] [CrossRef]
- Ebihara, M.; Sohn, W.Y.; Katayama, K. Lifetime Mapping of Photo-Excited Charge Carriers by the Transient Grating Imaging Technique for Nano-Particulate Semiconductor Films. Rev. Sci. Instrum. 2019, 90, 073905. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katayama, K.; Kawaguchi, K.; Egawa, Y.; Pan, Z. Local Charge Carrier Dynamics for Photocatalytic Materials Using Pattern-Illumination Time-Resolved Phase Microscopy. Energies 2022, 15, 9578. https://doi.org/10.3390/en15249578
Katayama K, Kawaguchi K, Egawa Y, Pan Z. Local Charge Carrier Dynamics for Photocatalytic Materials Using Pattern-Illumination Time-Resolved Phase Microscopy. Energies. 2022; 15(24):9578. https://doi.org/10.3390/en15249578
Chicago/Turabian StyleKatayama, Kenji, Kei Kawaguchi, Yuta Egawa, and Zhenhua Pan. 2022. "Local Charge Carrier Dynamics for Photocatalytic Materials Using Pattern-Illumination Time-Resolved Phase Microscopy" Energies 15, no. 24: 9578. https://doi.org/10.3390/en15249578
APA StyleKatayama, K., Kawaguchi, K., Egawa, Y., & Pan, Z. (2022). Local Charge Carrier Dynamics for Photocatalytic Materials Using Pattern-Illumination Time-Resolved Phase Microscopy. Energies, 15(24), 9578. https://doi.org/10.3390/en15249578







