Optimized Production of Second-Generation Bioethanol from a Spent C4 Grass: Vetiver (Chrysopogon zizanioides)
Abstract
:1. Introduction
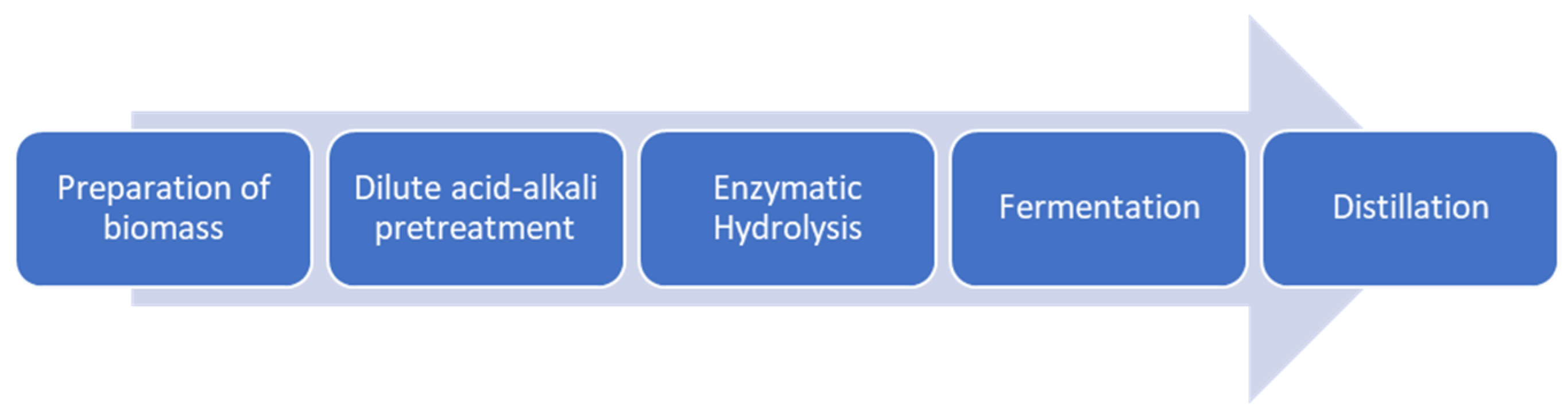
2. Materials and Methods
2.1. Biomass Sources and Preparation
2.2. Bioethanol Production
2.2.1. Dilute Acid-Alkali Pretreatment
2.2.2. Enzymatic Hydrolysis
2.2.3. Bioethanol Fermentation
2.2.4. Simultaneous Saccharification and Fermentation (SSF)
2.3. Determination of Sugars and Bioethanol
2.4. Quality Analysis of Bioethanol
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Vetiver Grass
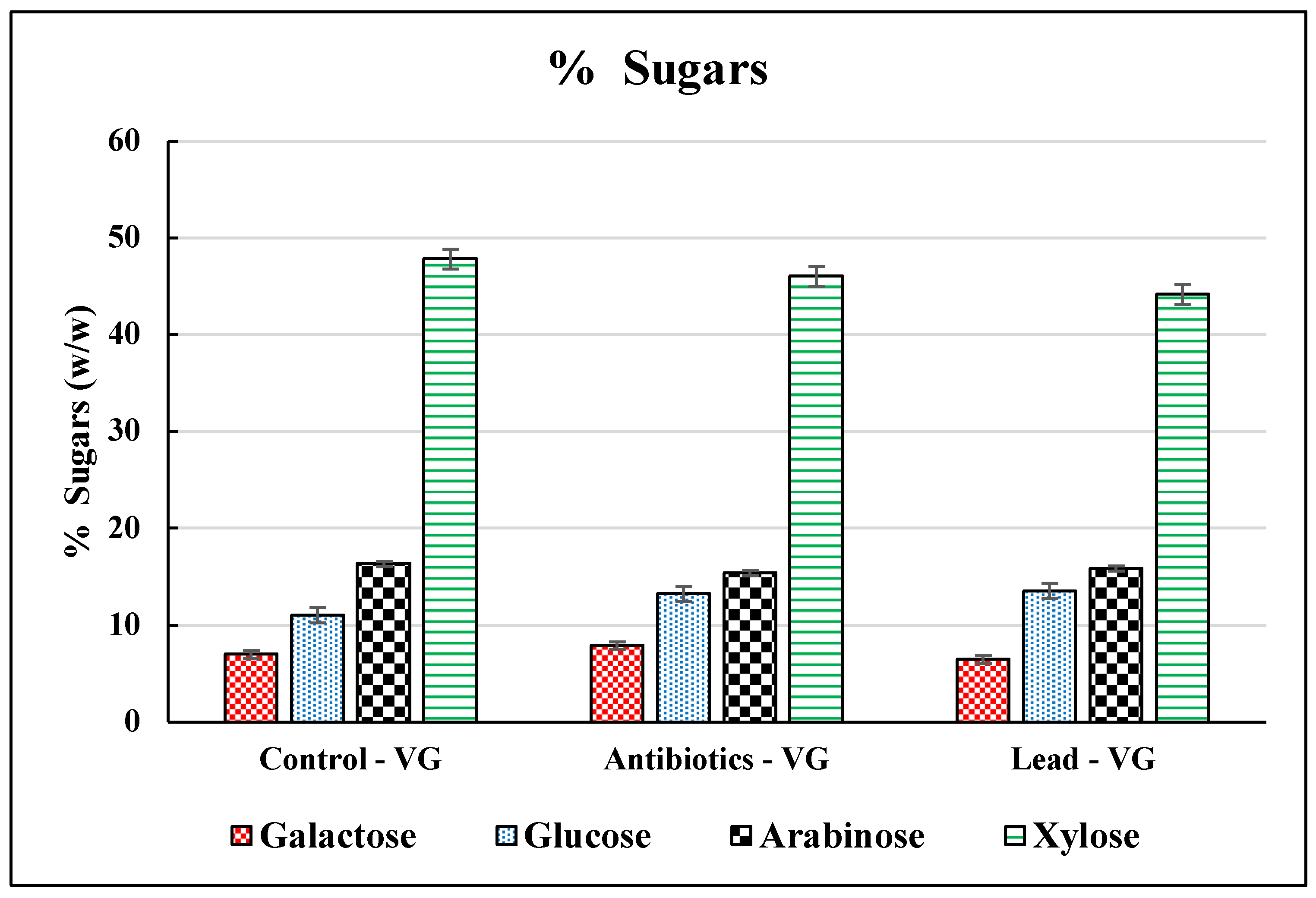
3.2. Sugar Release from Vetiver Grass
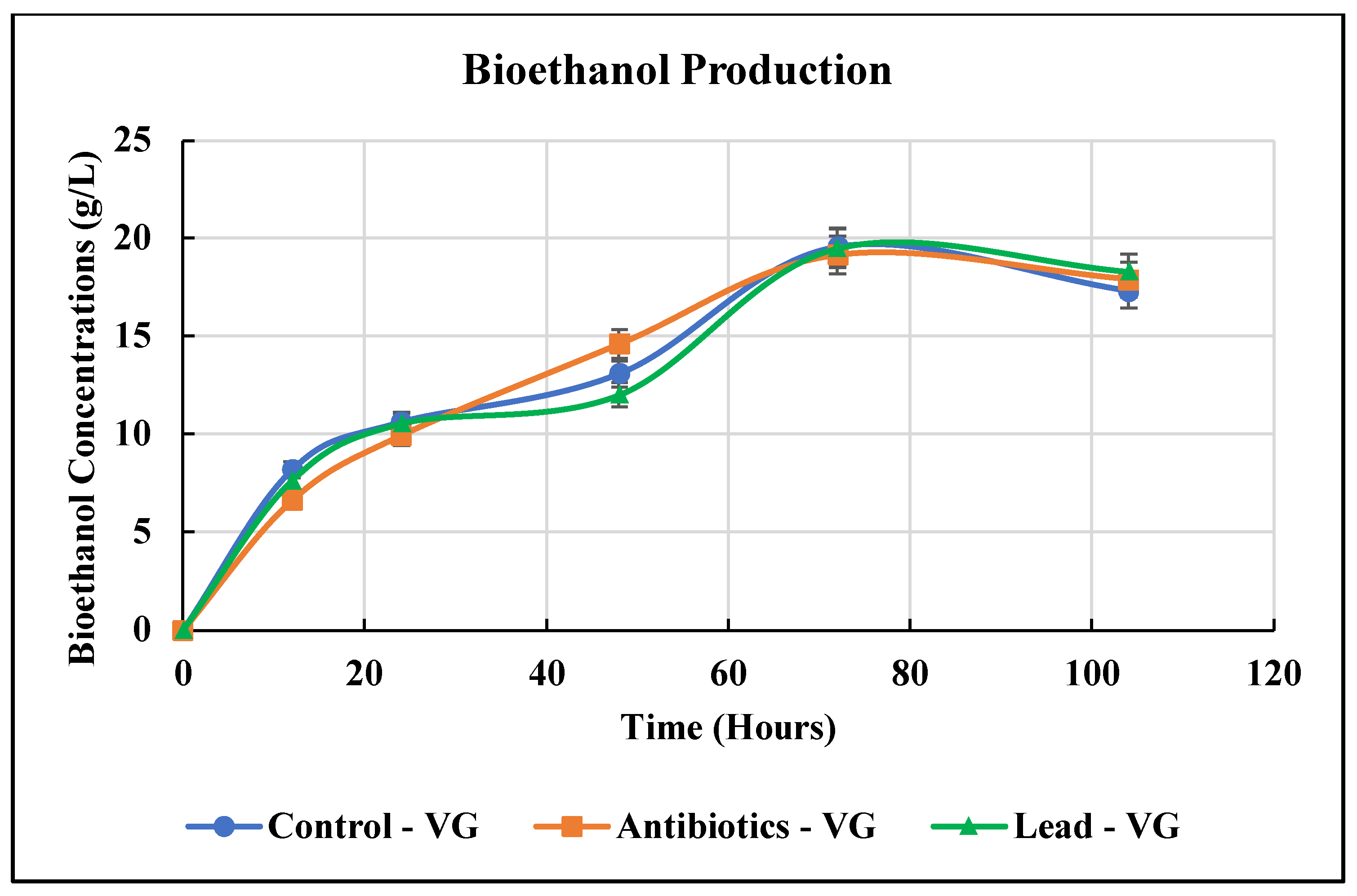
3.3. Bioethanol Production from Vetiver Grass
3.4. Quality Analysis of Bioethanol
3.5. Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing bioenergy production from the raw and defatted microalgal biomass using wastewater as the cultivation medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef]
- Solomon, B.D. Biofuels and sustainability. Ann. N. Y. Acad. Sci. 2010, 1185, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Ethanol explained—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/energyexplained/biofuels/ethanol.php (accessed on 13 November 2022).
- An Overview on the Application of the Vetiver Grass System in Asia-Pacific and Southern African Regions. Available online: https://www.vetiver.org/AUS_asiaoverview.htm (accessed on 13 November 2022).
- Zhang, Z.; Sarkar, D.; Sidhu, V.; Warke, M.; Datta, R. Impact of EDDS Dosage on Lead Phytoextraction in Contaminated Urban Residential Soils. Front. Sustain. Cities 2022, 3, 773467. [Google Scholar] [CrossRef]
- Raman, J.K.; Gnansounou, E. LCA of bioethanol and furfural production from vetiver. Bioresour. Technol. 2015, 185, 202–210. [Google Scholar] [CrossRef]
- Wongwatanapaiboon, J.; Kangvansaichol, K.; Burapatana, V.; Inochanon, R.; Winayanuwattikun, P.; Yongvanich, T.; Chulalaksananukul, W. The potential of cellulosic ethanol production from grasses in Thailand. J. Biomed. Biotechnol. 2012, 2012, 303748. [Google Scholar] [CrossRef] [Green Version]
- Panja, S.; Sarkar, D.; Datta, R. Removal of antibiotics and nutrients by Vetiver grass (Chrysopogon zizanioides) from secondary wastewater effluent. Int. J. Phytoremediat. 2020, 22, 764–773. [Google Scholar] [CrossRef]
- Pidatala, V.R.; Li, K.; Sarkar, D.; Wusurika, R.K.; Datta, R. Identification of biochemical pathways associated with lead tolerance and detoxification in Chrysopogon zizanioides L. Nash (vetiver) by metabolic profiling. Environ. Sci. Technol. 2016, 50, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Sarkar, D.; Das, P.; Panja, S.; Parikh, C.; Ramanathan, D.; Bagley, S.; Datta, R. Tetracycline uptake and metabolism by vetiver grass (Chrysopogon zizanioides L. Nash). Environ. Sci. Pollut. Res. 2016, 23, 24880–24889. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Sarkar, D.; Li, K.; Datta, R. Uptake and transformation of ciprofloxacin by vetiver grass (Chrysopogon zizanioides). Int. Biodeterior. Biodegrad. 2019, 142, 200–210. [Google Scholar] [CrossRef]
- Attinti, R.; Barrett, K.R.; Datta, R.; Sarkar, D. Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ. Pollut. 2017, 225, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Gnansounou, E.; Alves, C.M.; Raman, J.K. Multiple applications of vetiver grass—A review. Int. J. Environ. Sci. 2017, 2, 125–141. [Google Scholar]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Hou, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Neve, S.; Sarkar, D.; Datta, R. Effects of Pyrolysis Temperature and Residence Time on Physicochemical Properties of Biochar Derived from Spent Vetiver Roots. presented at the ASA, CSSA, SSSA International Annual Meeting, Nov. 2022. Available online: https://scisoc.confex.com/scisoc/2022am/meetingapp.cgi/Paper/142823 (accessed on 14 November 2022).
- Panja, S.; Sarkar, D.; Zhang, Z.; Datta, R. Removal of antibiotics and nutrients by vetiver grass (Chrysopogon zizanioides) from a plug flow reactor based constructed wetland model. Toxics 2021, 9, 84. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Sidhu, V.; Datta, R. Removal of Lead in Residential Soils of Jersey City Using Biodegradable Chelating Agent-Enhanced Phytoremediation. presented at the GSA 2020 Connects Online, Oct. 2020. Available online: https://gsa.confex.com/gsa/2020AM/webprogram/Paper356930.html (accessed on 14 November 2022).
- Nagara, V.N.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of heavy metals from stormwater runoff using granulated drinking water treatment residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Geiger, E.M.; Sarkar, D.; Datta, R. Evaluation of copper-contaminated marginal land for the cultivation of vetiver grass (Chrysopogon zizanioides) as a lignocellulosic feedstock and its impact on downstream bioethanol production. Appl. Sci. 2019, 9, 2685. [Google Scholar] [CrossRef]
- Restiawaty, E.; Dewi, A. Comparison of Pretreatment Methods on Vetiver Leaves for Efficient Processes of Simultaneous Saccharification and Fermentation by Neurospora sp. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2017; Volume 877, No. 1; p. 012048. [Google Scholar] [CrossRef]
- Sutjahjo, D.H. The characteristics of bioethanol fuel made of vegetable raw materials. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 296, No. 1; p. 012019. [Google Scholar] [CrossRef]
- Kvaalen, E. Alcohol Distillation: Basic Principles, Equipment, Performance Relationships, and Safety; Purdue University, Cooperative Extension Service: West Lafayette, IN, USA, 1984. [Google Scholar]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Bint-E-Naser, S.F.; Hossain, L.; Debnath, M.; Barua, P.P.; Khan, M.S. Analyzing Physico-Chemical Properties of Bioethanol and Bioethanol-Blended Fuels. J. Nat. Sci. Sustain. Technol. 2017, 11, 331–339. [Google Scholar]
- Maas, R.H.; Bakker, R.R.; Boersma, A.R.; Bisschops, I.; Pels, J.R.; de Jong, E.; Weusthuis, R.A.; Reith, H. Pilot-scale conversion of lime-treated wheat straw into bioethanol: Quality assessment of bioethanol and valorization of side streams by anaerobic digestion and combustion. Biotechnol. Biofuels 2008, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yüksel, F.; Yüksel, B. The use of ethanol–gasoline blend as a fuel in an SI engine. Renew. Energy 2004, 29, 1181–1191. [Google Scholar] [CrossRef]
- Methacanon, P.; Chaikumpollert, O.; Thavorniti, P.; Suchiva, K. Hemicellulosic polymer from Vetiver grass and its physicochemical properties. Carbohydr. Polym. 2003, 54, 335–342. [Google Scholar] [CrossRef]
- Sambusiti, C.; Ficara, E.; Rollini, M.; Manzoni, M.; Malpei, F. Sodium hydroxide pretreatment of ensiled sorghum forage and wheat straw to increase methane production. Water Sci. Technol. 2012, 66, 2447–2452. [Google Scholar] [CrossRef]
- Rooney, W.L.; Blumenthal, J.; Bean, B.; Mullet, J.E. Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioprod. Biorefining 2007, 1, 147–157. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, S.H.; Chung, I.M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Y.; Jiang, J.; Yi, Z.; Xiao, L.; Ai, X.; Chen, Z. Comparison of lignocellulose composition in four major species of Miscanthus. Afr. J. Biotechnol. 2012, 11, 12529–12537. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Huong, V.T.T.; Atjayutpokin, T.; Chinwatpaiboon, P.; Smith, S.M.; Boonyuen, S.; Luengnaruemitchai, A. Two-stage acid-alkali pretreatment of vetiver grass to enhance the subsequent sugar release by cellulase digestion. Renew. Energy 2022, 195, 755–765. [Google Scholar] [CrossRef]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Sun, X.F. Fractional and structural characterization of hemicelluloses isolated by alkali and alkaline peroxide from barley straw. Carbohydr. Polym. 2002, 49, 415–423. [Google Scholar] [CrossRef]
- Das, S.P.; Ghosh, A.; Gupta, A.; Goyal, A.; Das, D. Lignocellulosic fermentation of wild grass employing recombinant hydrolytic enzymes and fermentative microbes with effective bioethanol recovery. BioMed Res. Int. 2013, 2013, 386063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliana, C.; Jorge, R.; Juan, P.; Luis, R. Effects of the pretreatment method on enzymatic hydrolysis and ethanol fermentability of the cellulosic fraction from elephant grass. Fuel 2014, 118, 41–47. [Google Scholar] [CrossRef]
- Liang, X.; Hua, D.; Zhang, J.; Xu, H.; Li, Y.; Zhang, X.; Zhao, S. Cellulosic ethanol production using rice grass (Spartina spp.) with cellulase. Asian J. Chem. 2011, 23, 1815–1818. [Google Scholar]
- Yasuda, M.; Takenouchi, Y.; Nitta, Y.; Ishii, Y.; Ohta, K. Italian ryegrass (Lolium multiflorum Lam) as a high-potential bio-ethanol resource. Bioenergy Res. 2015, 8, 1303–1309. [Google Scholar] [CrossRef]
- Yasuda, M.; Ishii, Y.; Ohta, K. Napier grass (Pennisetum purpureum Schumach) as raw material for bioethanol production: Pretreatment, saccharification, and fermentation. Biotechnol. Bioprocess Eng. 2014, 19, 943–950. [Google Scholar] [CrossRef]
- Kataria, R.; Ghosh, S. Saccharification of Kans grass using enzyme mixture from Trichoderma reesei for bioethanol production. Bioresour. Technol. 2011, 102, 9970–9975. [Google Scholar] [CrossRef]
- Aiyejagbara, M.; Aderemi, B.; Ameh, A.; Ishidi, E.; Ibeneme, E.F.A.; Olakunle, M. Production of Bioethanol from Elephant Grass (Pennisetum purpureum) Stem. Int. J. Innov. Math. Stat. Energy Policies 2016, 4, 1–9. [Google Scholar]
- Prasertwasu, S.; Khumsupan, D.; Komolwanich, T.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Efficient process for ethanol production from Thai Mission grass (Pennisetum polystachion). Bioresour. Technol. 2014, 163, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Gokulakrishnan, R.; Kanagavel, M.; Thajuddin, N. Production of biofuel ethanol from pretreated seagrass by using Saccharomyces cerevisiae. Indian J. Sci. Technol. 2011, 4, 1087–1089. [Google Scholar] [CrossRef]
- Njoku, S.I.; Iversen, J.A.; Uellendahl, H.; Ahring, B.K. Production of ethanol from hemicellulose fraction of cocksfoot grass using Pichia stipitis. Sustain. Chem. Processes 2013, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- e Silva, C.F.L.; Schirmer, M.A.; Maeda, R.N.; Barcelos, C.A.; Pereira, N., Jr. Potential of giant reed (Arundo donax L.) for second generation ethanol production. Electron. J. Biotechnol. 2015, 18, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Bibi, F.; Yasmin, H.; Jamal, A.; Al-Harbi, M.S.; Ahmad, M.; Zafar, M.; Ahmad, B.; Samra, B.N.; Ahmed, A.F.; Ali, M.I. Deciphering role of technical bioprocess parameters for bioethanol production using microalgae. Saudi J. Biol. Sci. 2021, 28, 7595–7606. [Google Scholar] [CrossRef]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Šantek, B. Bioethanol production from renewable raw materials and its separation and purification: A review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Skiba, E.A.; Ovchinnikova, E.V.; Budaeva, V.V.; Banzaraktsaeva, S.P.; Kovgan, M.A.; Chumachenko, V.A.; Mironova, G.F.; Kortusov, A.N.; Parmon, V.N.; Sakovich, G.V. Miscanthus bioprocessing using HNO3-pretreatment to improve productivity and quality of bioethanol and downstream ethylene. Ind. Crops Prod. 2022, 177, 114448. [Google Scholar] [CrossRef]
- Azeke, E.M.; Eze, C.L.; Ubong, I.; Kuroshi, L. The Potential of Elephant Grass (Pennisetum purpureum Schum), a Nigerian Indigenous Grass. In Proceedings of the Bioethanol Production: A Decarbonization Alternative for the Maritime Industry, Southern African Transport Conference, Pretoria, South Africa, 8–11 July 2019. [Google Scholar]

| C4 Grasses | Cellulose (%) | Hemicellulose (%) | Lignin (%) | References |
|---|---|---|---|---|
| Vetiver | 34.48 | 35.07 | 14.34 | This study |
| Vetiver | 34.49 | 34.12 | 14.69 | [20] |
| Vetiver | 34.06 | 34.05 | 15.12 | [21] |
| Vetiver | 31.39 | 34.55 | 17.58 | [26] |
| Vetiver | 32.6 | 31.5 | 17.3 | [10] |
| Vetiver | 31.85−38.51 | 37.87–42.61 | 3.67–5.06 | [11] |
| Vetiver | 30–35 | 40 | 10 | [33] |
| Wheat straw | 49 | 34 | 6.5 | [34] |
| Sorghum | 26.3 | 20 | 7 | [35] |
| Switchgrass | 29.5–37.8 | 21.5–27.4 | 13.9–21.1 | [36] |
| Miscanthus | 32.71 | 34.86 | 8.9 | [37] |
| C4 Grasses | Microorganisms | Mechanisms | Bioethanol Yields | References |
|---|---|---|---|---|
| Vetiver (fresh) | S. cerevisiae | SSF | 279.76 mg/g or 19.58 g/L | This study |
| Vetiver (antibiotics contamination) | S. cerevisiae | SSF | 273.90 mg/g or 19.17 g/L | This study |
| Vetiver (lead contamination) | S. cerevisiae | SSF | 278.57 mg/g or 19.50 g/L | This study |
| Dwarf Napier grass (Schumach) | S. cerevisiae NBRC 2044 | SSF | 121 mg/g | [46] |
| Kans grass (Saccharum spontaneum) | S. cerevisiae | SAF | 460 mg/g | [47] |
| Switch grass (Panicum virgatum) | S. cerevisiae 424 A (LNH-ST) | SSCF | 32.1 g/L | [45] |
| Elephant grass (Pennisetun purpureum) | Aspergillus niger and S. cerevisiae | SSF | 23.4 g/L | [43] |
| Switchgrass (Panicum virgatum) | Kluyveromyces marxianus IMB3 | SSF | 22.5 g/L | [48] |
| Mission grass (Pennisetum polystachion) | S. cerevisiae TISTR 5596 | SHF | 16 g/L | [49] |
| Thatch grass (Hyparrhenia rufa) | Zymomonas mobilis | SSF | 8.8 g/L | [42] |
| Sea grass (Cymodocea serrulata) | S. cerevisiae | SSF | 0.047 mL/g | [50] |
| Cocksfoot grass (Dactylis glomerata) | Pichia stipitis CBS 6054 | SSF | 158 mL/kg | [51] |
| Giant miscanthus (Miscanthus giganteus) | Scheffersomyces (Pichia) stipitis CBS 6054 | SSF | 12.1 g/L | [52] |
| Rice grass (Spartina spp.) | Trichoderma reesei SEMCC 3.217 and S. cerevisiae SEMCC 2.157 | SSF | 28.1 g/L | [44] |
| Parameter | ASTM Test Methods | Control—VG | Lead—VG | Antibiotics—VG | Standard |
|---|---|---|---|---|---|
| Ethanol Content (%) | ASTM D 5501 | 98.89 | 98.85 | 98.83 | 99–100 |
| Density at 25°C (g/mL) | ASTM D 4052 | 0.766 | 0.784 | 0.774 | 0.79 |
| Freezing Point (°C) | ASTM D 2386 | −94 | −94 | −94 | −114 |
| Boiling Point (°C) | ASTM D 5399-09 | 78.3 | 78.4 | 78.4 | 78 |
| Flash Point (°C) | ASTM D 93 | 12.8 | 12.7 | 12.7 | 13 |
| API gravity (°) | ASTM D 4052 | 52.3 | 51.9 | 53.1 | 52 |
| Calorific Value (MJ/kg) | ASTM D 2014-96 | 31.26 | 30.68 | 33.09 | |
| Heat of vaporization (kJ/kg K) | ASTM E 2071 | 278 | 282 | 279 | 289 |
| Reid vapor pressure (kPa) | ASTM D 323-99a | 12.67 | 11.96 | 13.24 | 14.25 |
| Viscosity (cSt) | ASTM D 88-94 | 1.01 | 1.01 | 1.04 | 1.03 |
| Cu strip corrosion at 50 °C | ASTM D 130-04 | 1a | 1a | 1a | 1a |
| ASTM distillation IBP (°C) | ASTM D 86-04b | 80.5 | 80.6 | 81 | 81 |
| Sulfur content (wt%) | ASTM D 3177-89 | 0.03 | 0.03 | 0.03 | 0.03 |
| Water content (%) | ASTM D 95-70 | 1.11 | 1.15 | 1.17 | 0–1 |
| ASTM color | ASTM D 1500-03 | None | None | None | None |
| Research Octane Number | ASTM D 2699 | 107 | 108 | 108 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neve, S.; Sarkar, D.; Zhang, Z.; Datta, R. Optimized Production of Second-Generation Bioethanol from a Spent C4 Grass: Vetiver (Chrysopogon zizanioides). Energies 2022, 15, 9597. https://doi.org/10.3390/en15249597
Neve S, Sarkar D, Zhang Z, Datta R. Optimized Production of Second-Generation Bioethanol from a Spent C4 Grass: Vetiver (Chrysopogon zizanioides). Energies. 2022; 15(24):9597. https://doi.org/10.3390/en15249597
Chicago/Turabian StyleNeve, Sameer, Dibyendu Sarkar, Zhiming Zhang, and Rupali Datta. 2022. "Optimized Production of Second-Generation Bioethanol from a Spent C4 Grass: Vetiver (Chrysopogon zizanioides)" Energies 15, no. 24: 9597. https://doi.org/10.3390/en15249597
APA StyleNeve, S., Sarkar, D., Zhang, Z., & Datta, R. (2022). Optimized Production of Second-Generation Bioethanol from a Spent C4 Grass: Vetiver (Chrysopogon zizanioides). Energies, 15(24), 9597. https://doi.org/10.3390/en15249597









