Biodiesel and Bioplastic Production from Waste-Cooking-Oil Transesterification: An Environmentally Friendly Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Frying Oil Collection
2.2. Experimental Design
2.3. Chemical Procedures
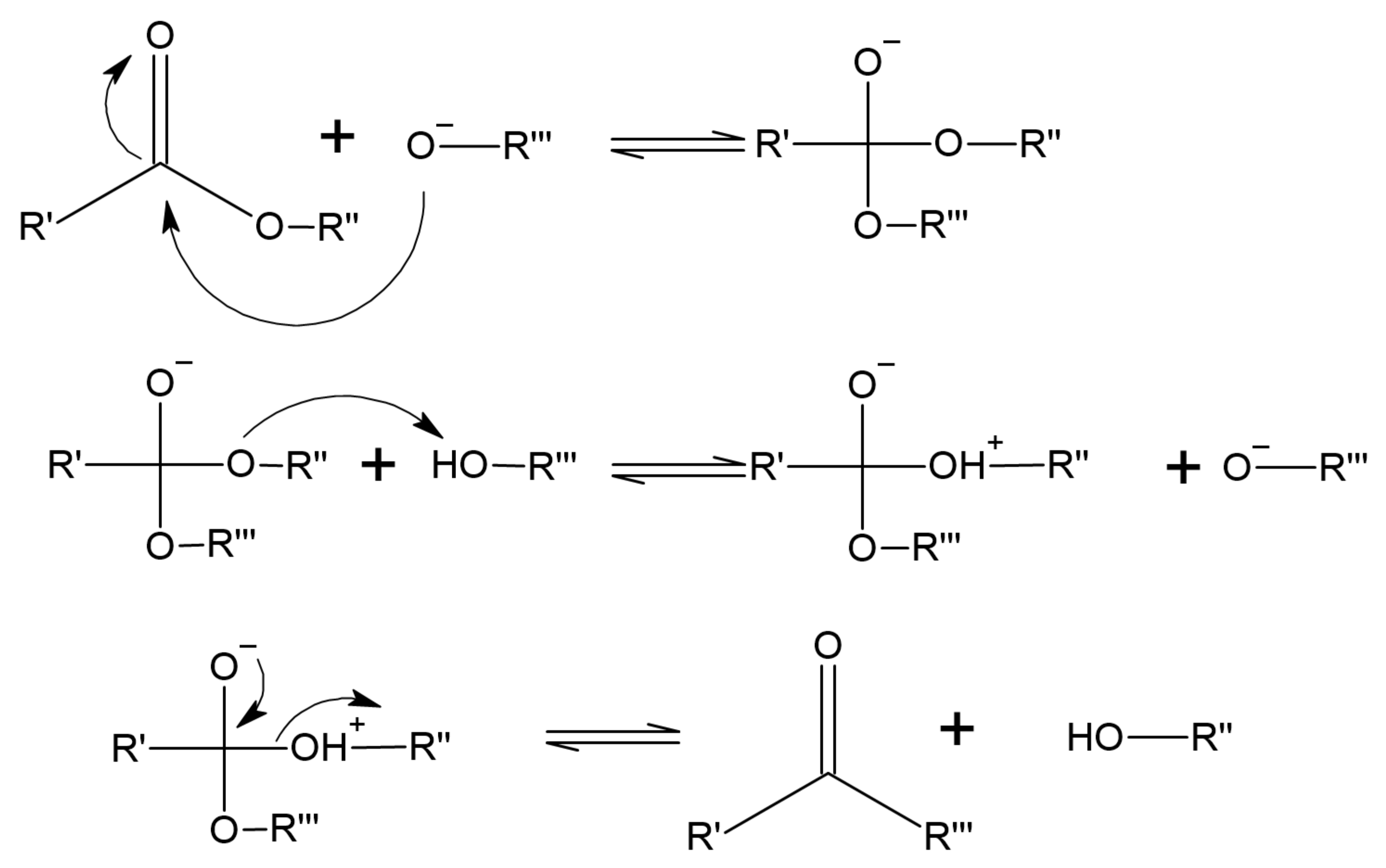
Transesterification Process
2.4. Determination of Biodiesel’s Production Yield
2.5. Physicochemical Characterizations
2.6. Thin Layer Chromatography (TLC)
2.7. Purification of Glycerol
2.8. Infrared Spectroscopy
2.9. Synthesis of Bioplastic
2.10. Moisture Content of the Bioplastic
2.11. Thickness and Degradation Analysis
3. Results
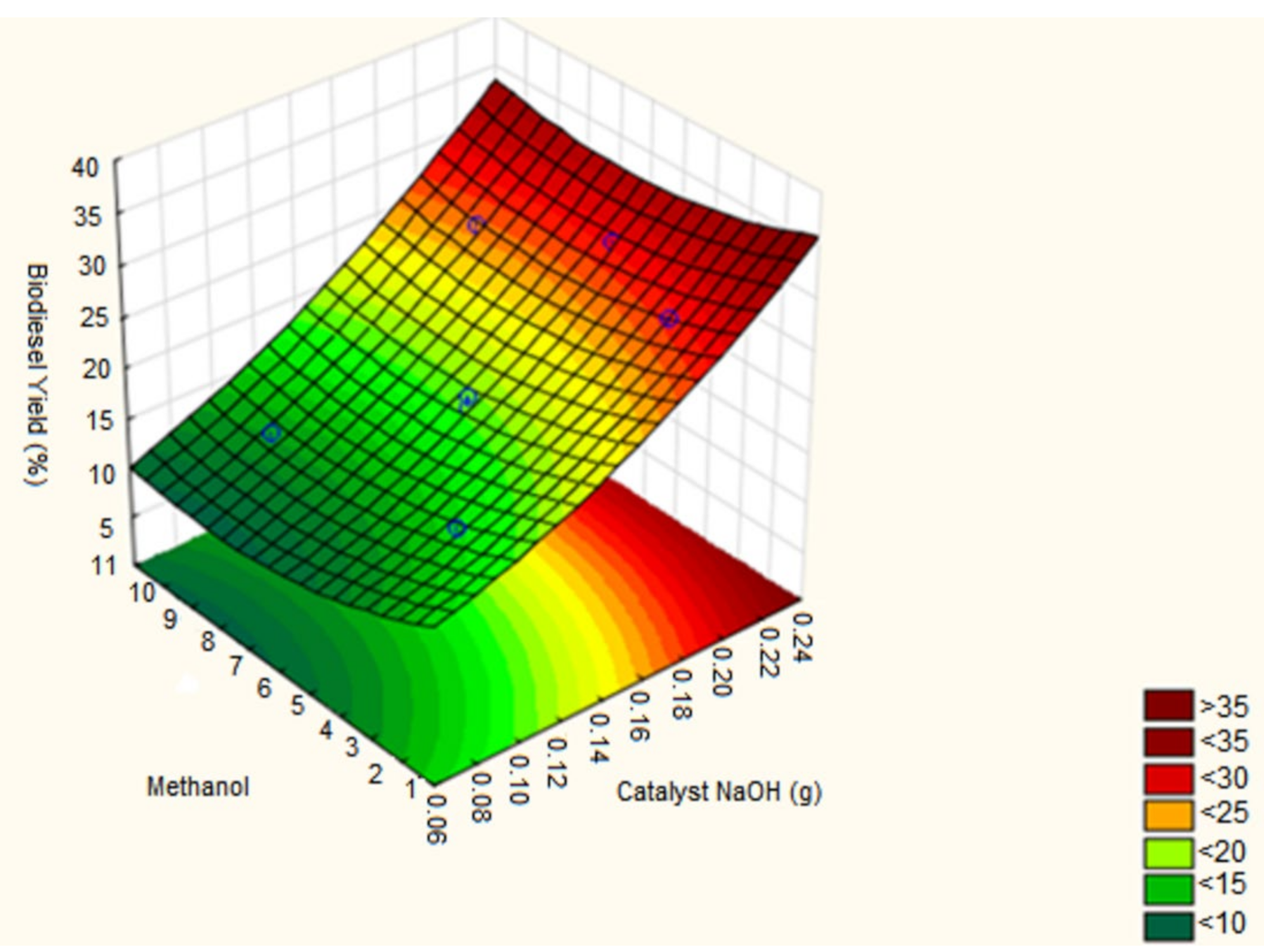
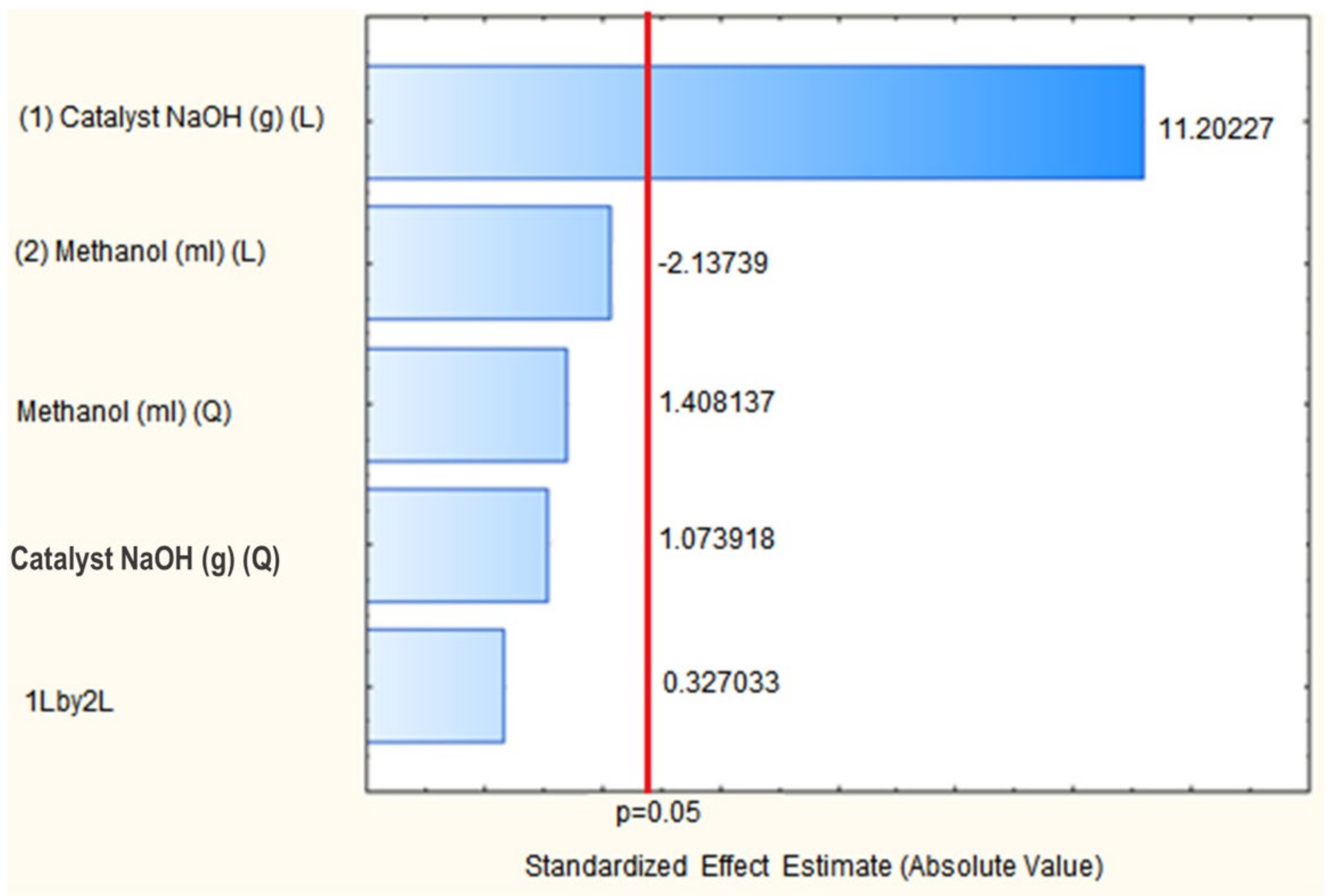
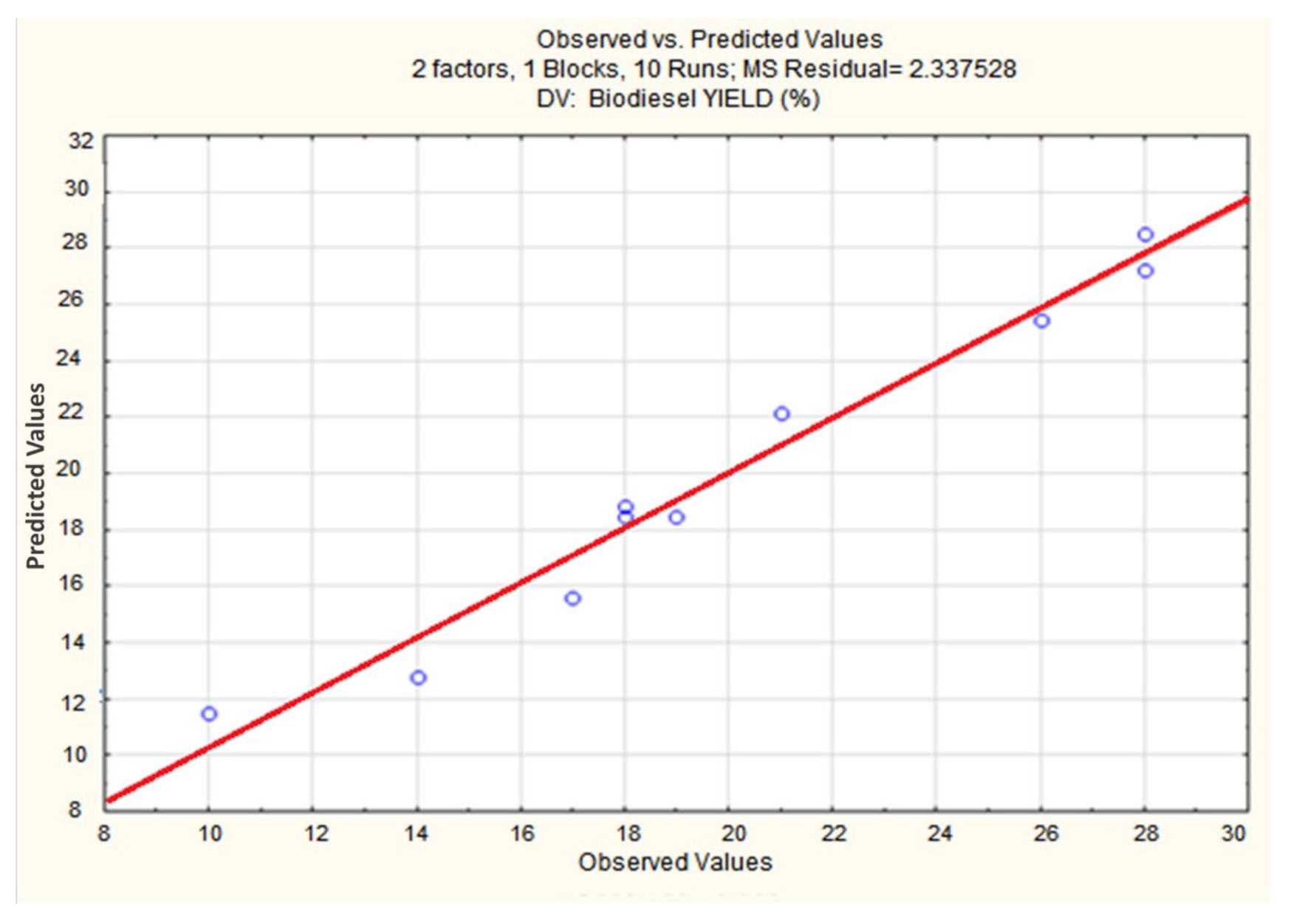
3.1. Biodiesel Production
3.2. Glycerol Purifications and Application
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajala, O.E.; Aberuagba, F.; Odetoye, T.E.; Ajala, A.M. Biodiesel: Sustainable energy replacement to petroleum-based diesel fuel—A review. Chem. Biol. Eng. Rev. 2015, 2, 145–156. [Google Scholar] [CrossRef]
- Schmidt, C.W. Biodiesel: Cultivating alternative fuels. Environ. Health Perspect. 2007, 115, A86–A91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, D.M.M.; Suarez, P.A.Z. From peanut oil to biodiesel—History and Brazilian policyfor the energetic use of fats and oils. Rev. Virtual Quim. 2017, 9, 39–51. [Google Scholar] [CrossRef]
- MME—Ministry of Mines and Energy. Resolution no 16 of 06.09.2021 of the National Energy Policy Council—CNPE. Available online: https://in.gov.br/en/web/dou/-/despacho-do-presidente-da-republica-344146044 (accessed on 30 November 2021).
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sust. Energ. Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by transesterification of rapeseed oil using ultrasound: A kinetic study of base-catalysed reactions. Energies 2018, 11, 2229. [Google Scholar] [CrossRef] [Green Version]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef] [Green Version]
- Zahed, M.A.; Zakeralhosseini, Z.; Mohajeri, L.; Bidhendi, G.N.; Mesgari, S. Multivariable analysis and optimization of biodiesel production from waste cooking oil. Environ. Processes 2018, 5, 303–312. [Google Scholar] [CrossRef]
- Devaraj, K.; Mani, Y.; Rawoof, S.A.A.; Thanarasu, A.; Dhanasekaran, A.; Subramanian, S. Feasibility of biodiesel production from waste cooking oil: Lab-scale to pilot-scale analysis. Environ. Sci. Pollut. Res. 2020, 27, 25828–25835. [Google Scholar] [CrossRef]
- Sinha, D.; Murugavelh, S. Biodiesel production from waste cotton seed oil using low cost catalyst: Engine performance and emission characteristics. Perspect. Sci. 2016, 8, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Rehman, H.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, J.J.; Sun, Y.Q.; Xiu, Z.L. Bioconversion of raw glycerol from waste cooking-oil-based biodiesel production to 1,3-propanediol and lactate by a microbial consortium. Front. Bioeng. Biotechnol. 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, S.; Silva, T.; Miranda, A.; Fernandes, M.; Felicio, H.; Calarge, F.; Santana, J.; Tambourgi, E. The Potential of biodiesel production from frying oil used in the restaurants of São Paulo city, Brazil. Chem. Eng. Trans. 2014, 37, 577–582. [Google Scholar]
- Abomohra, A.E.F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Jiang, K.; Hu, X. Energy demand and emissions in 2030 in China: Scenarios and policy options. Environ. Econ. Policy Stud. 2006, 7, 233–250. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Sireus, V.; Petretto, G.L.; Mulasa, G.; Garronid, S. Bentonite as a refining agent in waste cooking oils recycling: Flash point, density and color evaluation. Nat. Prod. Commun. 2018, 13, 613–616. [Google Scholar] [CrossRef] [Green Version]
- Cvengros, J.; Cvengrosova, Z. Used frying oil and fat and their utilization in the production of methyl esters of higher fatty acid. Biomass Bioenergy 2004, 27, 173–181. [Google Scholar] [CrossRef]
- Shashidhara, Y.M.; Jayaram, S.R. Vegetable oils as a potential cutting fluid—An evolution. Tribol. Int. 2010, 43, 1073–1081. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Applications of waste cooking oil other than biodiesel: A review. Iran. J. Chem. Eng. 2015, 12, 55–76. [Google Scholar]
- Singhabhandhu, A.; Tezuka, T. Prospective framework for collection and exploitation of waste cooking oil as feedstock for energy conversion. Energy 2010, 35, 1839–1847. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Bhattarai, K.; Stalick, W.M.; McKay, S.; Geme, G.; Bhattarai, N. Biofuel: An alternative to fossil fuel for alleviating world energy and economic crises. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2011, 46, 1424–1442. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef] [Green Version]
- Manzanera, M.; Molina-Muñoz, M.L.; González-López, J. Biodiesel: An alternative fuel. Recent Pat. Biotechnol. 2008, 2, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Dmytryshyn, S.L.; Dalai, A.K.; Chaudhari, S.T.; Mishra, H.K.; Reaney, M.J. Synthesis and characterization of vegetable oil derived esters: Evaluation for their diesel additive properties. Bioresour. Technol. 2004, 92, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.F.; Hernandez, A.; Mullan, B.P.; Moore, K.; Trezona-Murray, M.; King, R.H.; Pluske, J.R. A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim. Prod. Sci. 2009, 49, 154–161. [Google Scholar] [CrossRef]
- Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Dos Santos, R.G. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals—A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Novi, J.C.; Oliveira, S.V.W.B.; Salgado-Junior, A.P.; Oliveira, M.M.B. Analysis of glycerol management: Risks and opportunities regarding its destination in view of the regulatory gap and sustainable aspects. REAd. Rev. Eletrônica Adm. 2018, 24, 217–243. [Google Scholar]
- Xiao, Y.; Varma, A. Conversion of glycerol to hydrocarbon fuels via bifunctional catalysts. ACS Energy Lett. 2016, 1, 963–968. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, D. Toward glycerol biorefinery: Metabolic engineering for the production of biofuels and chemicals from glycerol. Biotechnol. Biofuels 2016, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Brockhaus, S.; Petersen, M.; Kersten, W. A crossroads for bioplastics: Exploring product developers’ challenges to move beyond petroleum-based plastics. J. Clean. Prod. 2016, 127, 84–95. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375. [Google Scholar] [CrossRef]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of vegetable oils: A review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Solomons, T.W.G.; Fryhle, C.B.; Snyder, S.A. Organic chemistry, 11th ed.; Wiley: Hoboken, NJ, USA, 2014; p. 1240. [Google Scholar]
- Canesin, E.A.; de Oliveira, C.C.; Matsushita, M.; Felicidade Dias, L.; Reghiany Pedrão, M.; de Souza, N.E. Characterization ofresidual oils for biodiesel production. Electron. J. Biotechnol. 2014, 17, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Brühl, L. Official Methods and Recommended Practices of the American Oil Chemist’s Society, Physical and Chemical Characteristics of Oils, Fats and Waxes, Section I. Ed. The AOCS Methods Editor and the AOCS Technical Department. 54 pages. AOCS Press, Champaign, 1996. Fett/Lipid 1997, 99, 197. [Google Scholar] [CrossRef]
- AOCS, American Oil Chemists Society. Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 2004. [Google Scholar]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Aquino, I.P.; Hernandez, R.P.B.; Chicoma, D.L.; Pinto, H.P.F.; Aoki, I.V. Influence of light, temperature and metallic ions on biodiesel degradation and corrosiveness to copper and brass. Fuel 2012, 102, 795–807. [Google Scholar] [CrossRef]
- Cursaru, D.L.; Nassreddine, S.; Riachi, B.; Neagu, M.; Mihai, S.; Matei, D.; Brănoiu, G. Impact of moisture on the corrosion behavior of copper and mild carbon steel in corn biodiesel. Corros. Rev. 2018, 36, 559–574. [Google Scholar] [CrossRef]
- ANP—National Agency for Petroleum, Natural Gas and Biofuels. ANP Resolution Number 07, of 03.19.2008 (Revoked by ANP Resolution Number 14, of 05/11/2012)—Official Gazette of the Union. Brasília, 2008. Available online: https://www.legisweb.com.br/legislacao/?id=109704 (accessed on 2 December 2021).
- Shehzad, A.; Ahmed, A.; Quazi, M.M.; Jamshaid, M.; Ashrafur Rahman, S.M.; Hassan, M.H.; Javed, H.M.A. Current research and development status of corrosion behavior of automotive materials in biofuels. Energies 2021, 14, 1440. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Brask, F.; Xua, X. Analysis of biodiesel conversion using thin layer chromatography and nonlinear calibration curves. J. Chromatogr. A 2011, 1218, 2785–2792. [Google Scholar] [CrossRef]
- Cavalcante, K.S.B.; Penha, M.N.C.; Mendonça, K.K.M.; Louzeiro, H.C.; Vasconcelos, A.C.S.; Maciel, A.P.; Souza, A.G.; Silva, F.C. Optimization of transesterification of castor oil with ethanol using a central composite rotatable design (CCRD). Fuel 2010, 89, 1172–1176. [Google Scholar] [CrossRef]
- Lima, A.P.; Lima, A.L.; Santos, D.Q.; Neto, W.B. Application of Factorial Design and Response Surface Methods to Optimize Ethyl Biodiesel Production from Corn Oil. Rev. Virtual Quim. 2013, 5, 817–827. [Google Scholar]
- Feddern, V.; Cunha, A., Jr.; Pra, M.C.; de Abreu, P.G.; dos Santos Filho, J.I.; Higarashi, M.M.; Sulenta, M.; Coldebella, A. Animal Fat Wastes for Biodiesel Production. Embrapa Suínos e Aves 2011. Available online: https://www.alice.cnptia.embrapa.br/bitstream/doc/908324/1/InTechAnimalfatVIVIANFEDERNwastesforbiodieselproduction.pdf (accessed on 2 December 2021).
- ANP—National Agency for Petroleum, Natural Gas and Biofuels. ANP Resolution number 45, Dated 8.25.2014—Official Gazette of the Union. Brasília, 2014. Available online: https://www.legisweb.com.br/legislacao/?id=274064 (accessed on 2 December 2021).
- Wu, G.; Ge, J.C.; Choi, N.J. A comprehensive review of the application characteristics of biodiesel blends in diesel engines. Appl. Sci. 2020, 10, 8015. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumaria, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef] [Green Version]
- Mahamuni, N.N.; Adewuyi, Y.G. Fourier transform infrared spectroscopy (FTIR) method to monitor soy biodiesel and soybean oil in transesterification reactions, petrodiesel-biodiesel blends, and blend adulteration with soy oil. Energy Fuels 2009, 23, 3773–3782. [Google Scholar] [CrossRef]
- Gunter, G.C.; Craciun, R.; Tam, M.S.; Jackson, J.E.; Miller, D.J. FTIR And 31P-NMR spectroscopic analyses of surface species in phosphate-catalyzed lactic acid conversion. J. Catal. 1996, 164, 207–219. [Google Scholar] [CrossRef]
- Pitt, F.D.; Domingos, A.M.; Barros, A.C. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Muller, C.M.O.; Yamashita, F.; Laurindo, J.B. Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydr. Polym. 2008, 72, 82–87. [Google Scholar] [CrossRef]
- Kumar, A.A.; Karthick, K.; Arumugam, K.P. Properties of biodegradable polymers and degradation for sustainable development. Int. J. Chem. Eng. Appl. 2011, 2, 164–167. [Google Scholar] [CrossRef]
- United Nations. Department of Economic and Social Affairs. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2017.html (accessed on 5 December 2021).
- Sharma, P.; Usman, M.; Salama, E.S.; Redina, M.; Thakur, N.; Li, X. Evaluation of various waste cooking oils for biodiesel production: A comprehensive analysis of feedstock. Waste Manag. 2021, 136, 219–229. [Google Scholar] [CrossRef]
- Shaghaghi, S.; Ghahderijani, M.; Dehrouyeh, M.H. Optimization of indicators pollutant emission following blending diesel fuel with waste oil-derived biodiesel. J. Oleo Sci. 2020, 69, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaharin, M.S.M.; Abdullah, N.R.; Najafi, G.; Sharudina, H.; Yusafc, T. Effects of physicochemical properties of biodiesel fuel blends with alcohol on diesel engine performance and exhaust emissions: A review. Renew. Sust. Energy Rev. 2017, 79, 475–493. [Google Scholar] [CrossRef]
- Hartini, S.; Puspitasari, D.; Aisy, R.; Widharto, Y. Eco-efficiency Level of Production Process of Waste Cooking Oil to be Biodiesel with Life Cycle. E3S Web Conf. 2020, 202, 10004. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Keskin, A.; Gürü, M. The optimization of the esterification reaction in biodiesel production from trap grease. Energ. Sources Part A 2012, 34, 1238–1248. [Google Scholar] [CrossRef]
- Jalkh, R.; El-Rassy, H.; Chehab, G.; Abiad, M. Assessment of the physico-chemical properties of waste cooking oil and spent coffee grounds oil for potential use as asphalt binder rejuvenators. Waste Biomass Valorization 2018, 9, 2125–2132. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilization of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, Falk. New chemical products on the basis of glycerol. Chimica Oggi 2008, 26, 32–36. [Google Scholar]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Lackner, M. Bioplastics-biobased plastics as renewable and/or biodegradable alternatives to petroplastics. In Kirk-Othmer Encyclopedia of Chemical Technology, 6th ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–41. [Google Scholar]
- Ibrahim, N.I.; Shahar, F.S.; Sultan, M.T.H.; Shah, A.U.M.; Safri, S.N.A.; Mat Yazik, M.H. Overview of bioplastic introduction and its applications in product packaging. Coatings 2021, 11, 1423. [Google Scholar] [CrossRef]
- Muhammad, A.; Rashidi, A.R.; Roslan, A.; Idris, S.A. Development of bio based plastic materials for packaging from soybeans waste. AIP Conf. Proc. 2017, 1885, 020230. [Google Scholar]
- European Bioplastics. Bioplastics: Facts and Figures. 2017. Available online: http://www.european-bioplastics.org/news/publications/ (accessed on 5 December 2021).
- Lee, C.L.Y.; Yeo, W.S. A basic characterisation study of bioplastics via gelatinization of corn starch. J. Appl. Sci. Process Eng. 2021, 8, 820–833. [Google Scholar] [CrossRef]




| Independent Variables | Inferior Limit | Central Limit | Upper Limit |
|---|---|---|---|
| Sodium hydroxide (g) | 0.10 | 0.15 | 0.20 |
| Methanol (ml) | 3.00 | 6.00 | 9.00 |
| Assay | Volume of Methanol (mL) | Mass of Sodium Hydroxide (g) |
|---|---|---|
| 1st | 3.00 | 0.10 |
| 2nd | 3.00 | 0.20 |
| 3rd | 9.00 | 0.10 |
| 4th | 9.00 | 0.20 |
| 5th | 1.75 | 0.15 |
| 6th | 10.24 | 0.15 |
| 7th | 6.00 | 0.08 |
| 8th | 6.00 | 0.22 |
| 9th | 6.00 | 0.15 |
| 10th | 6.00 | 0.15 |
| Test | NaOH (g) | Methanol (mL) | Oil (mL) | Biodiesel (mL) | Biodiesel (%) |
|---|---|---|---|---|---|
| 1st | 0.10 | 3.00 | 30.00 | 17.00 | 56.60 |
| 2nd | 0.20 | 3.00 | 30.00 | 28.00 | 93.30 |
| 3rd | 0.10 | 9.00 | 30.00 | 14.00 | 46.60 |
| 4th | 0.20 | 9.00 | 30.00 | 26.00 | 86.60 |
| 5th | 0.15 | 1.76 | 30.00 | 21.00 | 70.00 |
| 6th | 0.15 | 10.24 | 30.00 | 18.00 | 60.00 |
| 7th | 0.08 | 6.00 | 30.00 | 10.00 | 33.30 |
| 8th | 0.22 | 6.00 | 30.00 | 28.00 | 93.30 |
| 9th | 0.15 | 6.00 | 30.00 | 18.00 | 60.00 |
| 10th | 0.15 | 6.00 | 30.00 | 19.00 | 63.30 |
| Essay | Units | ANP 2014 | WCO Biodiesel |
|---|---|---|---|
| Density at 25 °C | g/cm3 | 0.85 to 0.90 | 0.88 |
| Acidity level | mg KOH/g | 0.50 | 0.51 |
| Saponification index | mg KOH/g | _ | 105.00 |
| Viscosity at 40 °C | cST | 3.00 to 6.00 | 3.50 |
| Corrosivity to copper | _ | 1.00 | 1.00 |
| Fog point | °C | to 10.00 | 8.00 |
| Aspect | _ | _ | Clear |
| Biodiesel | WCO | |
|---|---|---|
| Rf | 0.63 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, C.A.; dos Santos, R.N.; Oliveira, G.G.; de Souza Ferreira, T.P.; de Souza, N.L.G.D.; Soares, A.S.; de Melo, J.F.; Colares, C.J.G.; de Souza, U.J.B.; de Araújo-Filho, R.N.; et al. Biodiesel and Bioplastic Production from Waste-Cooking-Oil Transesterification: An Environmentally Friendly Approach. Energies 2022, 15, 1073. https://doi.org/10.3390/en15031073
da Silva CA, dos Santos RN, Oliveira GG, de Souza Ferreira TP, de Souza NLGD, Soares AS, de Melo JF, Colares CJG, de Souza UJB, de Araújo-Filho RN, et al. Biodiesel and Bioplastic Production from Waste-Cooking-Oil Transesterification: An Environmentally Friendly Approach. Energies. 2022; 15(3):1073. https://doi.org/10.3390/en15031073
Chicago/Turabian Styleda Silva, Cristina Almeida, Raíssa Nunes dos Santos, Geiser Gabriel Oliveira, Talita Pereira de Souza Ferreira, Nelson Luis Gonçalves Dias de Souza, Aline Souza Soares, Joece Ferreira de Melo, Carla Jovania Gomes Colares, Ueric José Borges de Souza, Renisson Neponuceno de Araújo-Filho, and et al. 2022. "Biodiesel and Bioplastic Production from Waste-Cooking-Oil Transesterification: An Environmentally Friendly Approach" Energies 15, no. 3: 1073. https://doi.org/10.3390/en15031073










