A Review on Recent Progress in the Integrated Green Hydrogen Production Processes
Abstract
:1. Introduction
2. Fossil Fuel-Based Technologies
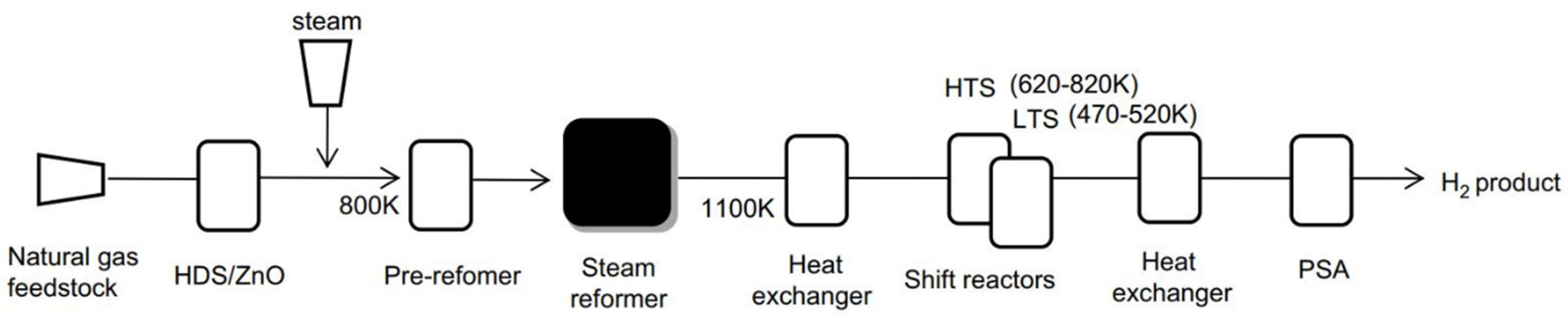
2.1. Steam Methane Reforming (SMR)
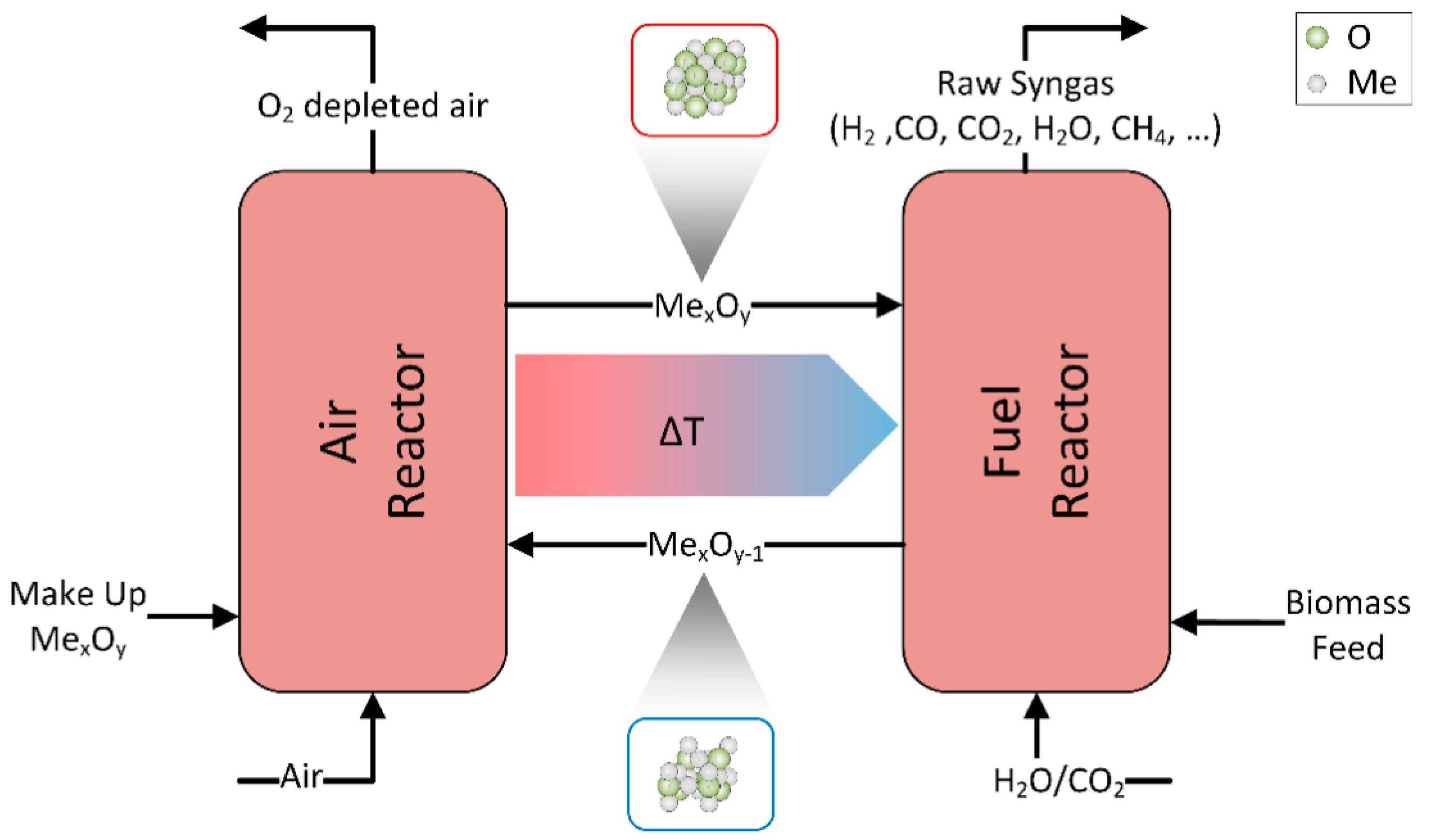
2.2. Chemical Looping
2.3. Integrated Gasification Combined Cycles (IGCC)
2.4. Biomass Gasification
2.5. Other Fossil Fuel-Based Technologies
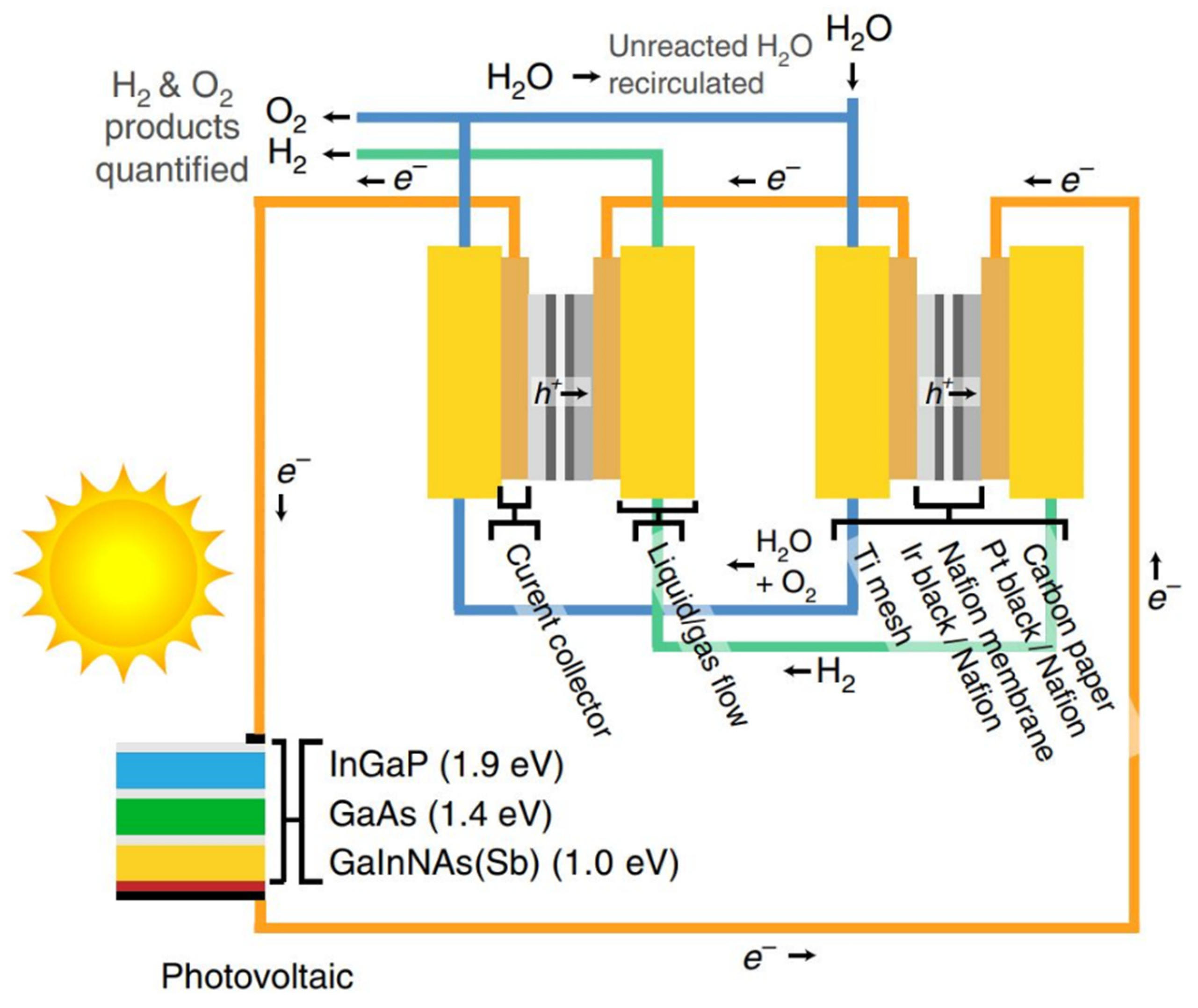
3. Green Hydrogen Technologies
- -
- Thermochemical routes such as single- and multi-step water splitting cycles;
- -
- Photochemical routes such as photoelectrochemical, photocatalytic, photobiological, etc.;
- -
- -
- Low-temperature cycles with an operating temperature below 1100 °C, such as sulfur-iodine, hybrid sulfur, hybrid copper chloride, etc.
- -
- High-temperature cycles with an operating temperature above 1100 °C, such as Zn/ZnO, FeO/Fe3O4, manganese oxide-based, ferrite cycles, etc. [98]
3.1. Low-Temperature Cycle
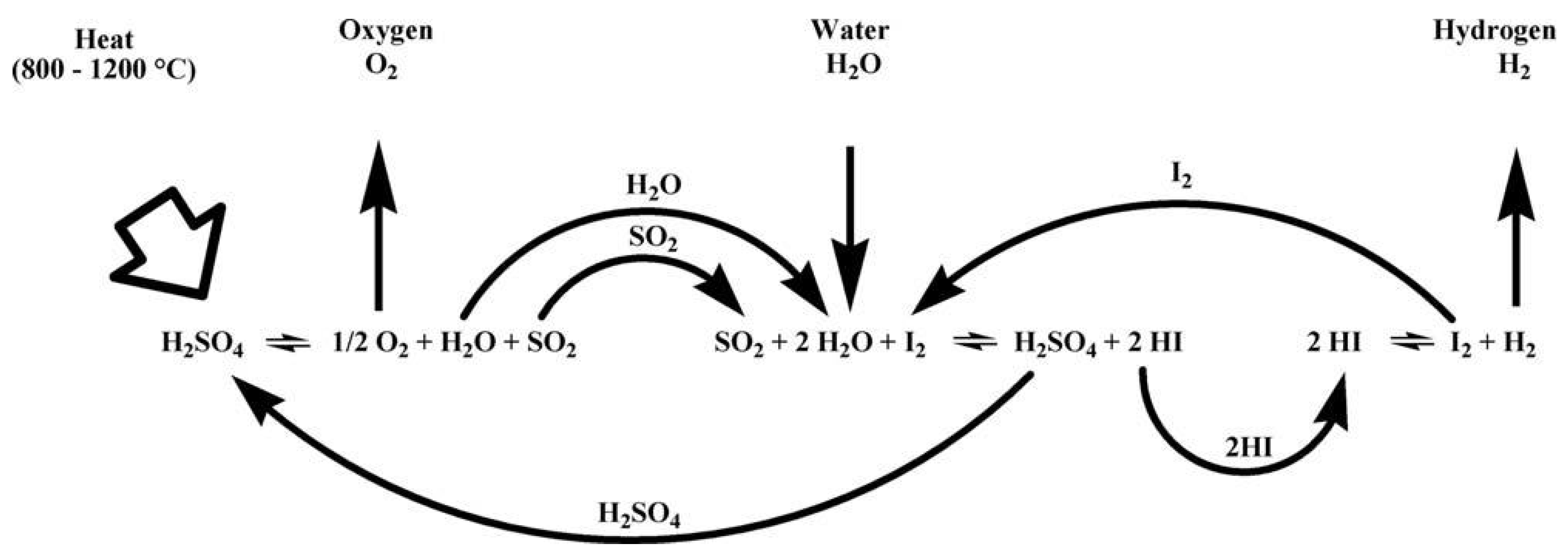
3.1.1. Sulfur-Iodine Cycle
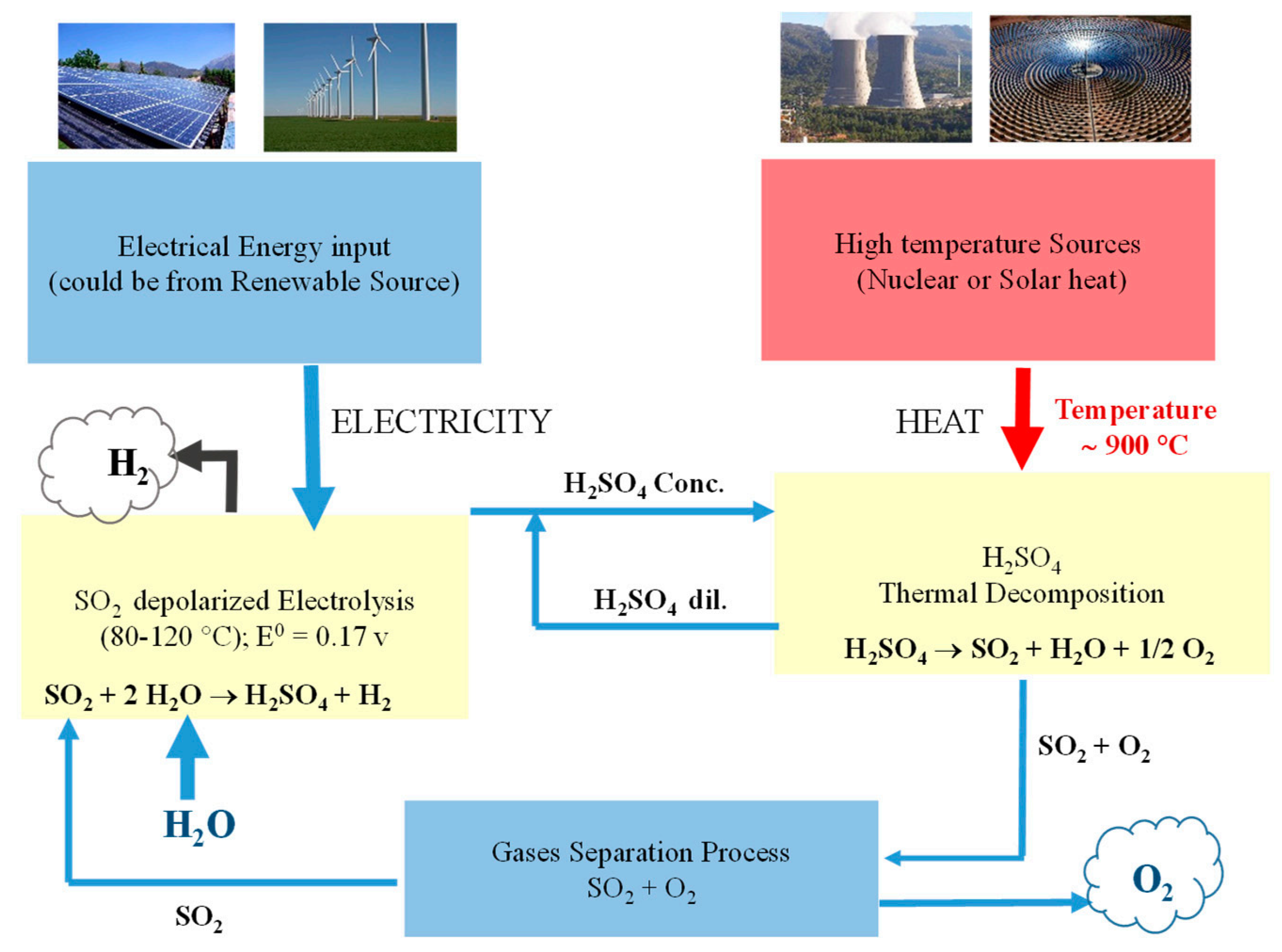
3.1.2. Hybrid Sulfur Cycle
- (a)
- The concentration of sulfuric acid by evaporating off the water;
- (b)
- Evaporating of concentrated and still liquid H2SO4;
- (c)
- Dissociation of gaseous acid into water vapour and SO3 at T = 350–400 °C;
- (d)
- Decomposition of SO3 to SO2 at T = 700–1000 °C.
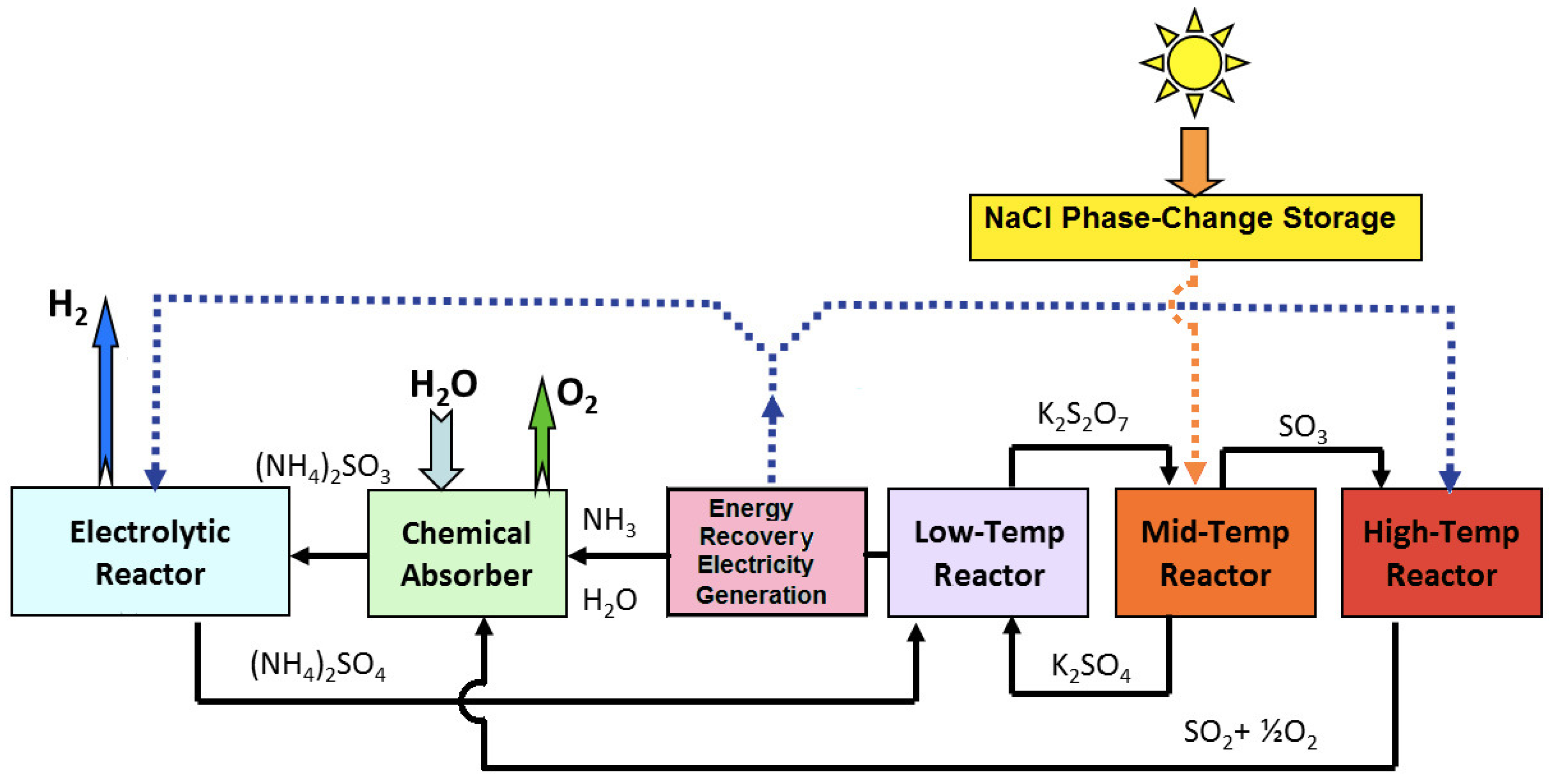
3.1.3. Sulfur Ammonia Cycle
- (a)
- Alkali metal sulphates such as sodium sulphate, potassium sulphate, caesium sulphates, etc.
- (b)
- Metal oxide such as ZnO, MnO, etc.
3.1.4. Cu-Cl Cycle
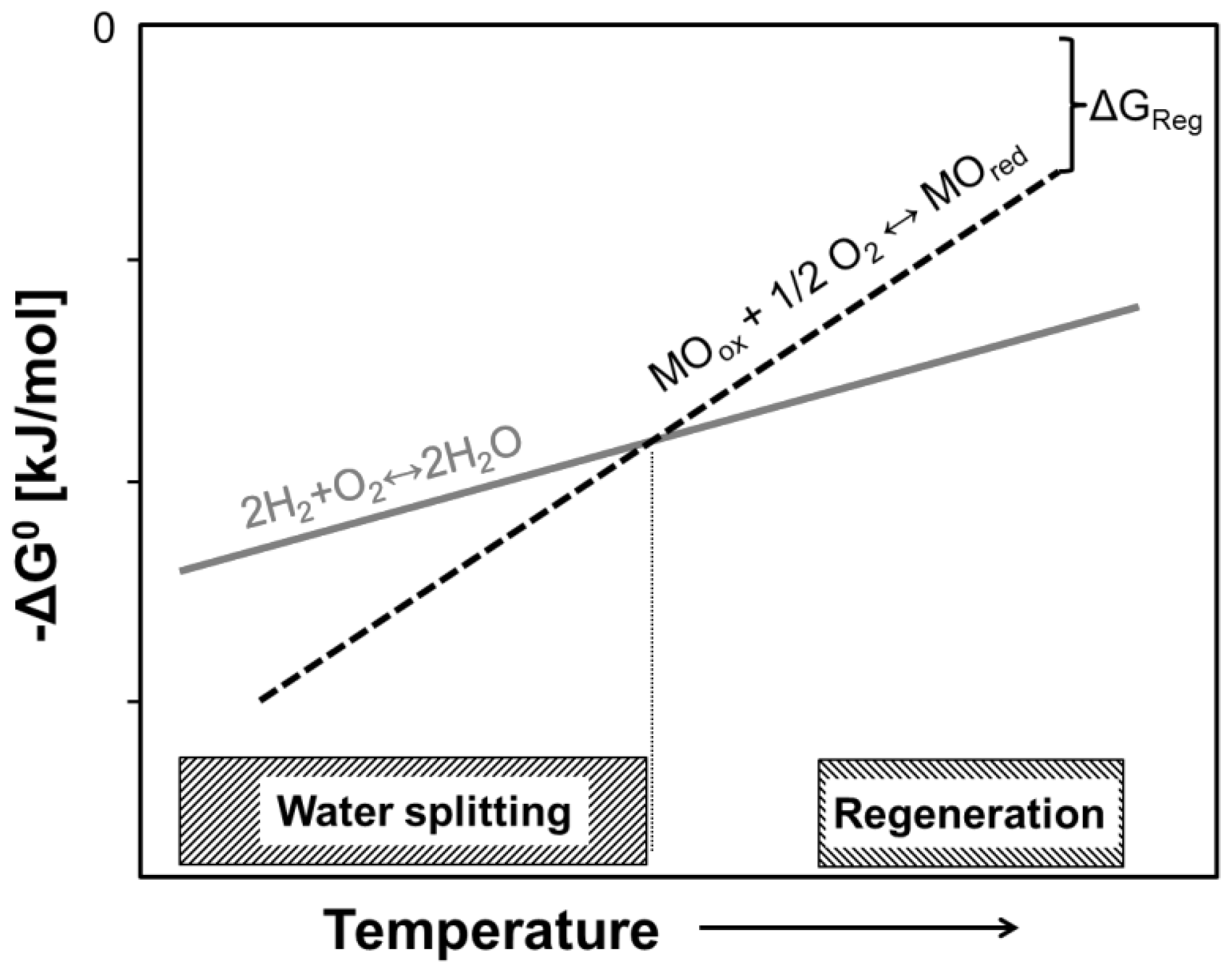
3.2. High-Temperature Cycles
3.2.1. ZnO Cycle
- (a)
- Limited reactor materials capable of enduring the reduction step high temperatures;
- (b)
- Limited efficiency of ZnO decomposition (theoretically about ~70% at 1750 °C);
- (c)
- Challenges with the hydrolysis step due to the formation of zinc oxide on zinc surface area which inhibits further oxidation of the underlying zinc;
- (d)
- Increased costs due to the utilization of a fluid wall reactor to counter particle deposition on reactor walls and loss of zinc metal to condensation;
- (e)
- Low visible-light absorption;
- (f)
3.2.2. Tin Oxide Cycle
3.2.3. Iron Oxide Cycle
3.2.4. Ferrite Cycle
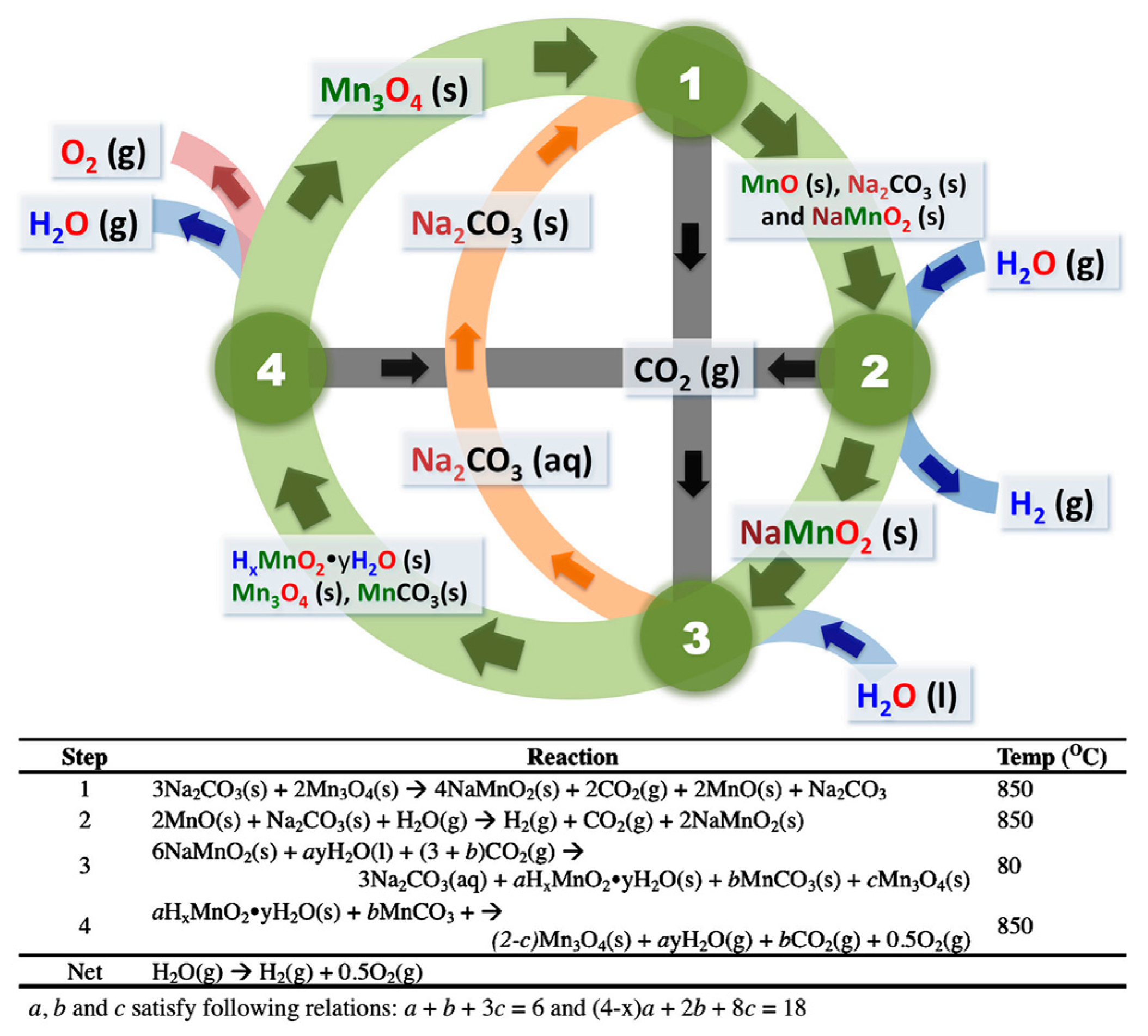
3.2.5. Manganese Oxide Cycles
3.2.6. Other Metal Oxide Cycles
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fallah Vostakola, M.; Mirkazemi, S.M.; Eftekhari Yekta, B. Structural, morphological, and optical properties of W-doped VO2 thin films prepared by sol-gel spin coating method. Int. J. Appl. Ceram. Technol. 2019, 16, 943–950. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Do, H.H.; Van Nguyen, T.; Singh, P.; Raizada, P.; Sharma, A.; Sana, S.S.; Grace, A.N.; Shokouhimehr, M.; Ahn, S.H.; et al. Perovskite oxide-based photocatalysts for solar-driven hydrogen production: Progress and perspectives. Sol. Energy 2020, 211, 584–599. [Google Scholar] [CrossRef]
- Fallah Vostakola, M.; Amini Horri, B. Progress in Material Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Potential options to greenize energy systems. Energy 2012, 46, 5–15. [Google Scholar] [CrossRef]
- Choolaei, M.; Bull, T.; Ramirez Reina, T.; Amini Horri, B. Synthesis and characterisation of nanocrystalline CuO–Fe2O3/GDC anode powders for solid oxide fuel cells. Ceram. Int. 2020, 46, 14776–14786. [Google Scholar] [CrossRef]
- Clemente, A.; Costa-Castelló, R. Redox flow batteries: A literature review oriented to automatic control. Energies 2020, 13, 4514. [Google Scholar] [CrossRef]
- Schoden, F.; Dotter, M.; Knefelkamp, D.; Blachowicz, T.; Hellkamp, E.S. Review of State of the Art Recycling Methods in the Context of Dye Sensitized Solar Cells. Energies 2021, 14, 3741. [Google Scholar] [CrossRef]
- Rajpar, A.H.; Ali, I.; Eladwi, A.E.; Bashir, M.B.A. Recent Development in the Design of Wind Deflectors for Vertical Axis Wind Turbine: A Review. Energies 2021, 14, 5140. [Google Scholar] [CrossRef]
- Moska, R.; Labus, K.; Kasza, P. Hydraulic Fracturing in Enhanced Geothermal Systems—Field, Tectonic and Rock Mechanics Conditions—A Review. Energies 2021, 14, 5725. [Google Scholar] [CrossRef]
- Le Saché, E.; Johnson, S.; Pastor-Pérez, L.; Horri, B.A.; Reina, T.R. Biogas upgrading via dry reforming over a Ni-Sn/CeO2-Al2O3 catalyst: Influence of the biogas source. Energies 2019, 12, 1007. [Google Scholar] [CrossRef] [Green Version]
- Price, C.A.H.; Arnold, W.; Pastor-Pérez, L.; Amini-Horri, B.; Reina, T.R. Catalytic Upgrading of a Biogas Model Mixture via Low Temperature DRM Using Multicomponent Catalysts. Top. Catal. 2020, 63, 281–293. [Google Scholar] [CrossRef]
- Rezaei, S.E.; Zebarjadi, M.; Esfarjani, K. Effect of exchange-correlation functional type and spin-orbit coupling on thermoelectric properties of ZrTe2. J. Solid State Chem. 2021, 302, 122414. [Google Scholar] [CrossRef]
- Amini Horri, B.; Choolaei, M.; Chaudhry, A.; Qaalib, H. A highly efficient hydrogen generation electrolysis system using alkaline zinc hydroxide solution. Int. J. Hydrogen Energy 2019, 44, 72–81. [Google Scholar] [CrossRef]
- Rafique, M.; Mubashar, R.; Irshad, M.; Gillani, S.S.A.; Tahir, M.B.; Khalid, N.R.; Yasmin, A.; Shehzad, M.A. A Comprehensive Study on Methods and Materials for Photocatalytic Water Splitting and Hydrogen Production as a Renewable Energy Resource. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3837–3861. [Google Scholar] [CrossRef]
- Hooshyari, K.; Amini Horri, B.; Abdoli, H.; Fallah Vostakola, M.; Kakavand, P.; Salarizadeh, P. A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells. Energies 2021, 14, 5440. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Brau, J.-F.; Morandin, M.; Berntsson, T. Hydrogen for oil refining via biomass indirect steam gasification: Energy and environmental targets. Clean Technol. Environ. Policy 2013, 15, 501–512. [Google Scholar] [CrossRef]
- Brau, J.F.; Morandin, M. Biomass-based hydrogen for oil refining: Integration and performances of two gasification concepts. Int. J. Hydrogen Energy 2014, 39, 2531–2542. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef] [Green Version]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef] [Green Version]
- Bellosta von Colbe, J.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C.; Fuiorescu, D. Hydrogen—An alternative fuel for automotive diesel engines used in transportation. Sustainability 2020, 12, 9321. [Google Scholar] [CrossRef]
- Renau, J.; García, V.; Domenech, L.; Verdejo, P.; Real, A.; Giménez, A.; Sánchez, F.; Lozano, A.; Barreras, F. Novel use of green hydrogen fuel cell-based combined heat and power systems to reduce primary energy intake and greenhouse emissions in the building sector. Sustainability 2021, 13, 1776. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. From hydrocarbon to hydrogen-carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Tang, J.; Liu, T.; Miao, S.; Cho, Y. Emerging energy harvesting technology for electro/photo-catalytic water splitting application. Catalysts 2021, 11, 142. [Google Scholar] [CrossRef]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. A review on solar-hydrogen/fuel cell hybrid energy systems for stationary applications. Prog. Energy Combust. Sci. 2009, 35, 231–244. [Google Scholar] [CrossRef]
- Quarton, C.J.; Tlili, O.; Welder, L.; Mansilla, C.; Blanco, H.; Heinrichs, H.; Leaver, J.; Samsatli, N.J.; Lucchese, P.; Robinius, M.; et al. The curious case of the conflicting roles of hydrogen in global energy scenarios. Sustain. Energy Fuels 2019, 4, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Minh, D.P.; Siang, T.J.; Vo, D.V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen production from biogas reforming: An overview of steam reforming, dry reforming, dual reforming, and tri-reforming of methane. In Hydrogen Supply Chain: Design, Deployment and Operation; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 111–166. ISBN 9780128111970. [Google Scholar]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Evers, A.A. A Proposal To Future Energy Supplies: Your Personal Power Provider (3P+) Virtual Power Plants with Direct Solar Hydrogen and Fuel Cells. I ECS Trans. 2009, 17, 691–696. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of hydrogen production using chemical-looping technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, W.; Zhang, G.; Dong, S. Exergy analysis of methane cracking thermally coupled with chemical looping combustion for hydrogen production. Appl. Energy 2016, 168, 1–12. [Google Scholar] [CrossRef]
- Yang, W.; Moon, J. Recent Advances in Earth-Abundant Photocathodes for Photoelectrochemical Water Splitting. ChemSusChem 2019, 12, 1889–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T. An overview of IGCC systems. In Integrated Gasification Combined Cycle (IGCC) Technologies; Wang, T., Stiegel, G., Eds.; Elsevier Ltd.: New Orleans, LA, USA, 2017; pp. 1–80. ISBN 9780081001851. [Google Scholar]
- Full, J.; Merseburg, S.; Miehe, R.; Sauer, A. A new perspective for climate change mitigation— introducing carbon-negative hydrogen production from biomass with carbon capture and storage (Hybeccs). Sustainability 2021, 13, 4026. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Korneev, D.V.; Bauman, Y.I.; Nalivaiko, A.Y.; Gromov, A.A. Selected Aspects of Hydrogen Production via Catalytic Decomposition of Hydrocarbons. Hydrogen 2021, 2, 122–133. [Google Scholar] [CrossRef]
- Speight, J.G. Synthesis gas and the Fischer–Tropsch process. In The Refinery of the Future; Speight, J.G., Ed.; Gulf Professional Publishing: Laramie, WY, USA, 2020; pp. 427–468. ISBN 9780128169940. [Google Scholar]
- Lamb, J.J.; Hillestad, M.; Rytter, E.; Bock, R.; Nordgård, A.S.R.; Lien, K.M.; Burheim, O.S.; Pollet, B.G. Traditional Routes for Hydrogen Production and Carbon Conversion. In Hydrogen, Biomass and Bioenergy; Lamb, J.J., Pollet, B.G., Eds.; Elsevier Ltd.: Trondheim, Norway, 2020; pp. 21–53. ISBN 9780081026298. [Google Scholar]
- Chen, G.; Tao, J.; Liu, C.; Yan, B.; Li, W.; Li, X. Hydrogen production via acetic acid steam reforming: A critical review on catalysts. Renew. Sustain. Energy Rev. 2017, 79, 1091–1098. [Google Scholar] [CrossRef]
- Go, K.S.; Son, S.R.; Kim, S.D.; Kang, K.S.; Park, C.S. Hydrogen production from two-step steam methane reforming in a fluidized bed reactor. Int. J. Hydrogen Energy 2009, 34, 1301–1309. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Sun, Z.; Zhang, Q.; Li, D.; Tang, D. Hydrogen production from chemical looping steam reforming of ethanol over perovskite-type oxygen carriers with bimetallic Co and Ni B-site substitution. Catalysts 2018, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Alobaid, F.; Ströhle, J. Special issue “thermochemical conversion processes for solid fuels and renewable energies”. Appl. Sci. 2021, 11, 1907. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production by sorption-enhanced chemical looping steam reforming of ethanol in an alternating fixed-bed reactor: Sorbent to catalyst ratio dependencies. Energy Convers. Manag. 2018, 155, 243–252. [Google Scholar] [CrossRef]
- Pujara, M.; Sheth, M.; Rachchh, N.; Bhoraniya, R.; Harichandan, A.B. Chemical Looping Reforming (CLR) System for H2 Production—A Review. In Renewable Energy and Climate Change-Proceedings of REC 2019; Deb, D., Dixit, A., Chandra, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 161, pp. 267–276. ISBN 9789813295773. [Google Scholar]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Dou, B.; Jiang, B.; Song, Y.; Zhang, C.; Zhang, Q.; Chen, H.; Xu, Y. Renewable hydrogen production from chemical looping steam reforming of ethanol using xCeNi/SBA-15 oxygen carriers in a fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12899–12909. [Google Scholar] [CrossRef]
- Muriungi, B.; Wang, L.; Shahbazi, A. Comparison of bimetallic Fe-Cu and Fe-Ca oxygen carriers for biomass gasification. Energies 2020, 13, 2019. [Google Scholar] [CrossRef] [Green Version]
- O’Malley, K.; Donat, F.; Whitty, K.J.; Sohn, H.Y. Scalable Preparation of Bimetallic Cu/Ni-Based Oxygen Carriers for Chemical Looping. Energy Fuels 2020, 34, 11227–11236. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Zhao, Z.; Huang, Z.; Zheng, A.; Zhao, K.; Li, H. Performance of Fe-Ni bimetallic oxygen carriers for chemical looping gasification of biomass in a 10 kWth interconnected circulating fluidized bed reactor. Int. J. Hydrogen Energy 2015, 40, 16021–16032. [Google Scholar] [CrossRef]
- Hossain, M.M.; Lasa, H.I. de Reactivity and Stability of Co-Ni/Al2O3 Oxygen Carrier in Multicycle CLC. React. Kinet. Catal. 2007, 53, 1817–1829. [Google Scholar] [CrossRef]
- Li, L.; Song, Y.; Jiang, B.; Wang, K.; Zhang, Q. A novel oxygen carrier for chemical looping reforming: LaNiO3 perovskite supported on montmorillonite. Energy 2017, 131, 58–66. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Li, Z.; Larring, Y.; Cai, N. Perovskite oxygen carrier with chemical memory under reversible chemical looping conditions with and without SO2 during reduction. Chem. Eng. J. 2021, 424, 130417. [Google Scholar] [CrossRef]
- Hamrang, F.; Seyed Mahmoudi, S.M.; Rosen, M.A. A novel electricity and freshwater production system: Performance analysis from reliability and exergoeconomic viewpoints with multi-objective optimization. Sustainability 2021, 13, 6448. [Google Scholar] [CrossRef]
- Miller, B.G. Clean Coal Technologies for Advanced Power Generation. In Clean Coal Engineering Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 261–308. ISBN 9780128113653. [Google Scholar]
- Huang, D.; Zhang, H.; Weng, S.; Su, M. Modeling and simulation of IGCC considering pressure and flow distribution of gasifier. Appl. Sci. 2016, 6, 292. [Google Scholar] [CrossRef] [Green Version]
- Cormos, C.C.; Cormos, A.M.; Serban Agachi, P. Techno-economical and environmental evaluations of IGCC power generation process with carbon capture and storage (CCS). In Computer Aided Chemical Engineering Computer; Pistikopoulos, E.N., Georgiadis, M.C., Kokossis, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 29, pp. 1678–1682. [Google Scholar]
- Hwang, B.; Kim, J.H.; Lee, D.; Nam, H.; Kim, H.N.; Baek, J.I.; Ryu, H.J. Investigation on the cause of the so2 generation during hot gas desulfurization (Hgd) process. Catalysts 2021, 11, 985. [Google Scholar] [CrossRef]
- Kaldis, S.P.; Pantoleontos, G.T.; Koutsonikolas, D.E. Membrane technology in IGCC processes for precombustion CO2 capture. In Current Trends and Future Developments on (Bio-) Membranes: Carbon Dioxide Separation/Capture by Using Membranes; Basile, A., Favvas, E.P., Eds.; Elsevier: Thessaloniki, Greece, 2018; pp. 329–357. ISBN 9780128136454. [Google Scholar]
- Tzimas, E.; Mercier, A.; Cormos, C.C.; Peteves, S.D. Trade-off in emissions of acid gas pollutants and of carbon dioxide in fossil fuel power plants with carbon capture. Energy Policy 2007, 35, 3991–3998. [Google Scholar] [CrossRef]
- Hossein Sahraei, M.; McCalden, D.; Hughes, R.; Ricardez-Sandoval, L.A. A survey on current advanced IGCC power plant technologies, sensors and control systems. Fuel 2014, 137, 245–259. [Google Scholar] [CrossRef]
- Gholamian, E.; Mahmoudi, S.M.S.; Zare, V. Proposal, exergy analysis and optimization of a new biomass-based cogeneration system. Appl. Therm. Eng. 2016, 93, 223–235. [Google Scholar] [CrossRef]
- Safarian, S.; Ebrahimi Saryazdi, S.M.; Unnthorsson, R.; Richter, C. Modeling of hydrogen production by applying biomass gasification: Artificial neural network modeling approach. Fermentation 2021, 7, 71. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Jia, X.; Feng, Y.; Dang, Q. Multi-Criteria Optimization of a Biomass-Based Hydrogen Production System Integrated With Organic Rankine Cycle. Front. Energy Res. 2020, 8, 584215. [Google Scholar] [CrossRef]
- Kaskun, S. An overview of hydrogen-rich gas production from biomass by using thermal technologies. IOP Conf. Ser. Earth Environ. Sci. 2020, 614, 012010. [Google Scholar] [CrossRef]
- Safarian, S.; Unnþórsson, R.; Richter, C. A review of biomass gasification modelling. Renew. Sustain. Energy Rev. 2019, 110, 378–391. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Puello, P.; Cabarcas, A. Process analysis of hydrogen production via biomass gasification under computer-aided safety and environmental assessments. ACS Omega 2020, 5, 19667–19681. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Performance analysis and environmental assessment of small-scale waste biomass gasification integrated CHP in Iceland. Energy 2020, 197, 117268. [Google Scholar] [CrossRef]
- Ji, P.; Feng, W.; Chen, B. Comprehensive simulation of an intensified process for H2 production from steam gasification of biomass. Ind. Eng. Chem. Res. 2009, 48, 3909–3920. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Bai, Z.; Xie, G.; Zheng, J.; Su, B. Thermodynamics analysis of a novel steam/air biomass gasification combined cooling, heating and power system with solar energy. Appl. Therm. Eng. 2020, 164, 114494. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, N.; Quan, C.; Wang, F.; Wu, C. Autothermal CaO looping biomass gasification to increase process energy efficiency and reduce ash sintering. Fuel 2020, 277, 118199. [Google Scholar] [CrossRef]
- Ishaq, H.; Islam, S.; Dincer, I.; Yilbas, B.S. Development and performance investigation of a biomass gasification based integrated system with thermoelectric generators. J. Clean. Prod. 2020, 256, 120625. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Ziabasharhagh, M. Energy and exergy analyses of a novel integrated process configuration for tri-generation heat, power and liquefied natural gas based on biomass gasification. Energy Convers. Manag. 2020, 209, 112624. [Google Scholar] [CrossRef]
- Megía, P.J.; Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Effect of the incorporation of reducibility promoters (Cu, Ce, Ag) in Co/CaSBA-15 catalysts for acetic acid steam reforming. Int. J. Energy Res. 2021, 45, 1685–1702. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Z.; Li, J.; Zhang, S.; Hu, S.; Xiang, J.; Wang, Y.; Liu, Q.; Hu, G.; Hu, X. Steam reforming of typical small organics derived from bio-oil: Correlation of their reaction behaviors with molecular structures. Fuel 2020, 259, 116214. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Cortese, M.; Martino, M. Bioalcohol reforming: An overview of the recent advances for the enhancement of catalyst stability. Catalysts 2020, 10, 665. [Google Scholar] [CrossRef]
- Hou, X.; Qing, S.; Liu, Y.; Li, L.; Gao, Z.; Qin, Y. Enhancing effect of MgO modification of Cu–Al spinel oxide catalyst for methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 477–489. [Google Scholar] [CrossRef]
- López-Martín, A.; Platero, F.; Caballero, A.; Colón, G. Thermo-Photocatalytic Methanol Reforming for Hydrogen Production over a CuPd−TiO2 Catalyst. ChemPhotoChem 2020, 4, 630–637. [Google Scholar] [CrossRef]
- Yu, C.L.; Sakthinathan, S.; Hwang, B.Y.; Lin, S.Y.; Chiu, T.W.; Yu, B.S.; Fan, Y.J.; Chuang, C. CuFeO2–CeO2 nanopowder catalyst prepared by self-combustion glycine nitrate process and applied for hydrogen production from methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 15752–15762. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Azenha, C.; Pacheco, P.T.; Sousa, J.M.; Mendes, A. The influence of the support composition on the physicochemical and catalytic properties of Cu catalysts supported on Zirconia-Alumina for methanol steam reforming. Appl. Catal. B Environ. 2020, 277, 119243. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A. Theoretical Prediction of the Efficiency of Hydrogen Production via Alkane Dehydrogenation in Catalytic Membrane Reactor. Hydrogen 2021, 2, 362–376. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A.; Mishakov, I.V.; Noskov, A.S. Mathematical modeling of the propane dehydrogenation process in the catalytic membrane reactor. Chem. Eng. J. 2011, 176–177, 151–157. [Google Scholar] [CrossRef]
- Shelepova, E.V.; Vedyagin, A.A.; Mishakov, I.V.; Noskov, A.S. Simulation of hydrogen and propylene coproduction in catalytic membrane reactor. Int. J. Hydrogen Energy 2015, 40, 3592–3598. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Jia, P.; Dong, D.; Wang, Y.; Hu, S.; Xiang, J.; Liu, Q.; Hu, X. Ethanol steam reforming over cobalt catalysts: Effect of a range of additives on the catalytic behaviors. J. Energy Inst. 2020, 93, 165–184. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Turczyniak-Surdacka, S. Enhanced catalytic performance of La2O3 promoted Co/CeO2 and Ni/CeO2 catalysts for effective hydrogen production by ethanol steam reforming: La2O3 promoted Co(Ni)/CeO2 catalysts in SRE. Renew. Energy 2020, 155, 378–395. [Google Scholar] [CrossRef]
- Martinelli, M.; Watson, C.D.; Jacobs, G. Sodium doping of Pt/m-ZrO2 promotes C–C scission and decarboxylation during ethanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 18490–18501. [Google Scholar] [CrossRef]
- Tyagi, D.; Shirsat, A.N.; Varma, S. Carbon derived from rice: Application as a support for platinum catalysts for hydrogen generation by HI decomposition. Bull. Mater. Sci. 2021, 44, 172. [Google Scholar] [CrossRef]
- Juárez-Martínez, L.C.; Espinosa-Paredes, G.; Vázquez-Rodríguez, A.; Romero-Paredes, H. Energy optimization of a Sulfur–Iodine thermochemical nuclear hydrogen production cycle. Nucl. Eng. Technol. 2021, 53, 2066–2073. [Google Scholar] [CrossRef]
- Lassouane, F.; Menia, S.; Khellaf, A. An overview of the hybrid sulfur cycle process for solar hydrogen production. In Proceedings of the 3rd International Symposium on Environment Friendly Energies and Applications, EFEA 2014; IEEE: Paris, France, 2014; pp. 1–6. [Google Scholar]
- Vagia, E.C.; Muradov, N.; Kalyva, A.; T-Raissi, A.; Qin, N.; Srinivasa, A.R.; Kakosimos, K.E. Solar hybrid photo-thermochemical sulfur-ammonia water-splitting cycle: Photocatalytic hydrogen production stage. Int. J. Hydrogen Energy 2017, 42, 20608–20624. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Other Hydrogen Production Methods. In Sustainable Hydrogen Production; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 411–439. ISBN 9780128015636. [Google Scholar]
- Acar, C.; Dincer, I. Hydrogen Production. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 1–40. ISBN 9780128095973. [Google Scholar]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Steinfeld, A. Solar thermochemical production of hydrogen℄A review. Sol. Energy 2005, 78, 603–615. [Google Scholar] [CrossRef]
- Perkins, C.; Weimer, A.W. Solar-Thermal Production of Renewable Hydrogen. AIChE J. 2009, 55, 286–293. [Google Scholar] [CrossRef]
- O’keefe, D.R.; Allen, C.L.; Besenbruch, G.; Brown, L.; Norman, J.H.; Sharp, R.; Mccorkle, K.H. Preliminary results from bench-scale testing of a sulfur-iodine thermochemical water-splitting cycle. Int. J. Hydrogen Energy 1982, 7, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, S.Y.; Lee, I.B. Study of alternative reactor-separator network in bunsen process of sulfur-iodine cycle for hydrogen production. J. Chem. Eng. Jpn. 2019, 52, 638–649. [Google Scholar] [CrossRef]
- Boretti, A.; Nayfeh, J.; Al-Maaitah, A. Hydrogen Production by Solar Thermochemical Water-Splitting Cycle via a Beam Down Concentrator. Front. Energy Res. 2021, 9, 666191. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Zhu, X.; Zhang, L. Effect of N-doping on the catalytic decomposition of hydrogen iodide over activated carbon: Experimental and DFT studies. Int. J. Hydrogen Energy 2020, 45, 4511–4520. [Google Scholar] [CrossRef]
- Grimes, C.A.; Varghese, O.; Ranjan, S. Hydrogen Generation by Water Splitting. In Light, Water, Hydrogen; Springer: New York, NY, USA, 2008; pp. 35–113. ISBN 9780126552812. [Google Scholar]
- Giaconia, A.; Caputo, G.; Ceroli, A.; Diamanti, M.; Barbarossa, V.; Tarquini, P.; Sau, S. Experimental study of two phase separation in the Bunsen section of the sulfur-iodine thermochemical cycle. Int. J. Hydrogen Energy 2007, 32, 531–536. [Google Scholar] [CrossRef]
- Dincer, I.; Bicer, Y. Solar Thermochemical Energy Conversion. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4b, pp. 895–946. ISBN 9780128095973. [Google Scholar]
- Perret, R. Solar Thermochemical Hydrogen Production Research (STCH) Thermochemical Cycle Selection and Investment Priority; Document number: SAND2011-3622; Sandia National Laboratories: Albuquerque, NM, USA, 2011; pp. 1–117.
- Zhu, Q.; Zhang, Y.; Zhou, C.; Wang, Z.; Zhou, J.; Cen, K. Optimization of liquid-liquid phase separation characteristics in the Bunsen section of the sulfur-iodine hydrogen production process. Int. J. Hydrogen Energy 2012, 37, 6407–6414. [Google Scholar] [CrossRef]
- Roeb, M.; Neises, M.; Monnerie, N.; Call, F.; Simon, H.; Sattler, C.; Schmücker, M.; Pitz-Paal, R. Materials-related aspects of thermochemical water and carbon dioxide splitting: A review. Materials 2012, 5, 2015–2054. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.; Kim, H.; Kim, Y.; Park, C.; Bae, K. The Role of Oxygen in Bunsen Reaction Section of Sulfur-Iodine Hydrogen Production Process. Trans. Korean Hydrog. New Energy Soc. 2010, 21, 278–285. [Google Scholar]
- Ying, Z.; Yang, J.; Zheng, X.; Wang, Y.; Dou, B. Energy and exergy analyses of a novel sulfur–iodine cycle assembled with HI–I2–H2O electrolysis for hydrogen production. Int. J. Hydrogen Energy 2021, 46, 23139–23148. [Google Scholar] [CrossRef]
- Singhania, A.; Bhaskarwar, A.N. Development of catalysts for hydrogen production from hydrogen iodide decomposition in thermo-chemical water-splitting sulfur-iodine cycle: A review. Catal. Rev.-Sci. Eng. 2017, 59, 446–489. [Google Scholar] [CrossRef]
- Ying, Z.; Zhang, Y.; Zheng, X.; Cui, G. Performance of electrochemical cell with various flow channels for Bunsen reaction in the sulfur–iodine hydrogen production process. Energy Convers. Manag. 2017, 151, 514–523. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, S.; Chang, L.; Wang, J.; Bao, W.; Wang, H. Direct electrolysis of Bunsen reaction product HI/H2SO4/H2O/toluene mixture for hydrogen production: Pt electrode characterization. Int. J. Hydrogen Energy 2018, 43, 13702–13710. [Google Scholar] [CrossRef]
- Zhang, K.; Bao, W.; Chang, L.; Wang, H. A review of recent researches on Bunsen reaction for hydrogen production via S–I water and H2S splitting cycles. J. Energy Chem. 2019, 33, 46–58. [Google Scholar] [CrossRef]
- Yu, X.; Meng, L.; Nagasawa, H.; Kanezashi, M.; Machida, M.; Tsuru, T. Evaluating the chemical stability of metal oxides in SO3 and applications of SiO2-based membranes to O2/SO3 separation. J. Am. Ceram. Soc. 2019, 102, 6946–6956. [Google Scholar] [CrossRef]
- Park, J.K.; Ifaei, P.; Ba-Alawi, A.H.; Safder, U.; Yoo, C.K. Hydrogen production through the sulfur–iodine cycle using a steam boiler heat source for risk and techno-socio-economic cost (RSTEC) reduction. Int. J. Hydrogen Energy 2020, 45, 14578–14593. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, R.; Zhu, X.; Zhang, M.; Li, J.; Zhang, L. Exploring the relationship between the physical properties of activated carbon catalysts and their efficiency in catalyzing hydrogen iodide decomposition to produce hydrogen. Int. J. Hydrogen Energy 2021, 46, 18207–18223. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, J.E.; Kim, Y.H.; Kang, K.S.; Kim, C.H.; Park, C.S.; Bae, K.K. Decomposition of hydrogen iodide on Pt/C-based catalysts for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4974–4980. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wang, L.J.; Zhang, P.; Chen, S.Z.; Xu, J.M.; Chen, J. Effect of preparation methods on Pt/alumina catalysts for the hydrogen iodide catalytic decomposition. Chinese Chem. Lett. 2009, 20, 102–105. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wang, Z.; Zhou, J.; Liu, J.; Cen, K. Effect of preparation method on platinum-ceria catalysts for hydrogen iodide decomposition in sulfur-iodine cycle. Int. J. Hydrogen Energy 2008, 33, 602–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhou, J.; Liu, J.; Cen, K. Experimental study of Ni/CeO2 catalytic properties and performance for hydrogen production in sulfur-iodine cycle. Int. J. Hydrogen Energy 2009, 34, 5637–5644. [Google Scholar] [CrossRef]
- Singhania, A.; Bhaskarwar, A.N. Catalytic performance of carbon nanotubes supported palladium catalyst for hydrogen production from hydrogen iodide decomposition in thermochemical sulfur iodine cycle. Renew. Energy 2018, 127, 509–513. [Google Scholar] [CrossRef]
- Tyagi, D.; Varma, S.; Bharadwaj, S.R. Pt/zirconia catalyst for hydrogen generation from HI decomposition reaction of S–I cycle. Int. J. Energy Res. 2015, 39, 484–493. [Google Scholar] [CrossRef]
- Tyagi, D.; Varma, S.; Bharadwaj, S.R. Pt/graphite catalyst for hydrogen generation by HI decomposition reaction in S–I thermochemical cycle. Int. J. Energy Res. 2015, 39, 2008–2018. [Google Scholar] [CrossRef]
- Petkovic, L.M.; Ginosar, D.M.; Rollins, H.W.; Burch, K.C.; Deiana, C.; Silva, H.S.; Sardella, M.F.; Granados, D. Activated carbon catalysts for the production of hydrogen via the sulfur-iodine thermochemical water splitting cycle. Int. J. Hydrogen Energy 2009, 34, 4057–4064. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Zhou, C.; Whiddon, R.; Zhang, Y.; Zhou, J.; Cen, K. Decomposition of hydrogen iodide via wood-based activated carbon catalysts for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 216–223. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Wang, R.; Wang, Z.; Zhou, J.; Cen, K. Influence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen iodide in the sulfur-iodine cycle for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 15003–15011. [Google Scholar] [CrossRef]
- Everson, R.C.; Stander, B.F.; Neomagus, H.W.J.P.; Van Der Merwe, A.F.; Le Grange, L.; Tietz, M.R. Sulphur trioxide decomposition with supported platinum/palladium on rutile catalysts: 1. Reaction kinetics of catalyst pellets. Int. J. Hydrogen Energy 2015, 40, 85–94. [Google Scholar] [CrossRef]
- Banerjee, A.M.; Pai, M.R.; Tewari, R.; Raje, N.; Tripathi, A.K.; Bharadwaj, S.R.; Das, D. A comprehensive study on Pt/Al2O3 granular catalyst used for sulfuric acid decomposition step in sulfur-iodine thermochemical cycle: Changes in catalyst structure, morphology and metal-support interaction. Appl. Catal. B Environ. 2015, 162, 327–337. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Matsukawa, T.; Hinokuma, S.; Machida, M. Catalytic SO3 decomposition activity and stability of Pt supported on anatase TiO2 for solar thermochemical water-splitting cycles. ACS Omega 2017, 2, 7057–7065. [Google Scholar] [CrossRef] [Green Version]
- Kawada, T.; Ikematsu, A.; Tajiri, T.; Takeshima, S.; Machida, M. Structure and SO3 decomposition activity of CeVO4/SiO2 catalysts for solar thermochemical water splitting cycles. Int. J. Hydrogen Energy 2015, 40, 10726–10733. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhu, Y.; Yang, H.; He, Y.; Xia, J.; Zhang, Y.; Wang, Z. SO3 decomposition over CuO–CeO2 based catalysts in the sulfur–iodine cycle for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 14876–14884. [Google Scholar] [CrossRef]
- Jianu, O.A.; Naterer, G.F.; Rosen, M.A. Hydrogen cogeneration with generation IV nuclear power plants. In Handbook of Generation IV Nuclear Reactors; Pioro, I.L., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 637–659. ISBN 9780081001622. [Google Scholar]
- Roeb, M.; Monnerie, N.; Houaijia, A.; Thomey, D.; Sattler, C. Solar Thermal Water Splitting. In Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Elsevier: Amsterdam, The Netherlands, 2013; pp. 63–86. ISBN 9780444563521. [Google Scholar]
- Corgnale, C.; Gorensek, M.B.; Summers, W.A. Review of sulfuric acid decomposition processes for sulfur-based thermochemical hydrogen production cycles. Processes 2020, 8, 1383. [Google Scholar] [CrossRef]
- Corgnale, C.; Summers, W.A. Solar hydrogen production by the Hybrid Sulfur process. Int. J. Hydrogen Energy 2011, 36, 11604–11619. [Google Scholar] [CrossRef]
- Staser, J.A.; Gorensek, M.B.; Weidner, J.W. Quantifying Individual Potential Contributions of the Hybrid Sulfur Electrolyzer. J. Electrochem. Soc. 2010, 157, B952. [Google Scholar] [CrossRef] [Green Version]
- Steimke, J.L.; Steeper, T.J.; Cólon-Mercado, H.R.; Gorensek, M.B. Development and testing of a PEM SO2-depolarized electrolyzer and an operating method that prevents sulfur accumulation. Int. J. Hydrogen Energy 2015, 40, 13281–13294. [Google Scholar] [CrossRef] [Green Version]
- Garrick, T.R.; Wilkins, C.H.; Pingitore, A.T.; Mehlhoff, J.; Gulledge, A.; Benicewicz, B.C.; Weidner, J.W. Characterizing Voltage Losses in an SO2 Depolarized Electrolyzer Using Sulfonated Polybenzimidazole Membranes. J. Electrochem. Soc. 2017, 164, F1591–F1595. [Google Scholar] [CrossRef] [Green Version]
- Meekins, B.H.; Thompson, A.B.; Gopal, V.; Mehrabadi, B.A.T.; Elvington, M.C.; Ganesan, P.; Newhouse-Illige, T.A.; Shepard, A.W.; Scipioni, L.E.; Greer, J.A.; et al. In-situ and ex-situ comparison of the electrochemical oxidation of SO2 on carbon supported Pt and Au catalysts. Int. J. Hydrogen Energy 2020, 45, 1940–1947. [Google Scholar] [CrossRef]
- Brown, N.R.; Revankar, S.T. A review of catalytic sulfur (VI) oxide decomposition experiments. Int. J. Hydrogen Energy 2012, 37, 2685–2698. [Google Scholar] [CrossRef]
- Colón-Mercado, H.R.; Hobbs, D.T. Catalyst evaluation for a sulfur dioxide-depolarized electrolyzer. Electrochem. commun. 2007, 9, 2649–2653. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Gong, G.T.; Lee, B.G.; Lee, K.Y.; Jeon, H.Y.; Shin, C.H.; Kim, H.; Jung, K.D. Catalytic decomposition of sulfur trioxide on the binary metal oxide catalysts of Fe/Al and Fe/Ti. Appl. Catal. A Gen. 2006, 305, 39–45. [Google Scholar] [CrossRef]
- Díaz-Abad, S.; Millán, M.; Rodrigo, M.A.; Lobato, J. Review of anodic catalysts for SO2 depolarized electrolysis for “green hydrogen” production. Catalysts 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.T.; Sawyer, D.T. Electrochemical oxidation of dissolved sulphur dioxide at platinum and gold electrodes. Electrochim. Acta 1965, 10, 239–252. [Google Scholar] [CrossRef]
- Falch, A.; Lates, V.; Kriek, R.J. Combinatorial Plasma Sputtering of PtxPdy Thin Film Electrocatalysts for Aqueous SO2 Electro-oxidation. Electrocatalysis 2015, 6, 322–330. [Google Scholar] [CrossRef]
- Falch, A.; Lates, V.A.; Kotzé, H.S.; Kriek, R.J. The Effect of Rapid Thermal Annealing on Sputtered Pt and Pt3Pd2 Thin Film Electrocatalysts for Aqueous SO2 Electro-Oxidation. Electrocatalysis 2016, 7, 33–41. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, B.; He, Y.; Zhu, Y.; Zhang, Y.; Wang, Z. Demetallized PtxNiy/C catalyst for SO2 electrochemical oxidation in the SI/HyS hydrogen production cycles. Int. J. Hydrogen Energy 2021, 46, 10161–10171. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, P.; Chen, S.; Wang, L. Pt-based bimetallic catalysts for SO2-depolarized electrolysis reaction in the hybrid sulfur process. Int. J. Hydrogen Energy 2014, 39, 14196–14203. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, K.; Yu, Y.; Mu, S. One-pot synthesis of Pt/CeO2/C catalyst for enhancing the SO2 electrooxidation. Electrochim. Acta 2017, 229, 253–260. [Google Scholar] [CrossRef]
- Jayakumar, J.V.; Gulledge, A.; Staser, J.A.; Kim, C.H.; Benicewicz, B.C.; Weidner, J.W. Polybenzimidazole membranes for hydrogen and sulfuric acid production in the hybrid sulfur electrolyzer. ECS Electrochem. Lett. 2012, 1, 44–48. [Google Scholar] [CrossRef]
- Taylor, R.; Davenport, R.; Talbot, J.; Herz, R.; Luc, W.; Genders, D.; Symons, P.; Brown, L. Status of the solar sulfur ammonia thermochemical hydrogen production system for splitting water. Energy Procedia 2014, 49, 2047–2058. [Google Scholar] [CrossRef] [Green Version]
- Shazed, A.R.; Kalyva, A.E.; Vagia, E.C.; Srinivasa, A.R.; Traissi, A.; Muradov, N.; Kakosimos, K.E. Chemical plant analysis of hydrogen production based on the hybrid sulfur-ammonia water splitting cycle. Chem. Eng. Trans. 2017, 61, 433–438. [Google Scholar] [CrossRef]
- Littlefield, J.; Wang, M.; Brown, L.C.; Herz, R.K.; Talbot, J.B. Process modeling and thermochemical experimental analysis of a solar sulfur ammonia hydrogen production cycle. Energy Procedia 2012, 29, 616–623. [Google Scholar] [CrossRef] [Green Version]
- T-Raissi, A.; Muradov, N.; Huang, C.; Adebiyi, O. Hydrogen from solar via light-assisted high-temperature water splitting cycles. J. Sol. Energy Eng. Trans. ASME 2007, 129, 184–189. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Corgnale, C.; Summers, W.A. Development of the hybrid sulfur cycle for use with concentrated solar heat. I. Conceptual design. Int. J. Hydrogen Energy 2017, 42, 20939–20954. [Google Scholar] [CrossRef]
- Niehoff, A.G.; Botero, N.B.; Acharya, A.; Thomey, D.; Roeb, M.; Sattler, C.; Pitz-Paal, R. Process modelling and heat management of the solar hybrid sulfur cycle. Int. J. Hydrogen Energy 2015, 40, 4461–4473. [Google Scholar] [CrossRef]
- Bilgen, E. Solar hydrogen production by hybrid thermochemical processes. Sol. Energy 1988, 41, 199–206. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Sutar, P.; Kumar, A.; Almomani, F.; Ali, M.H.; Ghosh, U.; Almuhtaseb, S.; Khraisheh, M. Solar hydrogen production via erbium oxide based thermochemical water splitting cycle. J. Renew. Sustain. Energy 2016, 8, 034702. [Google Scholar] [CrossRef]
- Yao, W.; Song, X.; Huang, C.; Xu, Q.; Wu, Q. Enhancing solar hydrogen production via modified photochemical treatment of Pt/CdS photocatalyst. Catal. Today 2013, 199, 42–47. [Google Scholar] [CrossRef]
- Yao, W.; Huang, C.; Muradov, N.; T-Raissi, A. A novel Pd-Cr2O3/CdS photocatalyst for solar hydrogen production using a regenerable sacrificial donor. Int. J. Hydrogen Energy 2011, 36, 4710–4715. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, D.; Cai, B.; Wang, S.; Niu, F.; Qin, L.; Huang, Y. Facile synthesis of CdS-ZnWO4 composite photocatalysts for efficient visible light driven hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 1962–1969. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nano particles with ZnS and CdS. Int. J. Hydrogen Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Chen, R.; Li, K.; Zhu, X.S.; Xie, S.L.; Dong, L.Z.; Li, S.L.; Lan, Y.Q. In situ synthesis of porous ZnO-embedded Zn1-xCdxS/CdS heterostructures for enhanced photocatalytic activity. CrystEngComm 2016, 18, 1446–1452. [Google Scholar] [CrossRef]
- Naterer, G.F.; Dincer, I.; Zamfirescu, C. Hybrid Copper–Chlorine Cycle. In Hydrogen Production from Nuclear Energy; Springer: London, UK, 2013; pp. 273–438. ISBN 9781447149385. [Google Scholar]
- Wu, W.; Hsu, F.T.; Chen, H.Y. Design and energy evaluation of a stand-alone copper-chlorine (Cu-Cl) thermochemical cycle system for trigeneration of electricity, hydrogen, and oxygen. Int. J. Energy Res. 2018, 42, 830–842. [Google Scholar] [CrossRef]
- Izanloo, M.; Mehrpooya, M. Investigation of a hybrid thermochemical Cu–Cl cycle, carbon capturing, and ammonia production process. J. Therm. Anal. Calorim. 2021, 144, 1907–1923. [Google Scholar] [CrossRef]
- Osuolale, F.; Ogunleye, O.; Fakunle, M.; Busari, A.; Abolanle, Y. Comparative studies of Cu-Cl thermochemical water decomposition cyles for hydrogen production. E3S Web Conf. 2018, 61, 9. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I.; Gabriel, K. Process Improvement and Analysis of an Integrated Four-Step Copper-Chlorine Cycle Modified with a Flash Vaporization Process for Hydrogen Production. Energy and Fuels 2021, 35, 9038–9046. [Google Scholar] [CrossRef]
- Yilmaz, F.; Selbaş, R. Thermodynamic performance assessment of solar based Sulfur-Iodine thermochemical cycle for hydrogen generation. Energy 2017, 140, 520–529. [Google Scholar] [CrossRef]
- Farsi, A.; Dincer, I.; Naterer, G.F. Review and evaluation of clean hydrogen production by the copper–chlorine thermochemical cycle. J. Clean. Prod. 2020, 276, 123833. [Google Scholar] [CrossRef]
- Farsi, A.; Dincer, I.; Naterer, G.F. Exergo-economic assessment by a specific exergy costing method for an experimental thermochemical hydrogen production system. Int. J. Energy Res. 2021, 45, 17358–17377. [Google Scholar] [CrossRef]
- Fan, G.; Ahmadi, A.; Ehyaei, M.A.; Das, B. Energy, exergy, economic and exergoenvironmental analyses of polygeneration system integrated gas cycle, absorption chiller, and Copper-Chlorine thermochemical cycle to produce power, cooling, and hydrogen. Energy 2021, 222, 120008. [Google Scholar] [CrossRef]
- Naterer, G.F.; Suppiah, S.; Rosen, M.A.; Gabriel, K.; Dincer, I.; Jianu, O.A.; Wang, Z.; Easton, E.B.; Ikeda, B.M.; Rizvi, G.; et al. Advances in unit operations and materials for the Cu–Cl cycle of hydrogen production. Int. J. Hydrogen Energy 2017, 42, 15708–15723. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Diver, R.B.; Miller, J.E.; Allendorf, M.D.; Siegel, N.P.; Hogan, R.E. Solar thermochemical water-splitting ferrite-cycle heat engines. J. Sol. Energy Eng. Trans. ASME 2008, 130, 0410011–0410018. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, R.; Kumar, A.; AlMomani, F.; Ghosh, U.; Anis, M.S.; Kakosimos, K.; Shende, R.; Rosen, M.A. Solar hydrogen production via a samarium oxide-based thermochemical water splitting cycle. Energies 2016, 9, 316. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int. J. Hydrogen Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. Tio2 as a photocatalyst for water splitting—an experimental and theoretical review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Xu, C.; Zhou, K.; Wang, Z.; Zhou, J.; Cen, K. A novel photo-thermochemical cycle of water-splitting for hydrogen production based on TiO2-x/TiO2. Int. J. Hydrogen Energy 2016, 41, 2215–2221. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.; Su, W.; Zhou, L. Experimental studies on the FeO/Fe3O4 cycle complemented with carbon gasification for producing hydrogen. Energy and Fuels 2013, 27, 4071–4076. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; Van Den Broeke, L.J.P.; Gharbia, S.; Dardor, D.; Jilani, M.; Folady, J.; Al-Fakih, M.S.; Tarsad, M.A. Solar hydrogen production via thermochemical iron oxide-iron sulfate water splitting cycle. Int. J. Hydrogen Energy 2015, 40, 1639–1650. [Google Scholar] [CrossRef]
- Bhosale, R.; Kumar, A.; Almomani, F. Solar Thermochemical Hydrogen Production via Terbium Oxide Based Redox Reactions. Int. J. Photoenergy 2016, 2016, 9727895. [Google Scholar] [CrossRef]
- Marugán, J.; Botas, J.A.; Martín, M.; Molina, R.; Herradón, C. Study of the first step of the Mn2O3/MnO thermochemical cycle for solar hydrogen production. Int. J. Hydrogen Energy 2012, 37, 7017–7025. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.; Ghosh, U.; Khraisheh, M. A comparative thermodynamic analysis of samarium and erbium oxide based solar thermochemical water splitting cycles. Int. J. Hydrogen Energy 2017, 42, 23416–23426. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Flamant, G.; Lemort, F. Two-step water splitting thermochemical cycle based on iron oxide redox pair for solar hydrogen production. Energy 2007, 32, 1124–1133. [Google Scholar] [CrossRef]
- D’Souza, L. Thermochemical hydrogen production from water using reducible oxide materials: A critical review. Mater. Renew. Sustain. Energy 2013, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Mehrpooya, M.; Raeesi, M.; Pourfayaz, F.; Delpisheh, M. Investigation of a hybrid solar thermochemical water-splitting hydrogen production cycle and coal-fueled molten carbonate fuel cell power plant. Sustain. Energy Technol. Assess. 2021, 47, 101458. [Google Scholar] [CrossRef]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- Haltiwanger, J.F.; Davidson, J.H.; Wilson, E.J. Renewable hydrogen from the Zn/ZnO solar thermochemical cycle: A cost and policy analysis. J. Sol. Energy Eng. Trans. ASME 2010, 132, 041011. [Google Scholar] [CrossRef]
- Bhosale, R.R. Solar hydrogen production via ZnO/Zn based thermochemical water splitting cycle: Effect of partial reduction of ZnO. Int. J. Hydrogen Energy 2021, 46, 4739–4748. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Meier, A.; Steinfeld, A. Review of the Two-Step H2O/CO2-Splitting solar thermochemical cycle based on Zn/ZnO redox reactions. Materials 2010, 3, 4922–4938. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, E.A. Solarthermal and solar quasi-electrolytic processing and separations: Zinc from zinc oxide as an example. Ind. Eng. Chem. Res. 1999, 38, 2275–2282. [Google Scholar] [CrossRef]
- Müller, R.; Steinfeld, A. H2O-splitting thermochemical cycle based on ZnO/Zn-redox: Quenching the effluents from the ZnO dissociation. Chem. Eng. Sci. 2008, 63, 217–227. [Google Scholar] [CrossRef]
- Wieckert, C.; Steinfeld, A. Solar thermal reduction of ZnO using CH4:ZnO and C:ZnO molar ratios less than 1. J. Sol. Energy Eng. Trans. ASME 2002, 124, 55–62. [Google Scholar] [CrossRef]
- Bhosale, R.R. Thermodynamic efficiency analysis of ZnO/Zn based solar thermochemical CH4 reforming and H2O splitting cycle. Int. J. Hydrogen Energy 2020, 45, 5760–5771. [Google Scholar] [CrossRef]
- Lindemer, M.D.; Advani, S.G.; Prasad, A.K. Experimental investigation of heterogeneous hydrolysis with Zn vapor under a temperature gradient. Int. J. Hydrogen Energy 2017, 42, 7847–7856. [Google Scholar] [CrossRef]
- Gstoehl, D.; Brambilla, A.; Schunk, L.O.; Steinfeld, A. A quenching apparatus for the gaseous products of the solar thermal dissociation of ZnO. J. Mater. Sci. 2008, 43, 4729–4736. [Google Scholar] [CrossRef]
- Alxneit, I. Assessing the feasibility of separating a stoichiometric mixture of zinc vapor and oxygen by a fast quench—Model calculations. Sol. Energy 2008, 82, 959–964. [Google Scholar] [CrossRef]
- Lindemer, M.D.; Advani, S.G.; Prasad, A.K. Hydrogen production via the heterogeneous hydrolysis of Zn vapor under a temperature gradient: Modeling and efficiency analysis. Int. J. Hydrogen Energy 2016, 41, 10557–10567. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, S.Y.; Li, Y.R. Advances in solar hydrogen production via two-step water-splitting thermochemical cycles based on metal redox reactions. Renew. Energy 2012, 41, 1–12. [Google Scholar] [CrossRef]
- Koepf, E.; Villasmil, W.; Meier, A. Pilot-scale solar reactor operation and characterization for fuel production via the Zn/ZnO thermochemical cycle. Appl. Energy 2016, 165, 1004–1023. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Lemont, F.; Flamant, G. Novel two-step SnO2/SnO water-splitting cycle for solar thermochemical production of hydrogen. Int. J. Hydrogen Energy 2008, 33, 6021–6030. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemont, F.; Flamant, G. Experimental Study of SnO2/SnO/Sn Thermochemical Systems for Solar Production of Hydrogen. AIChE J. 2008, 54, 2759–2767. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Solar thermal reduction of ZnO and SnO2: Characterization of the recombination reaction with O2. Chem. Eng. Sci. 2010, 65, 3671–3680. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S.; Jumas, J.C.; Olivier-Fourcade, J. Characterization of two-step tin-based redox system for thermochemical fuel production from solar-driven CO2 and H2O splitting cycle. Ind. Eng. Chem. Res. 2014, 53, 5668–5677. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; Sutar, P. Thermodynamic analysis of solar driven SnO2/SnO based thermochemical water splitting cycle. Energy Convers. Manag. 2017, 135, 226–235. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen production via a two-step water splitting thermochemical cycle based on metal oxide—A review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Kinetic investigation of hydrogen generation from hydrolysis of SnO and Zn solar nanopowders. Int. J. Hydrogen Energy 2009, 34, 5326–5336. [Google Scholar] [CrossRef]
- Vishnevetsky, I.; Epstein, M. Tin as a possible candidate for solar thermochemical redox process for hydrogen production. J. Sol. Energy Eng. Trans. ASME 2009, 131, 0210071–0210078. [Google Scholar] [CrossRef]
- Nakumura, T. Hydrogen Production From Water Utilizing. Sol. Energy 1977, 19, 467–475. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Yamamoto, R. Thermochemical two-step water splitting by ZrO2-supported NixFe3-xO4 for solar hydrogen production. Sol. Energy 2008, 82, 73–79. [Google Scholar] [CrossRef]
- Charvin, P.; Stéphane, A.; Florent, L.; Gilles, F. Analysis of solar chemical processes for hydrogen production from water splitting thermochemical cycles. Energy Convers. Manag. 2008, 49, 1547–1556. [Google Scholar] [CrossRef]
- Xu, R.; Wiesner, T.F. Conceptual design of a two-step solar hydrogen thermochemical cycle with thermal storage in a reaction intermediate. Int. J. Hydrogen Energy 2014, 39, 12457–12471. [Google Scholar] [CrossRef]
- Goikoetxea, N.B.; Gómez-Mancebo, M.B.; Fernández-Saavedra, R.; Borlaf, F.; García-Pérez, F.; Jiménez, J.A.; Llorente, I.; Rucandio, I.; Quejido, A.J. Understanding water-splitting thermochemical cycles based on nickel and cobalt ferrites for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 17578–17585. [Google Scholar] [CrossRef]
- Teknetzi, I.; Nessi, P.; Zaspalis, V.; Nalbandian, L. Ni-ferrite with structural stability for solar thermochemical H2O/CO2 splitting. Int. J. Hydrogen Energy 2017, 42, 26231–26242. [Google Scholar] [CrossRef]
- Amar, V.S.; Puszynski, J.A.; Shende, R.V. H2 generation from thermochemical water-splitting using yttria stabilized NiFe2O4 core-shell nanoparticles. J. Renew. Sustain. Energy 2015, 7, 023113. [Google Scholar] [CrossRef]
- Gokon, N.; Murayama, H.; Umeda, J.; Hatamachi, T.; Kodama, T. Monoclinic zirconia-supported Fe3O4 for the two-step water-splitting thermochemical cycle at high thermal reduction temperatures of 1400–1600 °C. Int. J. Hydrogen Energy 2009, 34, 1208–1217. [Google Scholar] [CrossRef]
- Kodama, T.; Kondoh, Y.; Yamamoto, R.; Andou, H.; Satou, N. Thermochemical hydrogen production by a redox system of ZrO2-supported Co(II)-ferrite. Sol. Energy 2005, 78, 623–631. [Google Scholar] [CrossRef]
- Reñones, P.; Alvarez-Galvan, M.C.; Ruiz-Matas, L.; Retuerto, M.; Navarro, R.M.; Fierro, J.L.G. Nickel ferrite supported on calcium-stabilized zirconia for solar hydrogen production by two-step thermochemical water splitting. Mater. Today Energy 2017, 6, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Saavedra, R.; Gómez-Mancebo, M.B.; Caravaca, C.; Sánchez, M.; Quejido, A.J.; Vidal, A. Hydrogen production by two-step thermochemical cycles based on commercial nickel ferrite: Kinetic and structural study. Int. J. Hydrogen Energy 2014, 39, 6819–6826. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Li, J.; Weimer, A.W. A spinel ferrite/hercynite water-splitting redox cycle. Int. J. Hydrogen Energy 2010, 35, 3333–3340. [Google Scholar] [CrossRef]
- Kreider, P.B.; Funke, H.H.; Cuche, K.; Schmidt, M.; Steinfeld, A.; Weimer, A.W. Manganese oxide based thermochemical hydrogen production cycle. Int. J. Hydrogen Energy 2011, 36, 7028–7037. [Google Scholar] [CrossRef]
- Marugán, J.; Botas, J.A.; Molina, R.; Herradón, C. Study of the hydrogen production step of the Mn2O3/MnO thermochemical cycle. Int. J. Hydrogen Energy 2014, 39, 5274–5282. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.S.; Askar, M.H.; Schöllhorn, R. Hydrated layered manganese dioxide: Part I. Synthesis and characterization of some hydrated layered manganese dioxides from α-NaMnO2. Solid State Ionics 2002, 150, 407–415. [Google Scholar] [CrossRef]
- Omomo, Y.; Sasaki, T.; Watanabe, M. Preparation of protonic layered manganates and their intercalation behavior. Solid State Ionics 2002, 151, 243–250. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Dey, S. Solar thermochemical splitting of water to generate hydrogen. Proc. Natl. Acad. Sci. USA 2017, 114, 13385–13393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, T.M.; Lichty, P.R.; Weimer, A.W. Manganese oxide dissociation kinetics for the Mn2O3 thermochemical water-splitting cycle. Part 1: Experimental. Chem. Eng. Sci. 2010, 65, 3709–3717. [Google Scholar] [CrossRef]
- Bayón, A.; De La Peña O’Shea, V.A.; Serrano, D.P.; Coronado, J.M. Influence of structural and morphological characteristics on the hydrogen production and sodium recovery in the NaOH-MnO thermochemical cycle. Int. J. Hydrogen Energy 2013, 38, 13143–13152. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Marugán, J.; Botas, J.Á.; Sanz, R. Hydrogen production by water splitting with Mn3-xCoxO4 mixed oxides thermochemical cycles: A thermodynamic analysis. Energy Convers. Manag. 2020, 216, 112945. [Google Scholar] [CrossRef]
- Xu, B.; Bhawe, Y.; Davis, M.E. Low-temperature, manganese oxide-based, thermochemical water splitting cycle. Proc. Natl. Acad. Sci. USA 2012, 109, 9260–9264. [Google Scholar] [CrossRef] [Green Version]
- Bayón, A.; De La Penã O’Shea, V.A.; Serrano, D.P.; Coronado, J.M. Exploring the alternative MnO-Na2CO3 thermochemical cycle for water splitting. J. CO2 Util. 2020, 42, 101264. [Google Scholar] [CrossRef]
- Bayón, A.; De La Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Role of the physicochemical properties of hausmannite on the hydrogen production via the Mn3O4-NaOH thermochemical cycle. Int. J. Hydrogen Energy 2016, 41, 113–122. [Google Scholar] [CrossRef]
- Murmura, M.A.; Varsano, F.; Padella, F.; La Barbera, A.; Alvani, C.; Annesini, M.C. Hydrogen production by the sodium manganese ferrite thermochemical cycle-experimental rate and modeling. Ind. Eng. Chem. Res. 2014, 53, 10310–10317. [Google Scholar] [CrossRef]
- Orfila, M.; Sanz, D.; Linares, M.; Molina, R.; Sanz, R.; Marugán, J.; Botas, J.Á. H2 production by thermochemical water splitting with reticulated porous structures of ceria-based mixed oxide materials. Int. J. Hydrogen Energy 2021, 46, 17458–17471. [Google Scholar] [CrossRef]
- Abanades, S.; Legal, A.; Cordier, A.; Peraudeau, G.; Flamant, G.; Julbe, A. Investigation of reactive cerium-based oxides for H2 production by thermochemical two-step water-splitting. J. Mater. Sci. 2010, 45, 4163–4173. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Karnaukhov, T.M.; Cherepanova, S.V.; Stoyanovskii, V.O.; Rogov, V.A.; Mishakov, I.V. Synthesis of binary Co–Mg–O oxide system and study of its behavior in reduction/oxidation cycling. Int. J. Hydrogen Energy 2019, 44, 20690–20699. [Google Scholar] [CrossRef]
- Karnaukhov, T.M.; Vedyagin, A.A.; Cherepanova, S.V.; Rogov, V.A.; Mishakov, I.V. Sol–gel synthesis and characterization of the binary Ni–Mg–O oxide system. J. Sol-Gel Sci. Technol. 2019, 92, 208–214. [Google Scholar] [CrossRef]
- Muhich, C.L.; Ehrhart, B.D.; Al-shankiti, I.; Ward, B.J.; Musgrave, C.B.; Weimer, A.W. A review and perspective of efficient hydrogen generation via solar thermal water splitting. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 261–287. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Botas, J.Á.; Marugán, J.; Sanz, R. Thermochemical hydrogen production using manganese cobalt spinels as redox materials. Int. J. Hydrogen Energy 2017, 42, 13532–13543. [Google Scholar] [CrossRef]
- Miller, J.E.; Allendorf, M.D.; Diver, R.B.; Evans, L.R.; Siegel, N.P.; Stuecker, J.N. Metal oxide composites and structures for ultra-high temperature solar thermochemical cycles. J. Mater. Sci. 2008, 43, 4714–4728. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N. Thermochemical cycles for high-temperature solar hydrogen production. Chem. Rev. 2007, 107, 4048–4077. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Q.; Tong, J.; Yang, M.; Jiang, Z.; Li, C. Influences of morphology and structure on Mn-based multi-step thermochemical H2O splitting cycle. Sol. Energy 2016, 129, 236–243. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. A comparative evaluation of three Cu-Cl cycles for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 7958–7968. [Google Scholar] [CrossRef]
- Murmura, M.A.; Vilardi, G. Energy and exergy analysis of the zinc/zinc oxide thermochemical cycle for hydrogen production and fuel cell power generation. Energy Convers. Manag. 2021, 247, 114761. [Google Scholar] [CrossRef]
- Wang, Y.; Sharma, A.; Duong, T.; Arandiyan, H.; Zhao, T.; Zhang, D.; Su, Z.; Garbrecht, M.; Beck, F.J.; Karuturi, S.; et al. Direct Solar Hydrogen Generation at 20% Efficiency Using Low-Cost Materials. Adv. Energy Mater. 2021, 11, 2101053. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef] [PubMed]
- Gorensek, M.; Summers, W.; Boltrunis, C.; Lahoda, E.; Allen, D.; Greyvenstein, R. Hybrid Sulfur Process Reference Design and Cost Analysis; Report No. SRNL-L1200-2008-00002; Savannah River National Laboratory: Jackson, SC, USA, 2009.
- Kromer, M.; Roth, K.; Takata, R.; Chin, P. Support for Cost Analyses on Solar-Driven High Temperature Thermochemical Water-Splitting Cycles; Final Report to Department of Energy, Order DE-DT0000951; TIAX LLC: Lexington, MA, USA, 2011. [Google Scholar]
- U.S. Department of Energy HydroGEN Program.
- Leybros, J.; Gilardi, T.; Saturnin, A.; Mansilla, C.; Carles, P. Plant sizing and evaluation of hydrogen production costs from advanced processes coupled to a nuclear heat source. Part I: Sulphur-iodine cycle. Int. J. Hydrogen Energy 2010, 35, 1008–1018. [Google Scholar] [CrossRef]
- Le Duigou, A.; Borgard, J.M.; Larousse, B.; Doizi, D.; Allen, R.; Ewan, B.C.; Priestman, G.H.; Elder, R.; Devonshire, R.; Ramos, V.; et al. HYTHEC: An EC funded search for a long term massive hydrogen production route using solar and nuclear technologies. Int. J. Hydrogen Energy 2007, 32, 1516–1529. [Google Scholar] [CrossRef]







| Metal | γa (%) | Oxidized Phase |
|---|---|---|
| Ni | 0.4 | NiO |
| Cd | 1.83 | CdO |
| Cu | 0 | Cu2O |
| Co | 2.27 | CoO |
| Sn | 40.82 | SnO2 |
| MnO | 0 | Mn3O4 |
| Fe | 74.79 | Fe3O4 |
| Fe | 74.79 | Fe2O3 |
| Years | HyS | CuCl | Ferrite | SA | ZnO | MnO | S-I |
|---|---|---|---|---|---|---|---|
| 2015 | 5.68 | 6.83 | 4.06 | 7.78 | 6.07 | - | - |
| 2025 | 3.85 | 5.39 | 2.42 | 4.71 | 4.18 | 4.63 | 4.68 |
| Cycle | Major Discovery | Ref. |
|---|---|---|
| S-I | Optimization of the Bunsen section for liquid–liquid separation. Increasing iodine content improved separation characteristics. The optimum I2/H2SO4 ratio was in the range of 2.45–3.99 at 70–85 °C | [107] |
| S-I | Developed a microporous membrane resistant to sulfur trioxide composed of α-alumina support, ZrO2-SiO2 intermediate layer, and organosilica sol top layer. High Si:Zr ratio and large pore of the ZrO2-SiO2 showed higher O2/SO3 selectivity and higher chemical stability against SO3 | [115] |
| S-I | Developed a modified cycle with fewer steps and used a steam boiler. The modified cycle has a higher HI decomposition rate, and the Bunsen reaction happened at lower temperatures. | [116] |
| S-I | Catalysts with hierarchical pore structure and higher specific surface area and micropore proportion of about 50% showed higher catalytic activity. | [117] |
| S-I | N doping promotes HI decomposition rate | [102] |
| HyS | Increasing Fe content in Fe/Al and Fe/Ti binary metal oxide catalysts improved catalytic activity | [143] |
| HyS | Cr, Ce, U, Mn, and Ni form stable sulphates and are not suitable catalysts for sulfuric acid decomposition. t/BaSO4-TiO2, Pt/TiO2, Pt/ZrO2, and Pt/SiO2 are the most suitable catalysts for the HyS cycle. | [135] |
| HyS | PtxPdy thin film deposited on a Si wafer showed high catalytic activity | [146] |
| HyS | Increasing Ni dopant in PtxNiy/C catalysts increased electron vacancies and improved catalytic performance | [148] |
| HyS | Incorporating ceria in Pt/C composite catalyst increased catalyst active area and improved its performance | [150] |
| Cu-Cl | Developing a novel integrated system for producing nitrogen, methane, ammonia, oxygen, and carbon dioxide | [168] |
| Cu-Cl | Optimizing the temperature of the hydrolysis step can minimize the number of byproducts. | [172] |
| Cu-Cl | Cu-Cl is the most promising cycle for large-scale hydrogen production. | [176] |
| ZnO/Zn | Partially reduced ZnO showed higher catalytic performance at elevated temperatures (R = 57.2%) | [193] |
| ZnO/Zn | Hindered recombination and lowered the reaction temperature of the first step (methane or carbon as reducing agent) | [197] |
| ZnO/Zn | A negative axial temperature gradient reduced the steam and inert gas proportion | [199,202] |
| SnO2/SnO | Reducing O2 partial pressure to about 10−3 bar decreased thermal reduction temperature | [209] |
| Fe3O4/FeO | Non-stoichiometric wustite have higher defect densities and showed higher reaction rates | [188] |
| Ferrite | Zirconia support enhances the energy radiation absorption and lowers the temperature | [186] |
| Ferrite | Ceramics nanoparticles can slightly increase hydrogen yield and hinder grain growth | [219] |
| Ferrite | ZrO2 can reduce the high-temperature sintering and improve catalytic activity | [218] |
| Ferrite | Calcium-stabilized zirconia forms calcium zirconate promotes high active phase dispersion and improves hydrogen yield | [222] |
| Ferrite | Sacrificial (carbon black and PEG) and zirconia plates improved the thermal stability of Ni-ferrite samples | [218] |
| Ferrite | Core-shell NiFe2O4/Y2O3 nanoparticles showed stable hydrogen volume, but lower rates of hydrogen production than that of mixed powders | [219] |
| Ferrite | Hercynite formation in alumina and cobalt ferrite decreased ferrite reduction temperature | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallah Vostakola, M.; Salamatinia, B.; Amini Horri, B. A Review on Recent Progress in the Integrated Green Hydrogen Production Processes. Energies 2022, 15, 1209. https://doi.org/10.3390/en15031209
Fallah Vostakola M, Salamatinia B, Amini Horri B. A Review on Recent Progress in the Integrated Green Hydrogen Production Processes. Energies. 2022; 15(3):1209. https://doi.org/10.3390/en15031209
Chicago/Turabian StyleFallah Vostakola, Mohsen, Babak Salamatinia, and Bahman Amini Horri. 2022. "A Review on Recent Progress in the Integrated Green Hydrogen Production Processes" Energies 15, no. 3: 1209. https://doi.org/10.3390/en15031209
APA StyleFallah Vostakola, M., Salamatinia, B., & Amini Horri, B. (2022). A Review on Recent Progress in the Integrated Green Hydrogen Production Processes. Energies, 15(3), 1209. https://doi.org/10.3390/en15031209







