Pollutant Emissions during Oxy-Fuel Combustion of Biomass in a Bench Scale CFB Combustor
Abstract
:1. Introduction
2. Literature Review
| Reference | Test Facility and Conditions | Fuels Tested | Composition of Oxidizing Medium | Emissions Reported | Remarks |
|---|---|---|---|---|---|
| Tan et al. [13] | CFB, 800 kWth, ~900 °C, limestone, Ca/S = 3 | Wood pellets, different coals, fraction of biomass in blends 20–50%wt. | O2/CO2 mixtures, 24–25% O2, recycled flue gas | NOx, SO2, CO, CO2, O2 | The addition of biomass did not have a significant influence on combustion conditions. Emissions of NO, SO2 and CO below the EU limits (200 ppm). |
| Duan et al. [5] | CFB, 10 kWth, 850 ± 10 °C | Rice husk, wood chips, dry wood flour, bituminous coal, biomass/coal ratio 0–100%wt. | Air, 70% O2/30% CO2 mixture | NO, NO2, CO, CO2, O2, SO2 | For single fuel combustion, emissions of NO were higher for biomass (the highest for rice husk) than for coal. Oxy- combustion produced less NO than combustion in air. NO emissions increased with increasing fraction of biomass in the fuel blend for all biomass fuels in air and oxy-fuel conditions. |
| Lupiáñez et al. [14] | BFB *, 50 kWth, 850–900 °C, limestone, Ca/S = 2.5 | Corn stover, anthracite, biomass fraction in blend 0.2 (on energy basis) | Air, 30% O2/70% CO2 mixture | NO, CO, CO2, O2, SO2 | No reduction in NO emissions during oxy-firing was observed. Influence of coal/biomass ratio on NO emissions was negligible. Emissions of SO2 higher in air than in oxy-combustion. |
| Lupiáñez et al. [15,16] | BFB *, 50 kWth, 850–925 °C, limestone, Ca/S ratio: 2.5, 4, 6 | Corn stover, lignite, biomass fraction in blend 0.1 and 0.2 | Air, 35% O2/65% CO2, 30% O2/70% CO2 | NO, SO2, CO, CO2, O2, HCl | Emissions of NO increased with increasing Ca/S ratio. They were more influenced by the excess O2 and concentration of CO than by the biomass share in the fuel blend. |
| Pu et al. [17] | BFB *, 850–950 °C | Pine powder, anthracite, biomass fraction in blend 0–30%wt. | 21–40% O2, CO2 | NO, NO2, CO, CO2, O2, SO2 | Concentration of NO increased with increasing initial concentration of O2 in the oxidizing medium. Addition of pine powder reduced the NO emission in the test at 950 °C with 35% O2 in the O2/CO2 mixture. |
| Wang et al. [18] | CFB, 10 kWth, 800–900 °C | Corn straw, wheat straw, coal, 30% biomass in blend | 50% O2/50% CO2, 50% O2/50% recycled flue gas | NO, N2O, CO, CO2, O2, HCN | NO and N2O emissions increased with increasing excess O2. An increase in the fraction of corn straw in the fuel blend caused an increase in emission factors of NO, N2O, and HCN. |
| Sher et al. [19] | BFB *, 20 kWth, 800 °C | Miscanthus, straw pellets, wood pellets | Air, O2/N2/CO2 mixtures: 21/22/57%, 24/25/52%, 27/28/45% | NOx, CO, CO2, O2 | Emissions of CO and NO decreased with increasing concentration of O2 in the oxy-fuel medium. At 30% O2, emissions of NO were like those for combustion in air. |
| Varol et al. [8] | CFB, 850 and 915 °C, limestone, Ca/S = 2 | Wood pellets, high-sulphur lignite, fraction of biomass in blend up to 60% | 25, 30% O2, CO2 | NOx, SO2, CO, CO2 | Increasing biomass share in the fuel blend had a negligible influence on NOx emissions. Emissions of CO and SO2 decreased with increasing fraction of biomass in the fuel blend. |
| Nguyen et al. [20] | CFB, 100 kWth | Wood pellets, lignite, fraction of biomass in blend 50–100%wt. | 21–29% O2, CO2 | NO, SO2, CO | An increase in biomass share caused a decrease in NO, SO2 and CO concentrations. Oxy-combustion of pure biomass can produce negative CO2 emissions of, approximately, –647 g/kWth. |
| Gao et al. [12] | TG-MS **, non-isothermal tests, 20–800 °C, heating rates 5–15 K/min | Microalgae Chlorella vulgaris, lignite, 50/50 blends | 21% O2/79% N2, 21% O2/79% CO2 | CO, CO2, COS, NO, NO2, SO2 | The presence of microalgae in the fuel blend during combustion in air resulted in lower emissions of CO2, CO and NO2 but in enhanced formation of NO, COS and SO2. Similar trend was observed during oxy-combustion. |
3. Materials and Methods
3.1. Fuel Tested
3.2. Experimental Setup and Procedure
4. Results and Discussion
4.1. Conventional Combustion
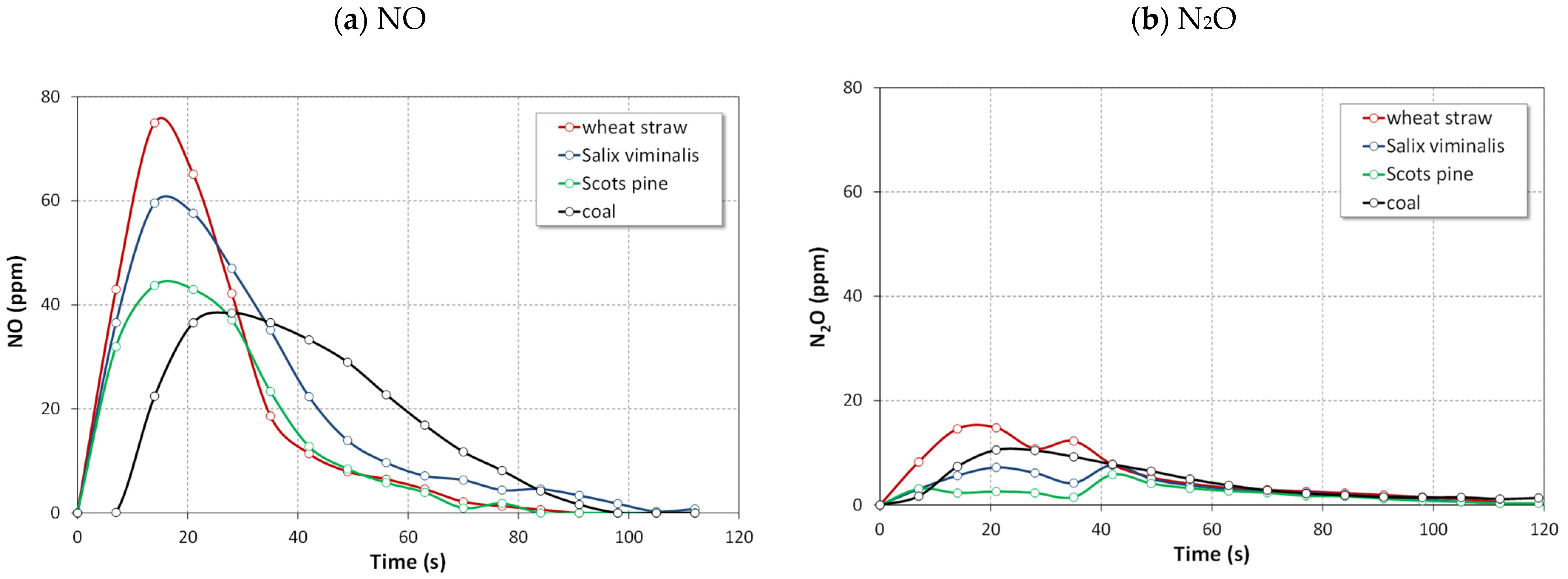
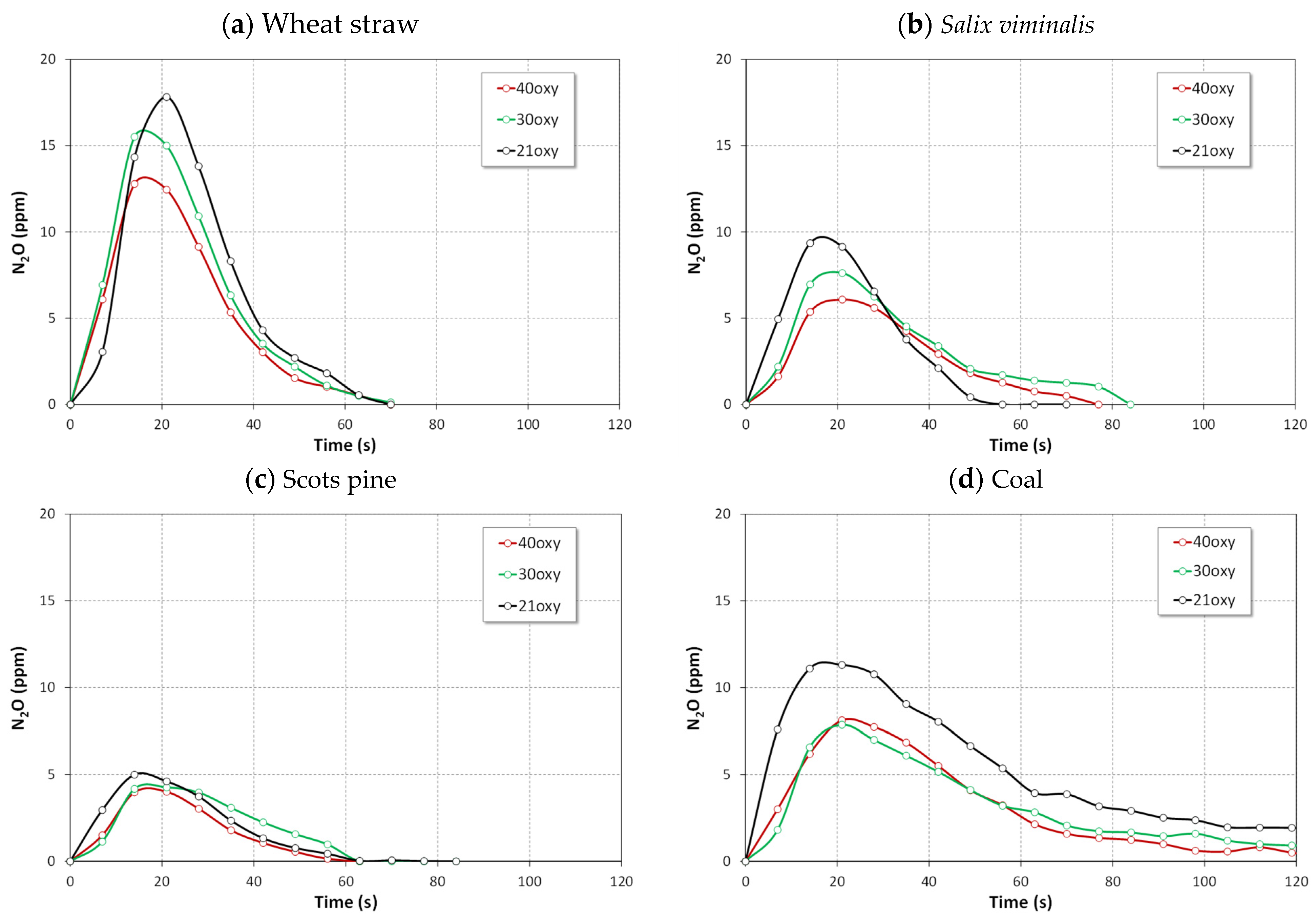
4.1.1. NO, NO2 and N2O Emissions
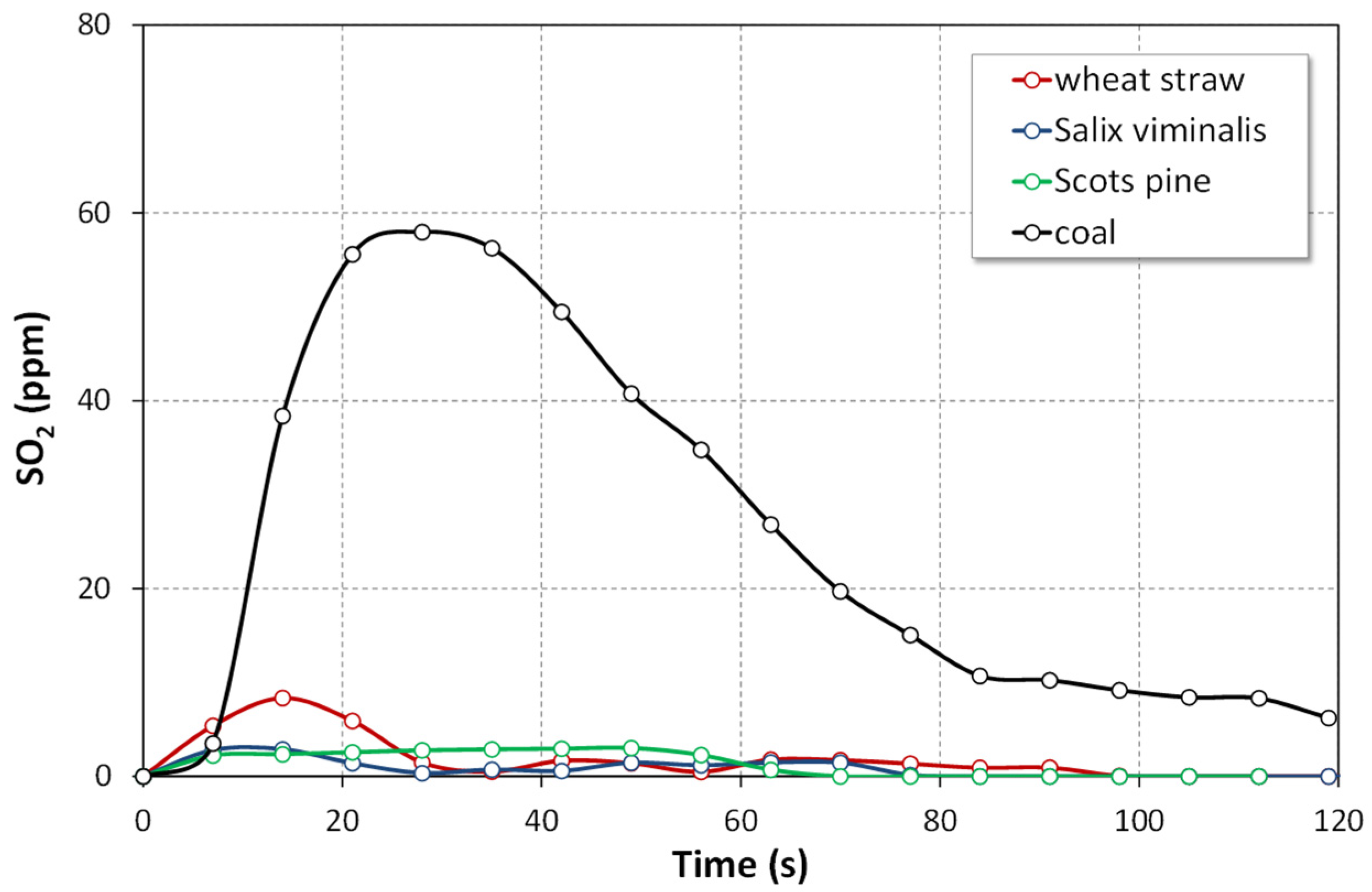
4.1.2. SO2 Emissions
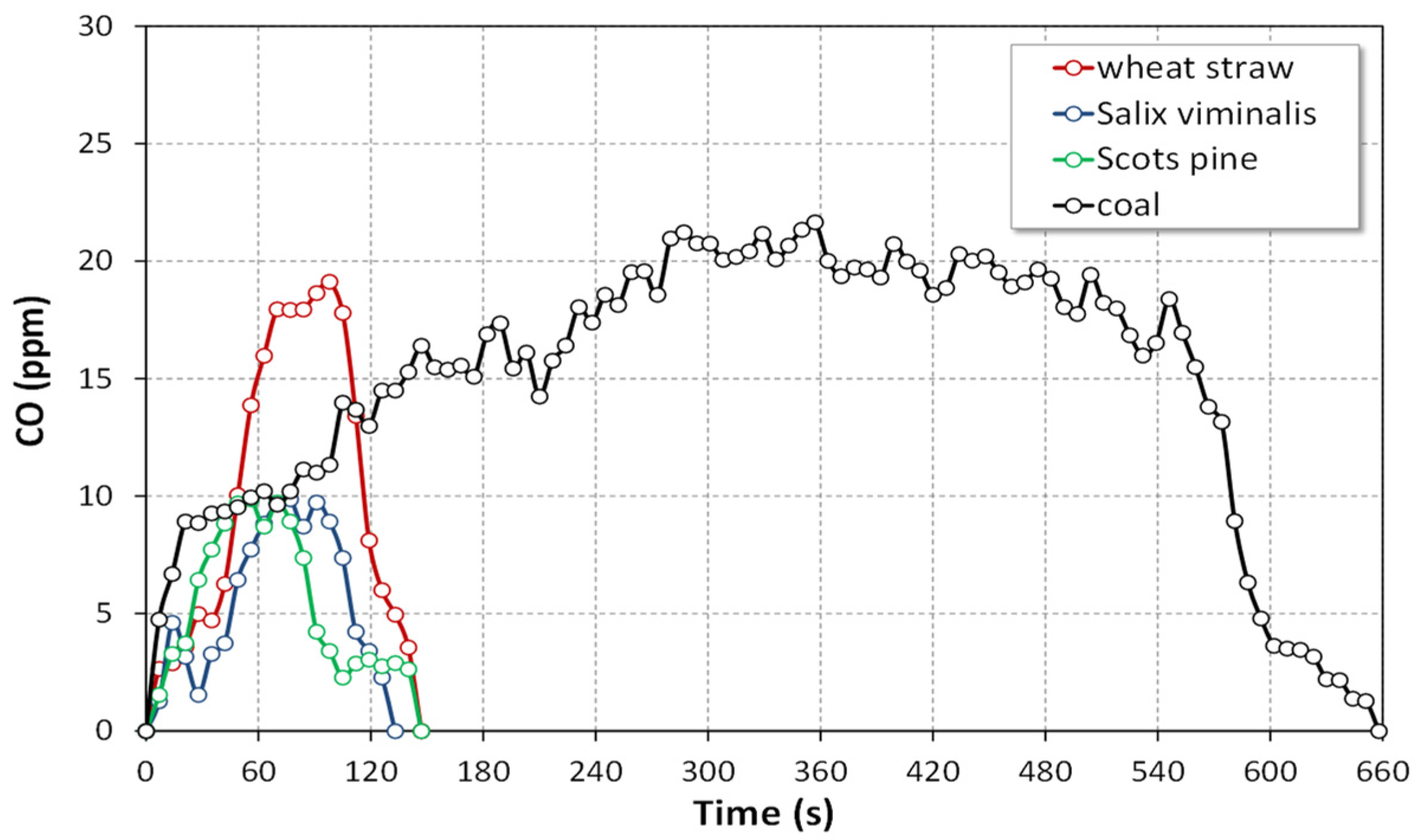
4.1.3. Emissions of CO
4.2. Oxy-Fuel Combustion
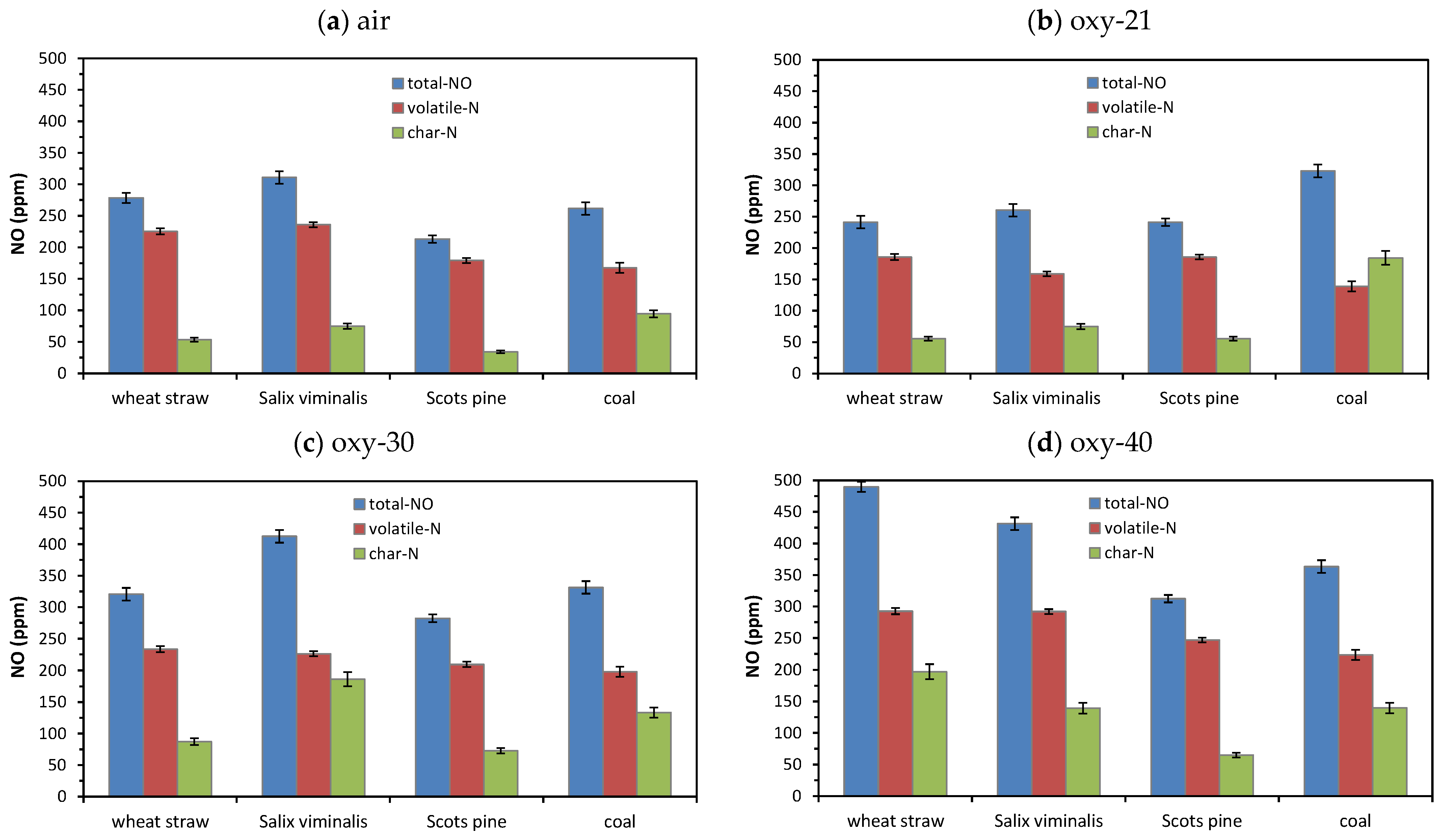
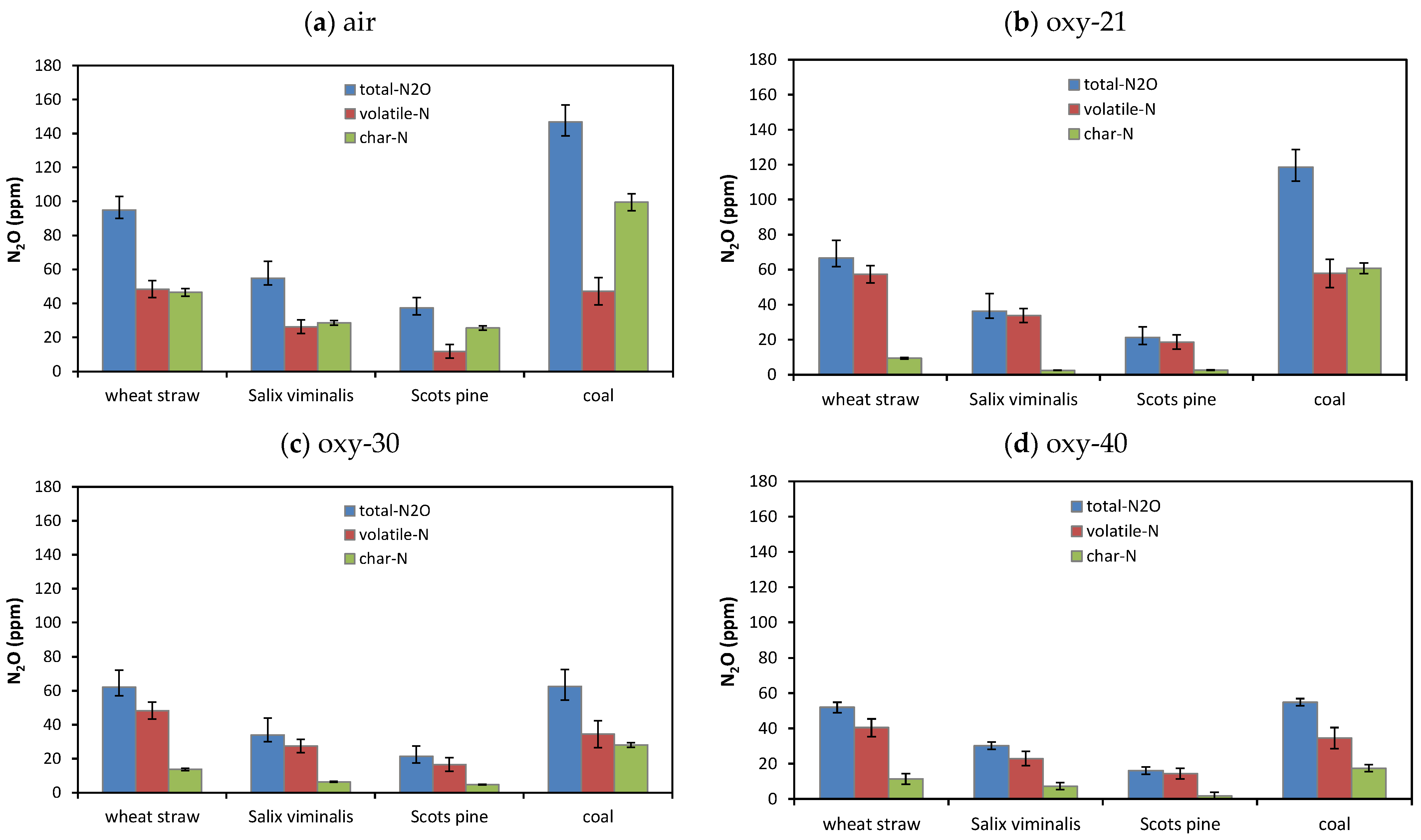
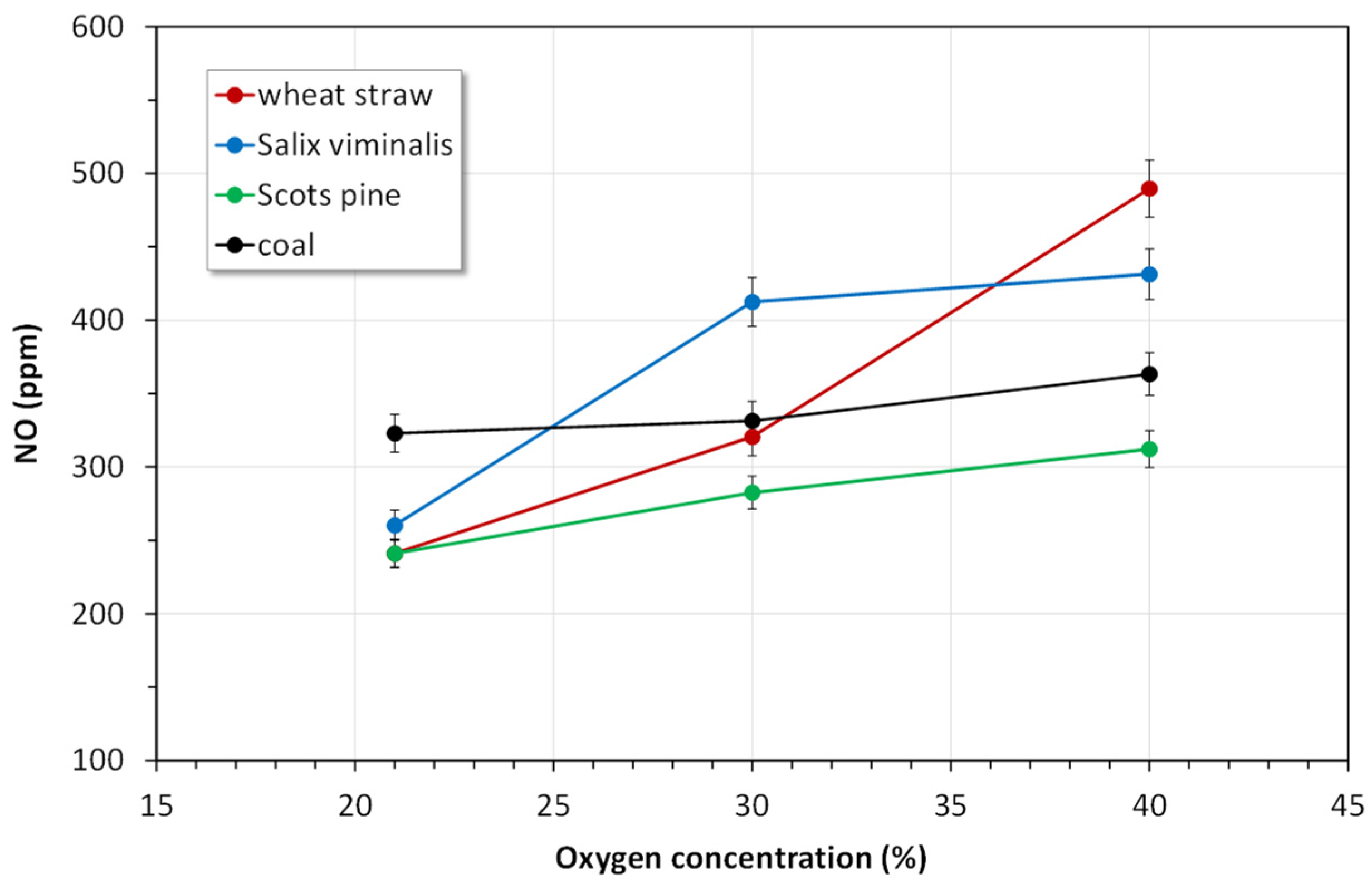
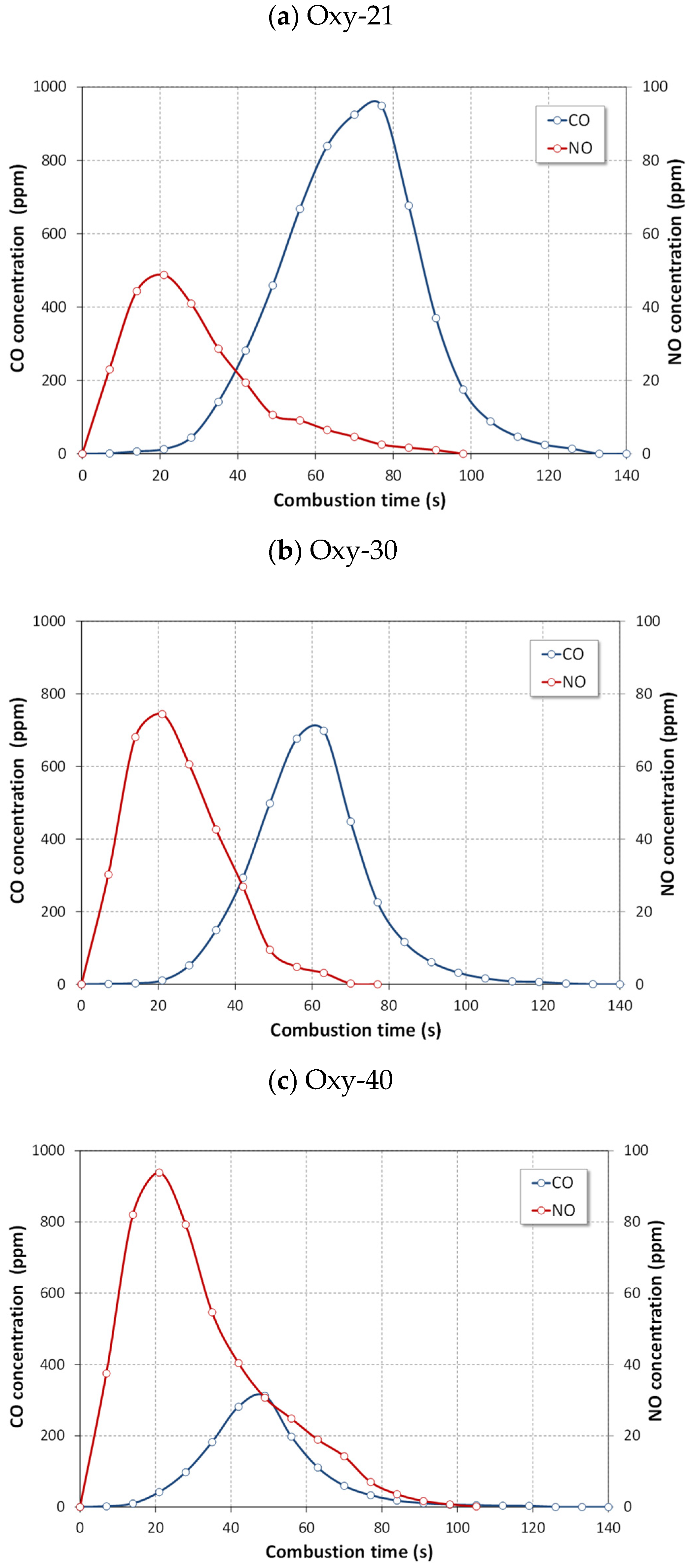
4.2.1. NOx Emissions
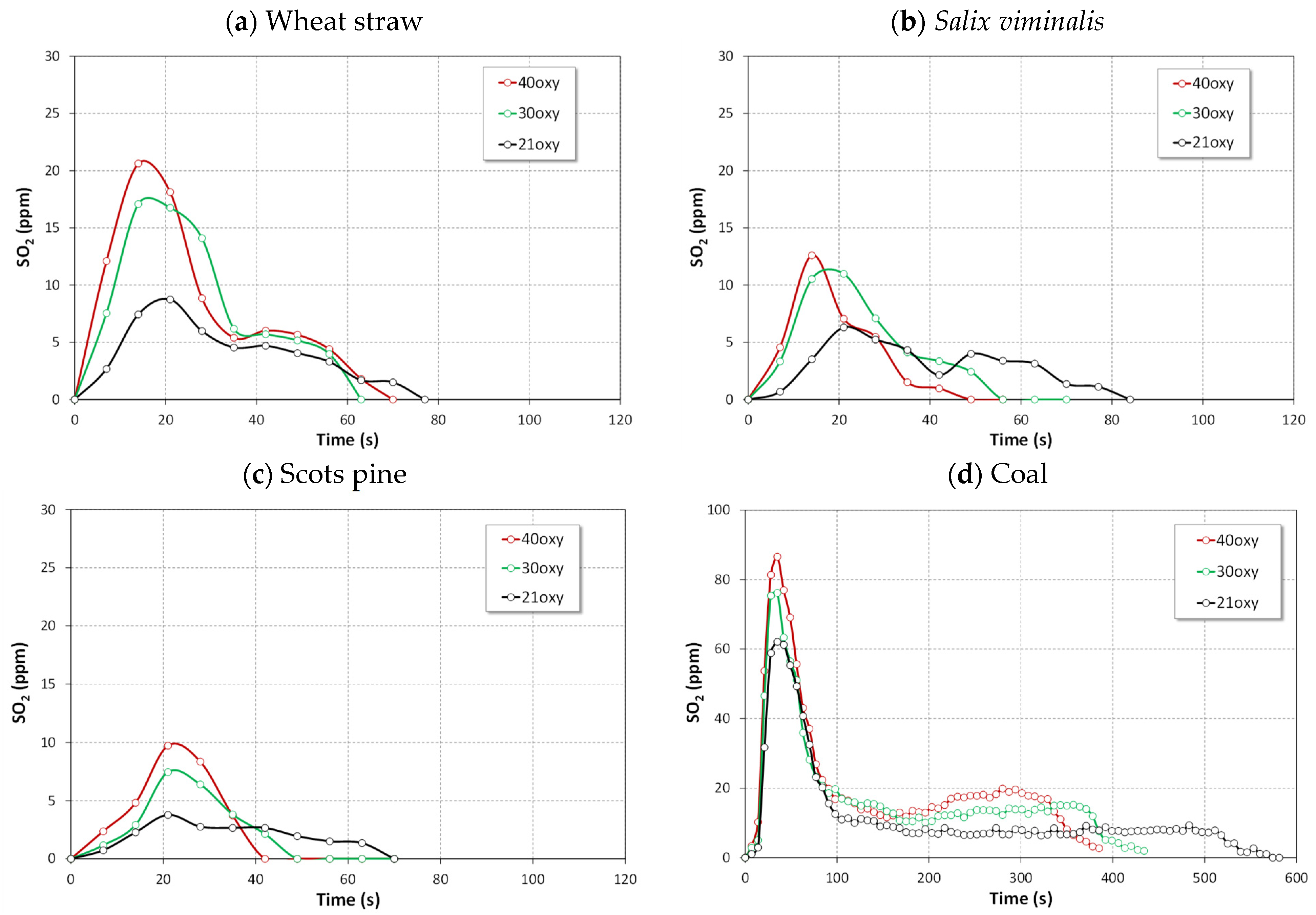
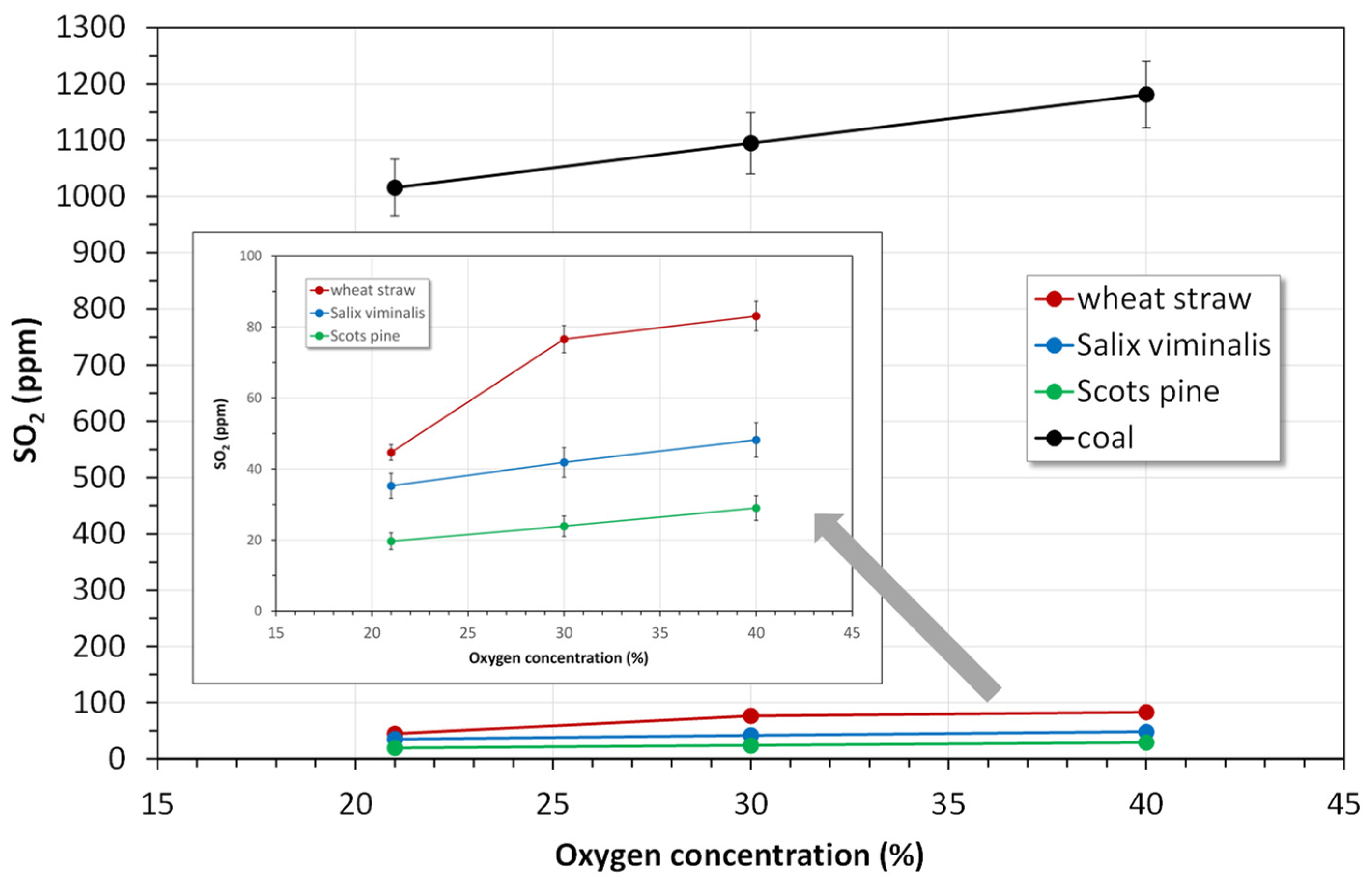
4.2.2. Emissions of SO2
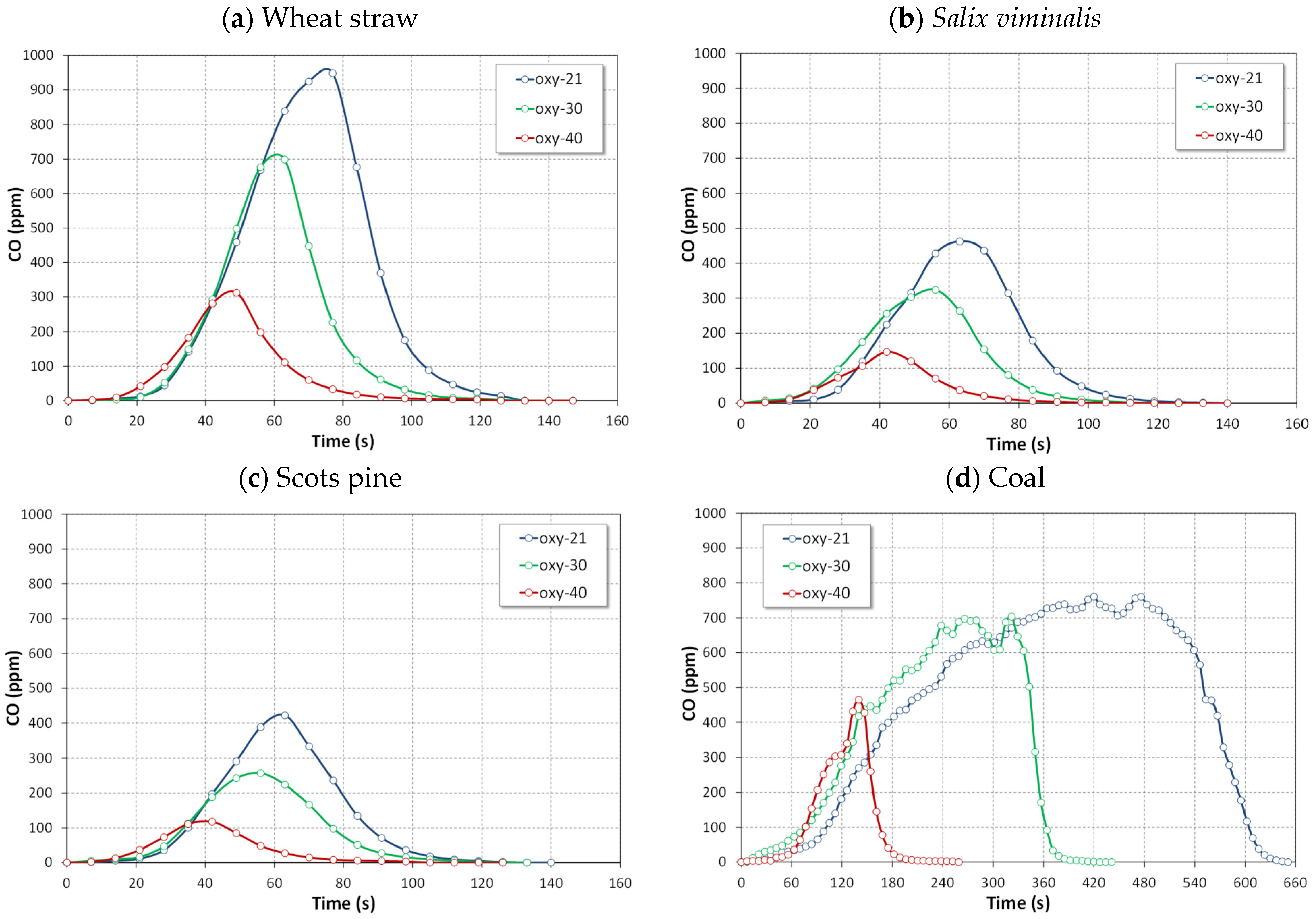
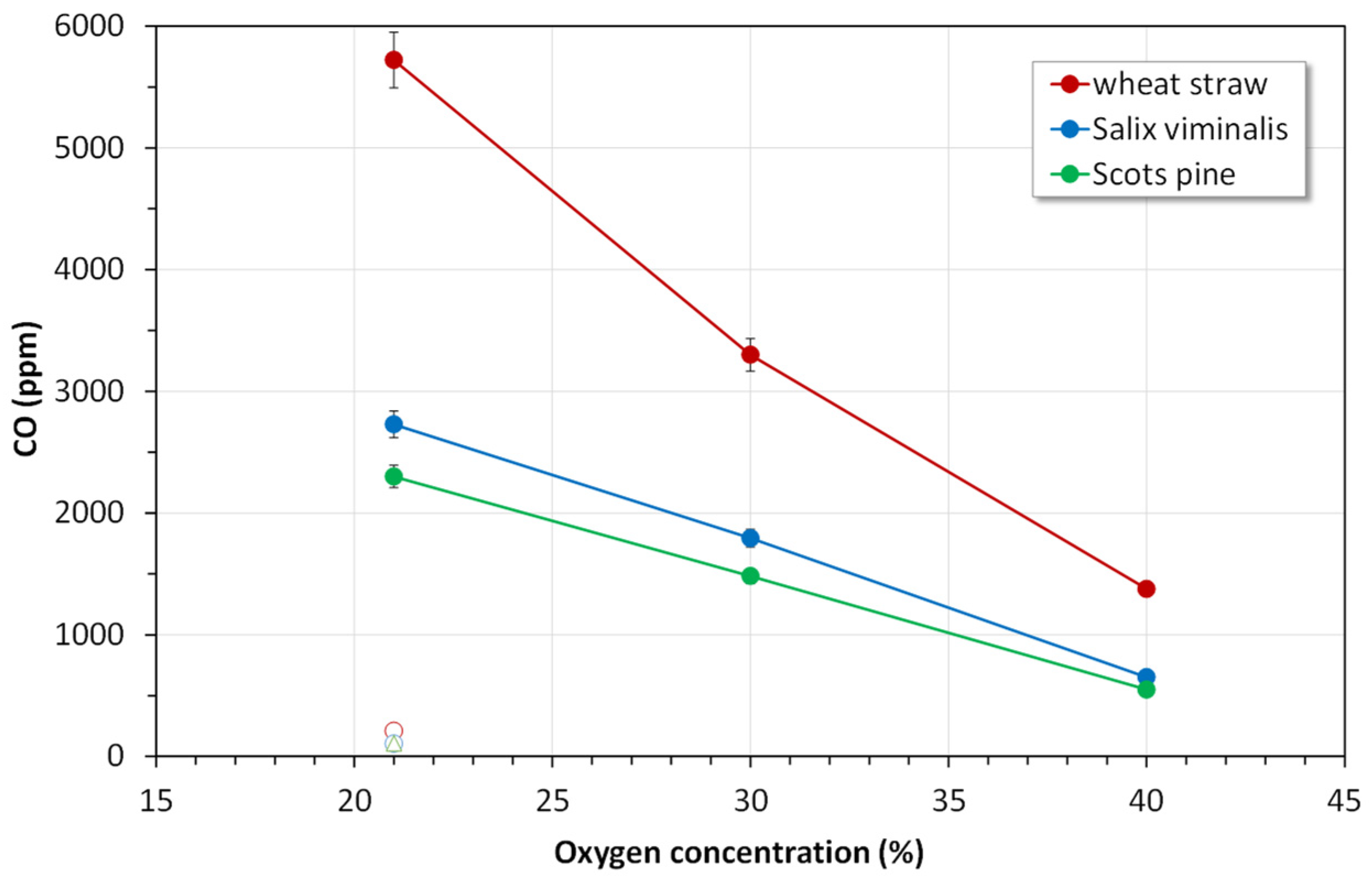
4.2.3. CO Emissions
5. Conclusions
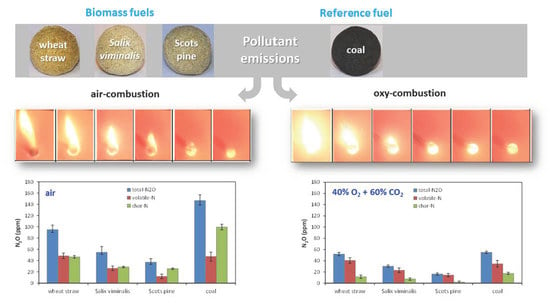
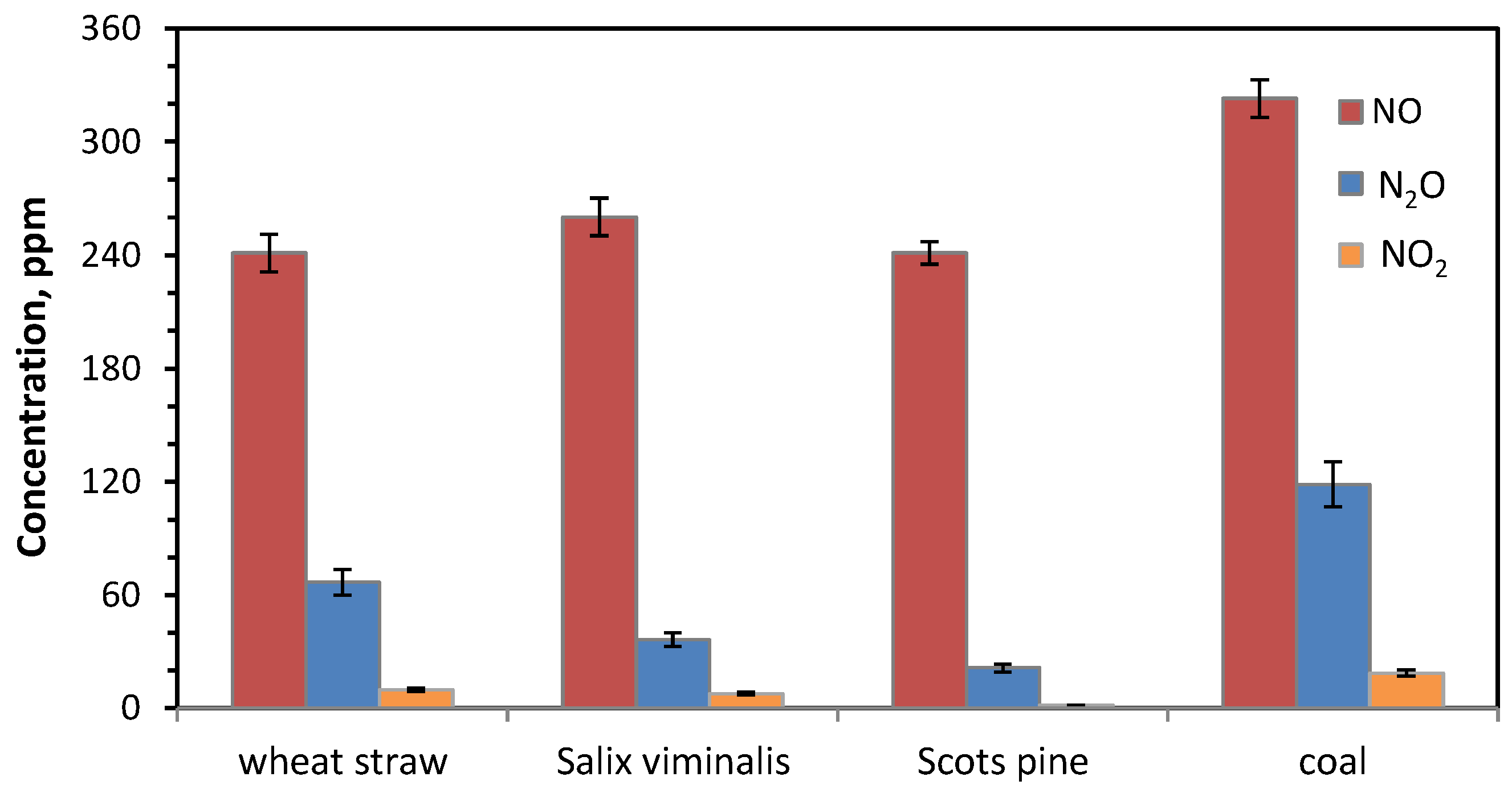
- The instantaneous emissions of SO2, N2O and CO for the combustion of biomass fuels in all atmospheres were lower than those for the combustion of bituminous coal.
- The instantaneous NO concentrations for the combustion of biomass fuels in all atmospheres were higher than those for the combustion of reference coal.
- Oxidation of nitrogen species released with volatile matter was responsible for high emissions of NOx during combustion of biomass fuels in air and mixtures of O2 and CO2.
- The highest instantaneous emissions of all gaseous pollutant during combustion of biomass fuels were observed for agro biomass (wheat straw) in both air and oxy-fuel atmospheres.
- The lowest emissions of all gaseous pollutant during combustion of biomass fuels were observed for woody biomass (Scots pine) in both air and oxy-fuel atmospheres.
- The lowest NO emissions and the highest N2O emissions for tested fuels were detected in oxy-21 atmosphere owing to lower temperature of fuel particles.
- The instantaneous CO emissions for the combustion of biomass in all atmospheres were much lower than those for the combustion of reference coal.
- Oxy-combustion of biomass or coal caused a significant increase in emissions of CO in comparison with air-combustion. This observation can be attributed to CO2 + C(char) reaction (the Boudouard reaction).
- Combustion of biomass in oxy-30 or oxy-40 atmospheres causes an increase in SO2 and NO emissions and a reduction in of CO and N2O emissions.
- Considering all pollutant emissions during oxy-combustion of biomass fuels, the optimal atmosphere is oxy-30.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, S.; Mondal, S.S. A review on the process and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plant. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Y.; Zhong, W.; Yu, A. Co-firing coal and biomass in oxy-fuel fluidized bed for CO2 capture: A review of recent advances. Chin. J. Chem. Eng. 2019, 27, 2261–2272. [Google Scholar] [CrossRef]
- Duan, L.; Duan, Y.; Zhao, C.; Anthony, E.J. NO emission during co-firing coal and biomass in an oxy-fuel circulating fluidized bed combustor. Fuel 2015, 150, 8–13. [Google Scholar] [CrossRef]
- Zhang, X.; Meloni, S. Technology Developments in the Cofiring of Biomass; IEA, Clean Coal Centre: London, UK, 2020. [Google Scholar]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M.M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Varol, M.; Symonds, R.; Anthony, E.J.; Lu, D.; Jia, L.; Tan, Y. Emissions from co-firing lignite and biomass in an oxy-fired CFBC. Fuel Processing Technol. 2018, 173, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.I.; Kumar, R. Current status and experimental investigation of oxy-fired fluidized bed. Renew. Sustain. Energy Rev. 2016, 61, 398–420. [Google Scholar] [CrossRef]
- Lupion, M.; Alvarez, I.; Otero, P.; Kuivalainen, R.; Lantto, J.; Hotta, A.; Hack, H. 30 MWth CIUDEN oxy-CFB boiler—First experience. Energy Procedia 2013, 37, 6179–6188. [Google Scholar] [CrossRef] [Green Version]
- Anthony, E.J.; Hack, H. Oxy-fired fluidized bed combustion: Technology prospects and new developments. In Fluidized Bed Technologies for Near-Zero Emission Combustion and Gasification; Scala, F., Ed.; Woodhead Publishing: London, UK, 2013; pp. 867–894. [Google Scholar]
- Gao, Y.; Tahmasebi, A.; Dou, J.; Yu, J. Combustion characteristics and air pollutant formation during oxy-fuel co-combustion of microalgae and lignite. Bioresour. Technol. 2016, 207, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Jia, L.; Wu, Y. Some combustion characteristics of biomass and coal cofiring under oxy-fuel conditions in a pilot-scale circulating fluidized combustor. Energy Fuels 2013, 27, 7000–7007. [Google Scholar] [CrossRef]
- Lupiáñez, C.; Carmen Mayoral, M.; Guedea, I.; Espatolero, S.; Díez, L.I.; Laguarta, S.; Manuel Andrés, J. Effect of co-firing on emissions and deposition during fluidized bed oxy-combustion. Fuel 2016, 184, 261–268. [Google Scholar] [CrossRef]
- Lupiáñez, C.; Carmen Mayoral, M.; Díez, L.I.; Pueyo, E.; Espatolero, S.; Díez, L.I.; Manuel Andrés, J. The role of limestone during fluidized bed oxy-combustion of coal and biomass. Appl. Energy 2016, 184, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Lupiáñez, C.; Carmen Mayoral, M.; Díez, L.I.; Pueyo, E.; Espatolero, S.; Manuel Andrés, J. On the oxy-combustion of lignite and corn stover in a lab-scale fluidized bed reactor. Biomass Bioenergy 2017, 96, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Pu, G.; Zang, H.; Du, J.; Zhang, X. Study on NO emission in the oxy-fuel combustion of cofiring coal and biomass in a bubbling fluidized bed combustor. BioResources 2017, 12, 1890–1902. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ren, Q.; Li, W.; Li, H.; Li, S.; Lu, Q. Nitrogenous gas emissions from coal/biomass co-combustion under a high oxygen concentration in a circulating fluidized bed. Energy Fuels 2017, 31, 3234–3242. [Google Scholar] [CrossRef]
- Sher, F.; Pans, M.A.; Sun, C.; Snape, C.; Liu, H. Oxy-fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel 2018, 215, 778–786. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Moon, J.-H.; Jo, S.-H.; Park, S.J.; Seo, M.W.; Ra, H.W.; Yoon, S.-J.; Yoon, S.-M.; Song, B.; Lee, U.; et al. Oxy-combustion characteristics as a function of oxygen concentration and biomass co-firing ratio in a 0.1 MWth circulating fluidized bed combustion test-rig. Energy 2020, 196, 117020. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A.; Kijo-Kleczkowska, A.; Musiał, T.; Wolski, K.; Środa, K. Gaseous emissions during oxy-fuel combustion of sewage sludge in a circulating fluidized bed. Powder Technol. 2020, 371, 209–216. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A.; Musiał, T.; Kijo-Kleczkowska, A.; Wolski, K.; Środa, K. NOx and N2O emissions during oxy-fuel combustion of bituminous coal and lignite in a circulating fluidized bed combustor. In Proceedings of the 13th International Conference on Fluidized Bed Technology, Vancouver, BC, Canada, 10–14 May 2020; Bi, X., Briens, C., Ellis, N., Wormsbecker, M., Eds.; GLAB Reactor and Fluidization Technologies: Vancouver, BC, Canada, 2021; pp. 587–592. [Google Scholar]
- Kosowska-Golachowska, M.; Kijo-Kleczkowska, A.; Luckos, A.; Wolski, K.; Musiał, T. Oxy-combustion of biomass in a circulating fluidized bed. Arch. Thermodyn. 2016, 37, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Kosowska-Golachowska, M.; Otwinowski, H.; Wolski, K.; Kijo-Kleczkowska, A.; Musiał, T.; Środa, K.; Richter, D. Oxy-fuel combustion of wheat straw pellets in a lab-scale fluidized bed combustor. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer Proceedings in Energy; Mudryk, K., Werle, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 375–386. [Google Scholar]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Shah, I.A.; Gou, X.; Zhang, Q.; Wu, J.; Wang, E.; Liu, Y. Experimental study on NOx emission characteristics of oxy-biomass combustion. J. Clean. Prod. 2018, 199, 400–410. [Google Scholar] [CrossRef]
- Chyang, C.S.; Wu, K.T.; Lin, C.S. Emission of nitrogen oxides in a vortexing fluidized bed combustor. Fuel 2007, 86, 234–243. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C. Conversion of char-N into NOx and N2O during combustion of biomass char. Fuel 2019, 242, 389–398. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Xie, G.; Yu, Y. NO emission characteristic during fluidized combustion of biomass with limestone addition. Fuel 2021, 291, 120264. [Google Scholar]
- Roknia, E.; Renb, X.; Panahia, A.; Levendis, Y.A. Emissions of SO2, NOx, CO2, and HCl from co-firing of coals with raw and torrefied biomass fuels. Fuel 2018, 211, 363–374. [Google Scholar] [CrossRef]
- Lasek, J.A.; Janusz, M.; Zuwała, J.; Głód, K.; Iluk, A. Oxy-fuel combustion of selected solid fuels under atmospheric and elevated pressures. Energy 2013, 62, 105–112. [Google Scholar] [CrossRef]
- Czakiert, T.; Sztekler, K.; Karski, S.; Markiewicz, D.; Nowak, W. Oxy-fuel circulating fluidized bed combustion in a small pilot-scale test rig. Fuel Processing Technol. 2010, 91, 1617–1623. [Google Scholar] [CrossRef]
- Jankowska, S.; Czakiert, T.; Krawczyk, G.; Borecki, P. model of fuel combustion in oxygen-enriched CFB environment. Fuel Processing Technol. 2010, 91, 290–295. [Google Scholar]
- Jia, L.; Tan, Y.; Anthony, E.J. Oxy-emissions of SO2 and NOx during fuel CFB combustion tests in a mini-circulating fluidized bed combustion reactor. Energy Fuel 2010, 24, 910–915. [Google Scholar] [CrossRef]
- Ahn, J.; Okerlund, R.; Fry, A.; Eddings, E.G. Sulfur trioxide formation during oxy-coal combustion. Int. J. Greenh. Gas Control 2011, 5, S127–S135. [Google Scholar] [CrossRef]
- Krzywanski, J.; Czakiert, T.; Muskala, W.; Sekret, R.; Nowak, W. Modeling of solid fuels combustion in oxygen-enriched atmosphere in circulating fluidized bed boiler: Part 1. The mathematical model of fuel combustion in oxygen-enriched CFB environment. Fuel Processing Technol. 2010, 91, 290–295. [Google Scholar] [CrossRef]
- Normann, F.; Andersson, K.; Leckner, B.; Johnsson, F. Emission control of nitrogen oxides in the oxy-fuel process. Prog. Energy Combust. Sci. 2009, 35, 385–397. [Google Scholar] [CrossRef]
- Meng, X.; Rokni, E.; Zhou, W.; Qi, H.; Sun, R.; Levendis, Y.A. Emissions from oxy-combustion of raw and torrefied biomass. J. Energy Resour. Technol. 2020, 142, 122307. [Google Scholar] [CrossRef]
- Allen, D.; Hayhurst, A.N. The chemical reactions of nitric oxide with solid carbon and catalytically with gaseous carbon monoxide. Fuel 2015, 142, 260–267. [Google Scholar] [CrossRef]


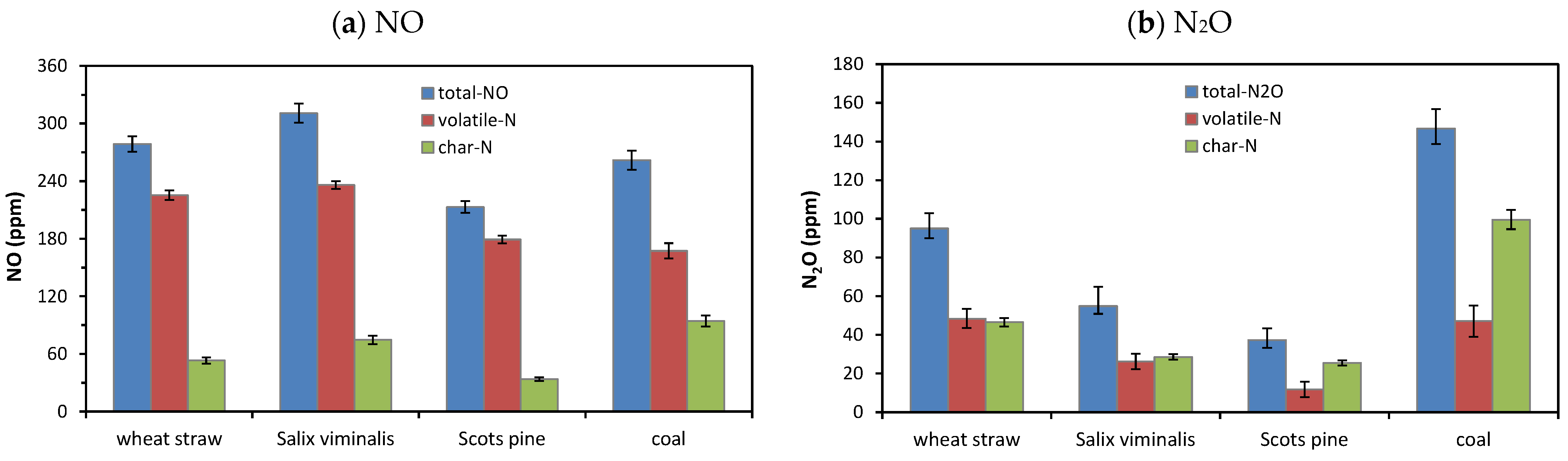



| Wheat Straw | Salix viminalis | Scots Pine | Bituminous Coal [21] | |
|---|---|---|---|---|
| Proximate analysis (air-dry basis), wt.% | ||||
| Moisture (M) | 8.4 | 6.9 | 7.0 | 8.7 |
| Ash yield (A) | 6.1 | 1.4 | 0.6 | 18.9 |
| Volatile matter (VM) | 68.3 | 76.3 | 76.8 | 26.8 |
| Fixed carbon (FC), (by difference) | 17.2 | 15.4 | 15.6 | 45.6 |
| Higher heating value (HHV), MJ/kg | 17.84 | 18.20 | 18.90 | 22.75 |
| Ultimate analysis (dry, ash-free basis), wt.% | ||||
| Carbon (C) | 50.20 | 49.60 | 50.90 | 73.30 |
| Hydrogen (H) | 5.80 | 6.00 | 5.70 | 4.30 |
| Sulphur (S) | 0.08 | 0.03 | 0.01 | 2.30 |
| Nitrogen (N) | 0.80 | 0.30 | 0.10 | 1.10 |
| Chlorine (Cl) | 0.15 | 0.01 | 0.01 | 0.70 |
| Oxygen (O), (by difference) | 42.97 | 44.06 | 43.28 | 18.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowska-Golachowska, M.; Luckos, A.; Kijo-Kleczkowska, A. Pollutant Emissions during Oxy-Fuel Combustion of Biomass in a Bench Scale CFB Combustor. Energies 2022, 15, 706. https://doi.org/10.3390/en15030706
Kosowska-Golachowska M, Luckos A, Kijo-Kleczkowska A. Pollutant Emissions during Oxy-Fuel Combustion of Biomass in a Bench Scale CFB Combustor. Energies. 2022; 15(3):706. https://doi.org/10.3390/en15030706
Chicago/Turabian StyleKosowska-Golachowska, Monika, Adam Luckos, and Agnieszka Kijo-Kleczkowska. 2022. "Pollutant Emissions during Oxy-Fuel Combustion of Biomass in a Bench Scale CFB Combustor" Energies 15, no. 3: 706. https://doi.org/10.3390/en15030706
APA StyleKosowska-Golachowska, M., Luckos, A., & Kijo-Kleczkowska, A. (2022). Pollutant Emissions during Oxy-Fuel Combustion of Biomass in a Bench Scale CFB Combustor. Energies, 15(3), 706. https://doi.org/10.3390/en15030706