Laboratory Studies on Permeability of Coals Using Briquettes: Understanding Underground Storage of CO2
Abstract
:1. Introduction
2. Measuring Apparatus
- The confining pressure regulation in the range of 0.1–40 MPa, and the stabilization accuracy equal to ±0.02 MPa;
- The gas pressure regulation at the sample inlet and outlet in the range of 0.1–1.6 MPa and 0.1–1.0 MPa, respectively, and the stabilization accuracy of 0.12% of the full scale;
- The flow rate measurement at the outlet of the sample in the range of 0–5 cm3/min, and the accuracy of 1.0% of the full scale.
3. Research Material
4. Methodology
5. Results
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Godage, N.; Gionfriddo, E. Use of natural sorbents as alternative and green extractive materials: A critical review. Anal. Chim. Acta 2020, 1125, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, M.; Pajdak, A.; Baran, P.; Zarębska, K. Isosteric heat of sorption of methane on selected hard coals. Przemysł Chem. 2019, 98, 625–629. [Google Scholar]
- Skiba, M.; Młynarczuk, M. Estimation of coal’s sorption parameters using artificial neural networks. Materials 2020, 13, 5422. [Google Scholar] [CrossRef] [PubMed]
- Godec, M.; Koperna, G.; Gale, J. CO2-ECBM: A Review of its Status and Global Potential. Energy Procedia 2014, 63, 5858–5869. [Google Scholar] [CrossRef] [Green Version]
- Gabruś, E.; Wojtacha-Rychter, K.; Aleksandrzak, T.; Smoliński, A.; Król, M. The feasibility of CO2 emission reduction by adsorptive storage on Polish hard coals in the Upper Silesia Coal Basin: An experimental and modeling study of equilibrium, kinetics and thermodynamics. Sci. Total Environ. 2021, 796, 149064. [Google Scholar] [CrossRef]
- Arrhenius, S. On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground. Philos. Mag. J. Sci. 1896, 41, 237–276. [Google Scholar] [CrossRef] [Green Version]
- Stephens, J.C. Growing interest in carbon capture and storage (CCS) for climate change mitigation. Sustain. Sci. Pract. Policy 2006, 2, 4–13. [Google Scholar] [CrossRef]
- Fulton, P.F.; Parente, C.A.; Rogers, B.A.; Shah, N.; Reznik, A.A. A laboratory investigation of enhanced recovery of methane from coal by carbon dioxide injection. In SPE Unconventional Gas Recovery Symposium; Society of Petroleum Engineers: Pittsburgh, PA, USA, 1980; pp. 65–72. [Google Scholar]
- Reznik, A.; Singh, P.; Foley, W. Enhanced Recovery of In Situ Methane by Carbon Dioxide Injection: An Experimental Feasibility Study; Chemical and Petroleum Engineering Department, University of Pittsburgh: Pittsburgh, PA, USA, 1982. [Google Scholar]
- Pajdak, A.; Kudasik, M.; Skoczylas, N.; Wierzbicki, M.; Braga, L.T.P.B. Studies on the competitive sorption of CO2 and CH4 on hard coal. Int. J. Greenh. Gas Control 2019, 90, 102789. [Google Scholar] [CrossRef]
- Krooss, B.M.; van Bergen, F.; Gensterblum, Y.; Siemons, N.; Pagnier, H.J.M.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Mazumder, S.; Wolf, K.H. Differential swelling and permeability change of coal in response to CO2 injection for ECBM. Int. J. Coal Geol. 2008, 74, 123–138. [Google Scholar] [CrossRef]
- Skoczylas, N.; Pajdak, A.; Kudasik, M.; Braga, L.T.P. CH4 and CO2 sorption and diffusion carried out in various temperatures on hard coal samples of various degrees of coalification. J. Nat. Gas Sci. Eng. 2020, 81, 103449. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4 and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Wolf, K.H.A.A.; Siemons, N.; Bruining, J. Multiphase flow experiments in order to understand the behavior of (partly) saturated coals as a gas reservoir: Examples. Geol. Belg. 2004, 7, 115–121. [Google Scholar]
- Yu, H.; Yuan, J.; Guo, W.; Cheng, J.; Hu, Q. A preliminary laboratory experiment on coalbed methane displacement with carbon dioxide injection. Int. J. Coal Geol. 2008, 73, 156–166. [Google Scholar] [CrossRef]
- Baran, P.; Rogozińska, J.; Zarębska, K.; Porada, S. Analysis of the coal-gas system for intensification of methane recovery with carbon dioxide. Przemysł Chem. 2014, 93, 2008–2012. [Google Scholar]
- Baran, P.; Zarębska, K.; Krzystolik, P.; Hadro, J.; Nunn, A. CO2-ECBM and CO2 Sequestration in Polish Coal Seam—Experimental Study. J. Sustain. Min. 2014, 13, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.; Tang, G.Q.; Kovscek, A.R. Laboratory and simulation investigation of enhanced coalbed methane recovery by gas injection. Transp. Porous Media 2008, 73, 141–159. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Wu, D.; Dusseault, M. Experiments on methane displacement by carbon dioxide in large coal specimens. Rock Mech. Rock Eng. 2011, 44, 579–589. [Google Scholar] [CrossRef]
- Bhowmik, S.; Dutta, P. Investigation into the methane displacement behavior by cyclic, pure carbon dioxide injection in dry, powdered, bituminous Indian coals. Energy Fuels 2011, 25, 2730–2740. [Google Scholar] [CrossRef]
- Lutynski, M.A.; Battistutta, E.; Bruining, H.; Wolf, K.H.A.A. Discrepancies in the assessment of CO2 storage capacity and methane recovery from coal with selected equations of state Part I. Experimental isotherm calculation. Physicochem. Probl. Miner. Process. 2011, 47, 159–168. [Google Scholar]
- Dutka, B.; Kudasik, M.; Topolnicki, J. Pore pressure changes accompanying exchange sorption of CO2/CH4 in a coal briquette. Fuel Process. Technol. 2012, 100, 30–34. [Google Scholar] [CrossRef]
- Dutka, B.; Kudasik, M.; Pokryszka, Z.; Skoczylas, N.; Topolnicki, J.; Wierzbicki, M. Balance of CO2/CH4 exchange sorption in a coal briquette. Fuel Process. Technol. 2013, 106, 95–101. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhu, Z.H.; Massarotto, P.; Rudolph, V. Mass transfer in coal seams for CO2 sequestration. Am. Inst. Chem. Eng. J. 2007, 53, 1028–1049. [Google Scholar] [CrossRef]
- Li, X.; Fang, Z. Current status and technical challenges of CO2 storage in coal seams and enhanced coalbed methane recovery: An overview. Int. J. Coal Sci. Technol. 2014, 1, 93–102. [Google Scholar] [CrossRef] [Green Version]
- International Energy Agency. 20 Years of Carbon Capture and Storage: Accelerating Future Deployment; IEA: Paris, France, 2016. [Google Scholar] [CrossRef]
- Sloss, L.L. Potential for Enhanced Coalbed Methane Recovery; CCC/252.; IEA Clean Coal Centre: London, UK, 2015. [Google Scholar]
- Kedzior, S. The occurrence of a secondary zone of coal-bed methane in the southern part of the Upper Silesian Coal Basin (southern Poland): Potential for methane exploitation. Int. J. Coal Geol. 2011, 86, 157–168. [Google Scholar] [CrossRef]
- Siemek, J.; Łukańko, Ł.; Macuda, J.; Maruta, M. Impact of hydraulic fracturing operations of coal seams on the acoustic climate. Arch. Min. Sci. 2019, 64, 51–64. [Google Scholar]
- Kudasik, M. Investigating Permeability of Coal Samples of Various Porosities under Stress Conditions. Energies 2019, 12, 762. [Google Scholar] [CrossRef] [Green Version]
- Braga, L.T.P.; Kudasik, M. Permeability measurements of raw and briquette coal of various porosities at different temperatures. Mater. Res. Express 2019, 6, 105609. [Google Scholar] [CrossRef]
- Durucan, S.; Edwards, J.S. The effects of stress and fracturing on permeability of coal. Min. Sci. Technol. 1986, 3, 205–216. [Google Scholar] [CrossRef]
- Huy, P.Q.; Sasaki, K.; Sugai, Y.; Ichikawa, S. Carbon dioxide gas permeability of coal core samples and estimation of fracture aperture width. Int. J. Coal Geol. 2010, 83, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Tang, D.; Xu, H.; Meng, Y.; Li, J. Experimental research on coal permeability: The roles of effective stress and gas slippage. J. Nat. Gas Sci. Eng. 2014, 21, 481–488. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, Z.; Liu, J.; Connell, L.D.; Elsworth, D. Effect of the effective stress coefficient and sorption-induced strain on the evolution of coal permeability: Experimental observations. Int. J. Greenh. Gas Control 2011, 5, 1284–1293. [Google Scholar] [CrossRef]
- Konecny, P.; Kozusnikova, A. Influence of stress on the permeability of coal and sedimentary rocks of the Upper Silesian basin. Int. J. Rock Mech. Min. Sci. 2011, 48, 347–352. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K. Coal cleat permeability for gas movement under triaxial, non-zero lateral strain condition: Atheoretical and experimental study. Fuel 2013, 109, 389–399. [Google Scholar] [CrossRef]
- Zou, J.; Chen, W.; Yang, D.; Yu, H.; Yuan, J. The impact of effective stress and gas slippage on coal permeability under cyclic loading. J. Nat. Gas Sci. Eng. 2016, 31, 236–248. [Google Scholar] [CrossRef]
- Yin, G.; Jiang, C.; Wang, J.G.; Xu, J. Combined Effect of Stress, Pore Pressure and Temperature on Methane Permeability in Anthracite Coal: An Experimental Study. Transp. Porous Media 2013, 100, 1–16. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Lai, F. Experimental study on porosity and permeability of anthracite coal under different stresses. J. Pet. Sci. Eng. 2015, 133, 810–817. [Google Scholar] [CrossRef]
- Wang, G.; Qin, Y.; Shen, J.; Chen, S.; Han, B.; Zhou, X. Dynamic-Change Laws of the Porosity and Permeability of Low- to Medium-Rank Coals under Heating and Pressurization Treatments in the Eastern Junggar Basin, China. J. Earth Sci. 2018, 29, 607–615. [Google Scholar] [CrossRef]
- Zhou, D.; Li, K.; Wang, H.; Jin, Y.; Ru, Z.; Xu, X.; Wu, C.; Jian, K. Permeability experiments with overburden pressure of coal measure reservoirs and numerical analysis of its stress sensitivity: A case study of Qinshui Basin, China. Energy Explor. Exploit. 2020, 38, 1690–1705. [Google Scholar] [CrossRef]
- Shilova, T.; Serdyukov, S. Permeability of Coking Coals and Patterns of Its Change in Leninsky Area, Kuznetsk Coal Basin, Russia. Appl. Sci. 2021, 11, 3969. [Google Scholar] [CrossRef]
- Kumar, H.; Mishra, M.K.; Mishra, S. Laboratory investigation of gas permeability and its impact on CBM potential. J. Pet. Explor. Prod. Technol. 2017, 8, 1183–1197. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, D.; Cai, Y.; Yao, Y. Fracture permeability evaluation of a coal reservoir using geophysical logging: A case study in the Zhengzhuang area, southern Qinshui Basin. Energy Explor. Exploit. 2016, 34, 378–399. [Google Scholar] [CrossRef] [Green Version]
- Somerton, W.H.; Söylemezoglu, I.M.; Dudley, R.C. Effect of stress on permeability of coal. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1975, 12, 129–145. [Google Scholar] [CrossRef]
- Kudasik, M.; Skoczylas, N.; Pajdak, A. Innovative apparatus for testing filtration, sorption and CO2/CH4 exchange sorption processes under isobaric conditions on sorbent subjected to confining pressure, in terms of laboratory tests of CO2-ECBM technology. Sensors 2020, 20, 5823. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.; da Costa, L.F.; Garcia, C.; Juan, R.; de Sousa, M.L.; Marques, M.; Pinheiro, H.J.; Rodrigues, C.; Ruiz, M.C. International Classification of In-Seam Coal; UN-ECE ENERGY/1998/50; UN ECE: Geneva, Switzerland, 1998. [Google Scholar]
- Skoczylas, N.; Dutka, B.; Sobczyk, J. Mechanical and gaseous properties of coal briquettes in terms of outburst risk. Fuel 2014, 134, 45–52. [Google Scholar] [CrossRef]
- Scheidegger, A.E. The Physics of Flow through Porous Media, 3rd ed.; University of Toronto Press: Toronto, ON, Canada, 1974. [Google Scholar]
- Klinkenberg, L.J. The permeability of porous media to liquids and gases. In Proceedings of the API Drilling and Production Practice, New York, NY, USA, 1 January 1941; pp. 200–213. [Google Scholar]
- Abuamarah, B.A.; Nabawy, B.S.; Shehata, A.M.; Kassem, O.M.K.; Ghrefat, H. Integrated geological and petrophysical characterization of oligocene deep marine unconventional poor to tight sandstone gas reservoir. Mar. Pet. Geol. 2019, 109, 868–885. [Google Scholar] [CrossRef]
- Gawor, M.; Skoczylas, N. Sorption Rate of Carbon Dioxide on Coal. Transp. Porous Media 2014, 101, 269–279. [Google Scholar] [CrossRef] [Green Version]

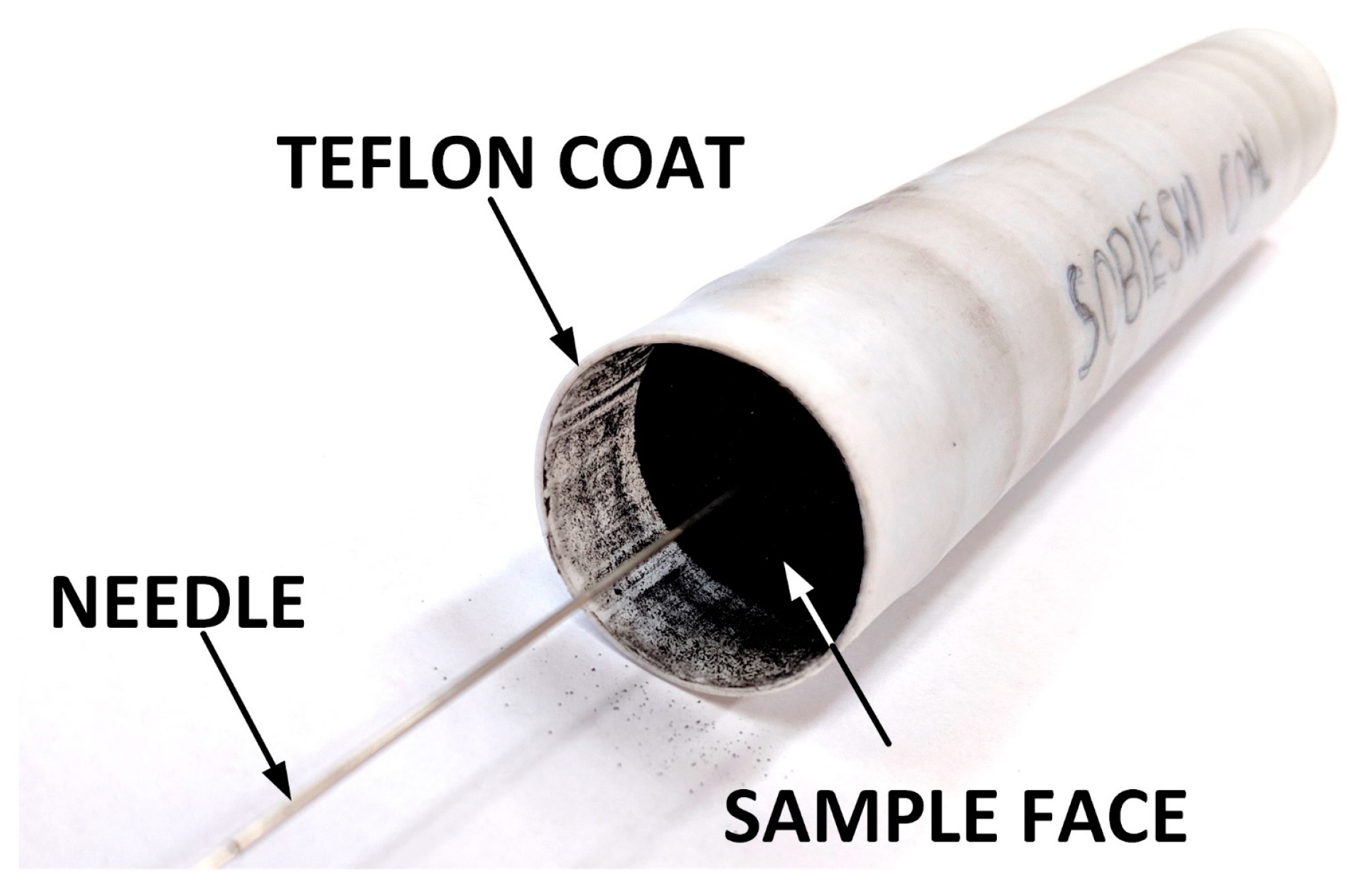
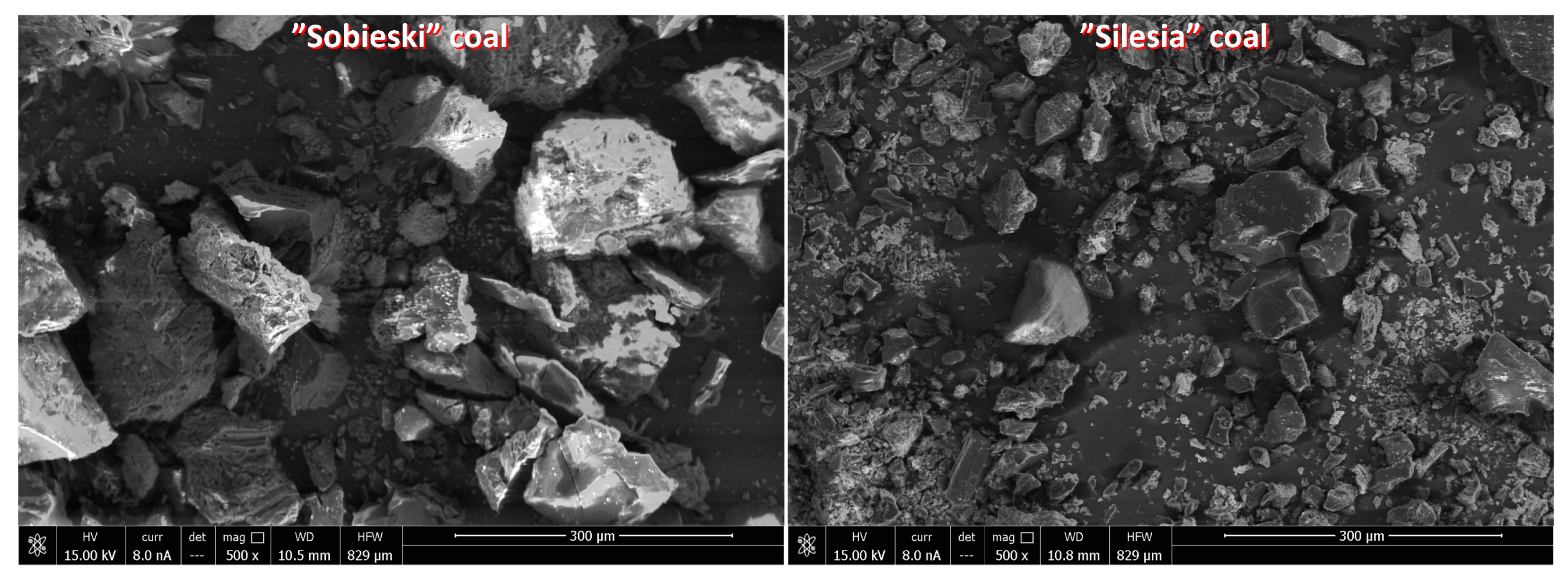
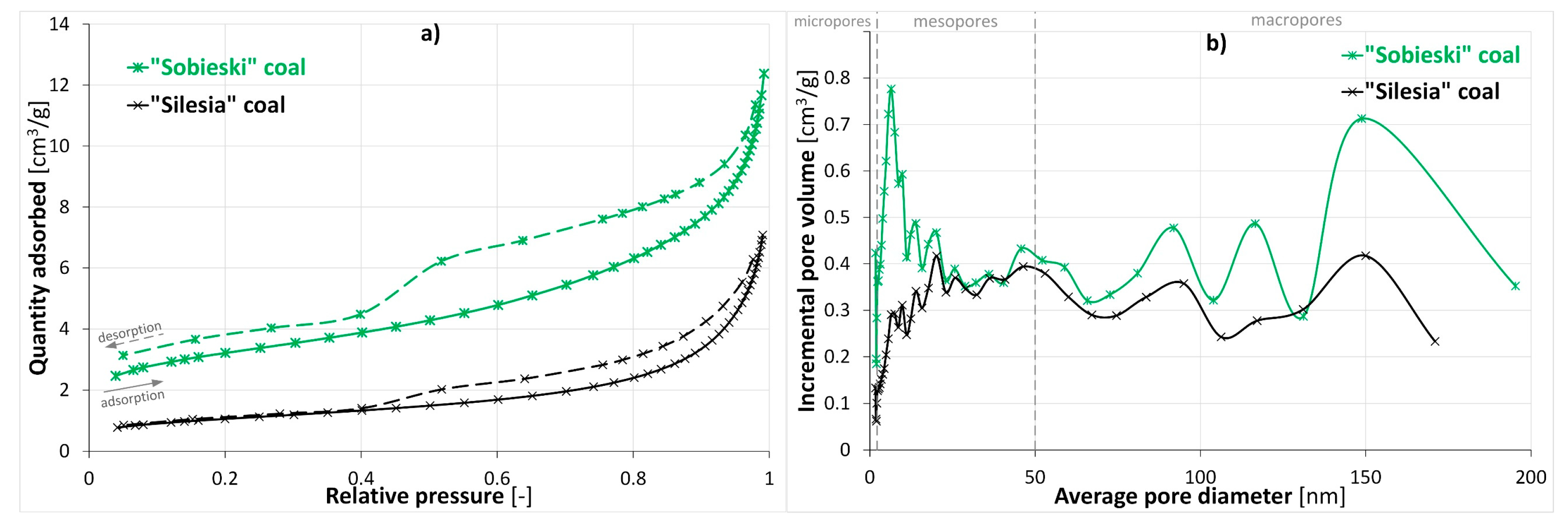



| Origin | Coal Rank | Ro [%] | Vdaf [%] | Ad [%] | Wt [%] | ρsk [g/cm3] | ϕ [%] |
|---|---|---|---|---|---|---|---|
| Sobieski mine | medium-rank D Para-bituminous coal | 0.565 | 39.63 | 8.41 | 5.35 | 1.410 | 32.3 |
| Silesia mine | medium-rank C Ortho-bituminous coal | 0.678 | 39.32 | 12.00 | 2.65 | 1.411 | 24.3 |
| Coal Sample | Maximum Quantity Adsorbed [cm3/g] | BET Surface Area [m2/g] | Langmuir Surface Area [m2/g] | BJH Adsorption Cumulative Volume of Pores [cm3/g] |
|---|---|---|---|---|
| “Sobieski” | 12.37 | 10.92 | 16.49 | 0.019 |
| “Silesia” | 7.08 | 3.72 | 5.71 | 0.011 |
| Sample | Confining Pressure [MPa] | Klinkenberg Permeability Coefficients in Relation to: | |||
|---|---|---|---|---|---|
| He | CO2 | ||||
| “Sobieski” | 1.5 | 81.032 | 0.004 | 71.696 | 0.003 |
| 10 | 33.798 | 0.027 | 23.435 | 0.049 | |
| 20 | 26.029 | 0.029 | 18.563 | 0.040 | |
| 30 | 21.902 | 0.023 | 16.218 | 0.033 | |
| “Silesia” | 1.5 | 6.615 | 0.005 | 2.038 | 0.020 |
| 10 | 2.248 | 0.039 | 0.657 | 0.011 | |
| 20 | 1.413 | 0.030 | 0.427 | 0.011 | |
| 30 | 0.758 | 0.020 | 0.258 | 0.016 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudasik, M.; Skoczylas, N.; Braga, L.T.P. Laboratory Studies on Permeability of Coals Using Briquettes: Understanding Underground Storage of CO2. Energies 2022, 15, 715. https://doi.org/10.3390/en15030715
Kudasik M, Skoczylas N, Braga LTP. Laboratory Studies on Permeability of Coals Using Briquettes: Understanding Underground Storage of CO2. Energies. 2022; 15(3):715. https://doi.org/10.3390/en15030715
Chicago/Turabian StyleKudasik, Mateusz, Norbert Skoczylas, and Letícia Teixeira Palla Braga. 2022. "Laboratory Studies on Permeability of Coals Using Briquettes: Understanding Underground Storage of CO2" Energies 15, no. 3: 715. https://doi.org/10.3390/en15030715






