Study of Parameters and Theory of Sucrose Dust Explosion
Abstract
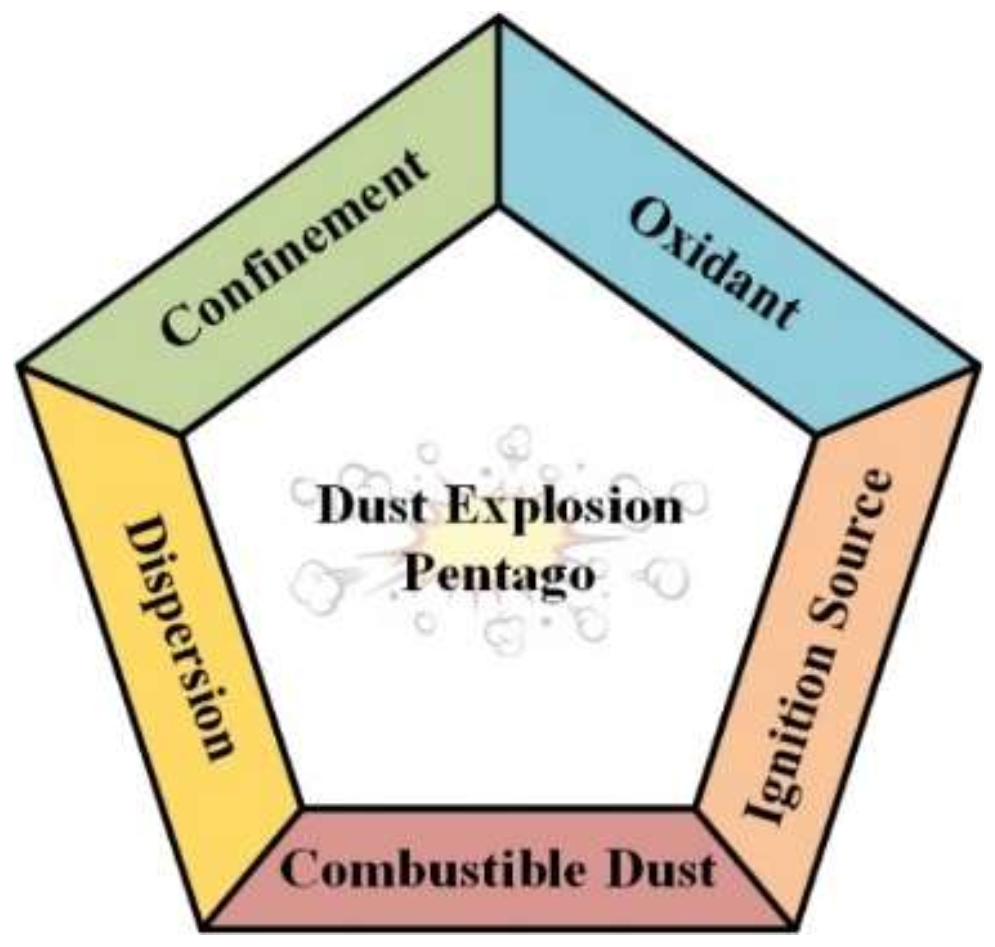
:1. Introduction
2. Experimental Devices and Sample
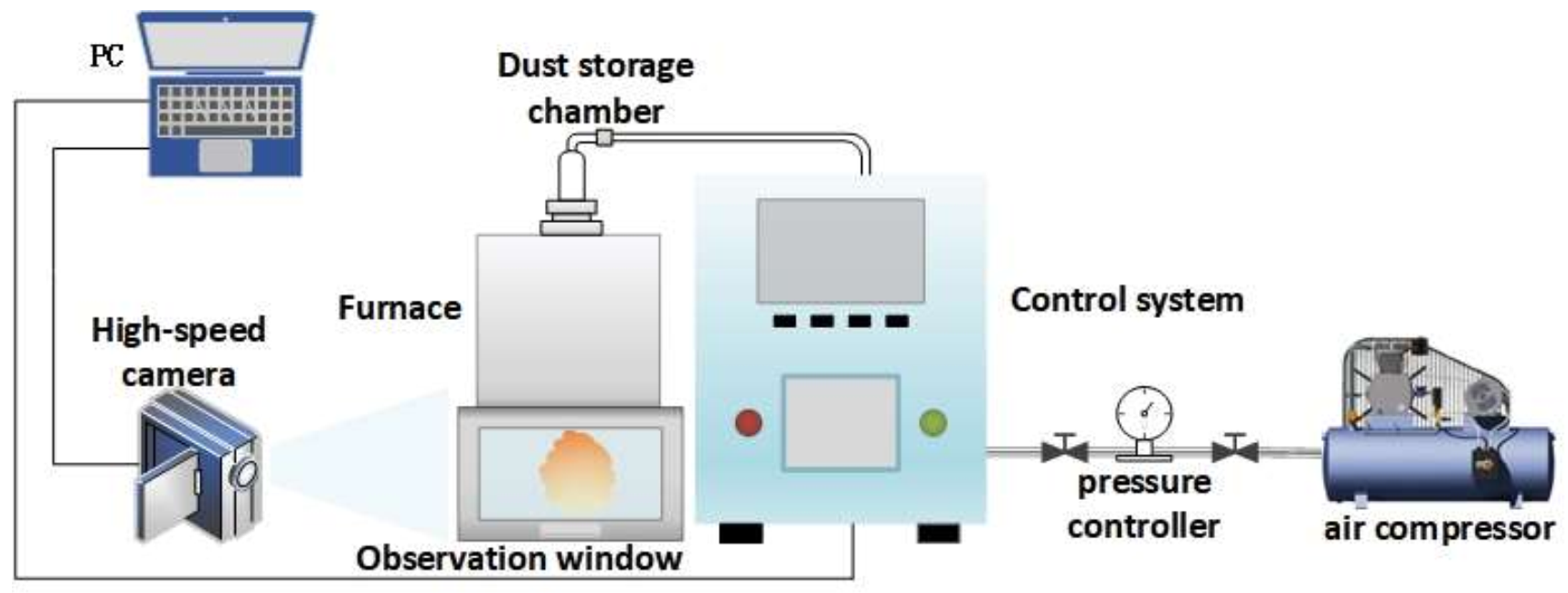
2.1. Experimental Device of MIE of Dust Cloud
2.2. Experimental Device of MIT of Powder Cloud
2.3. Experimental Materials
3. Results and Discussion
3.1. Effect of Parameters on MIE of Sucrose Dust Cloud
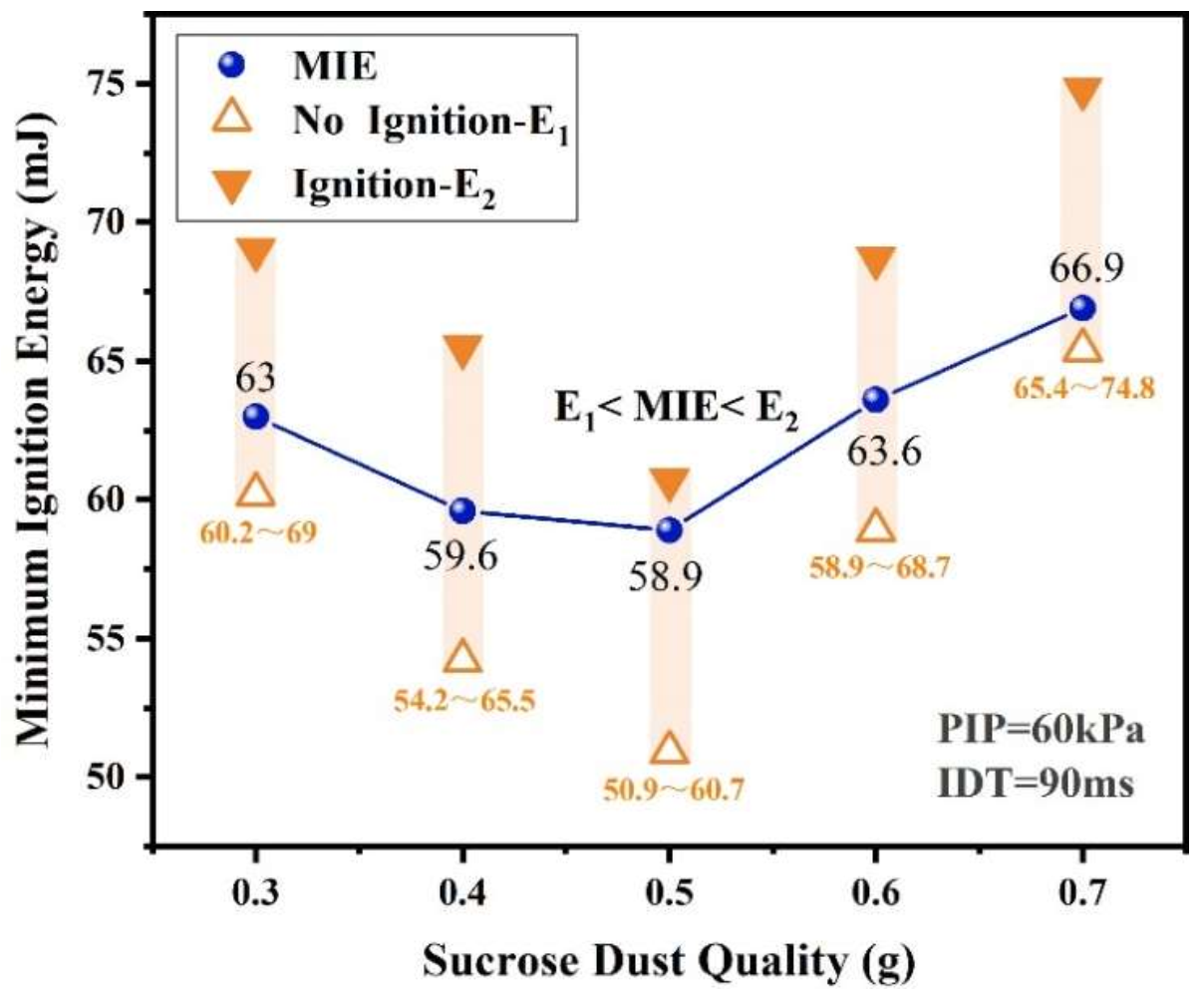
3.1.1. Effect of Quantity of Dust on MIE
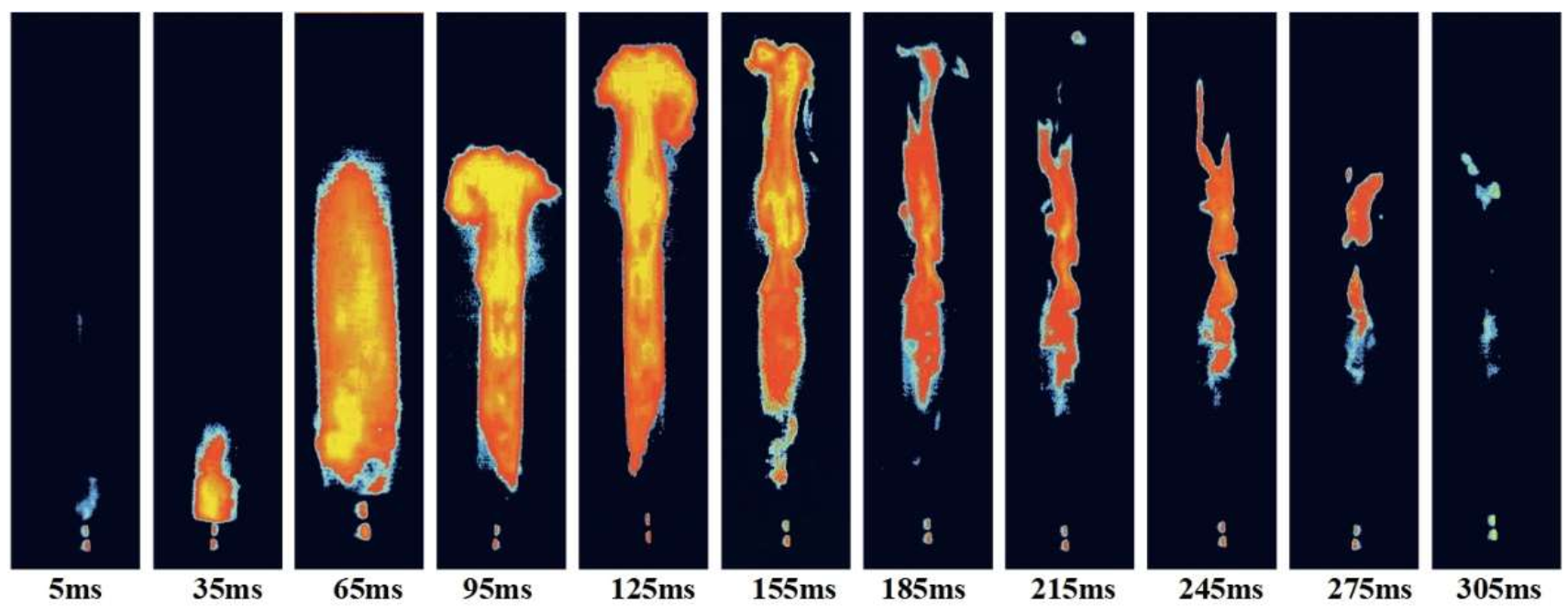
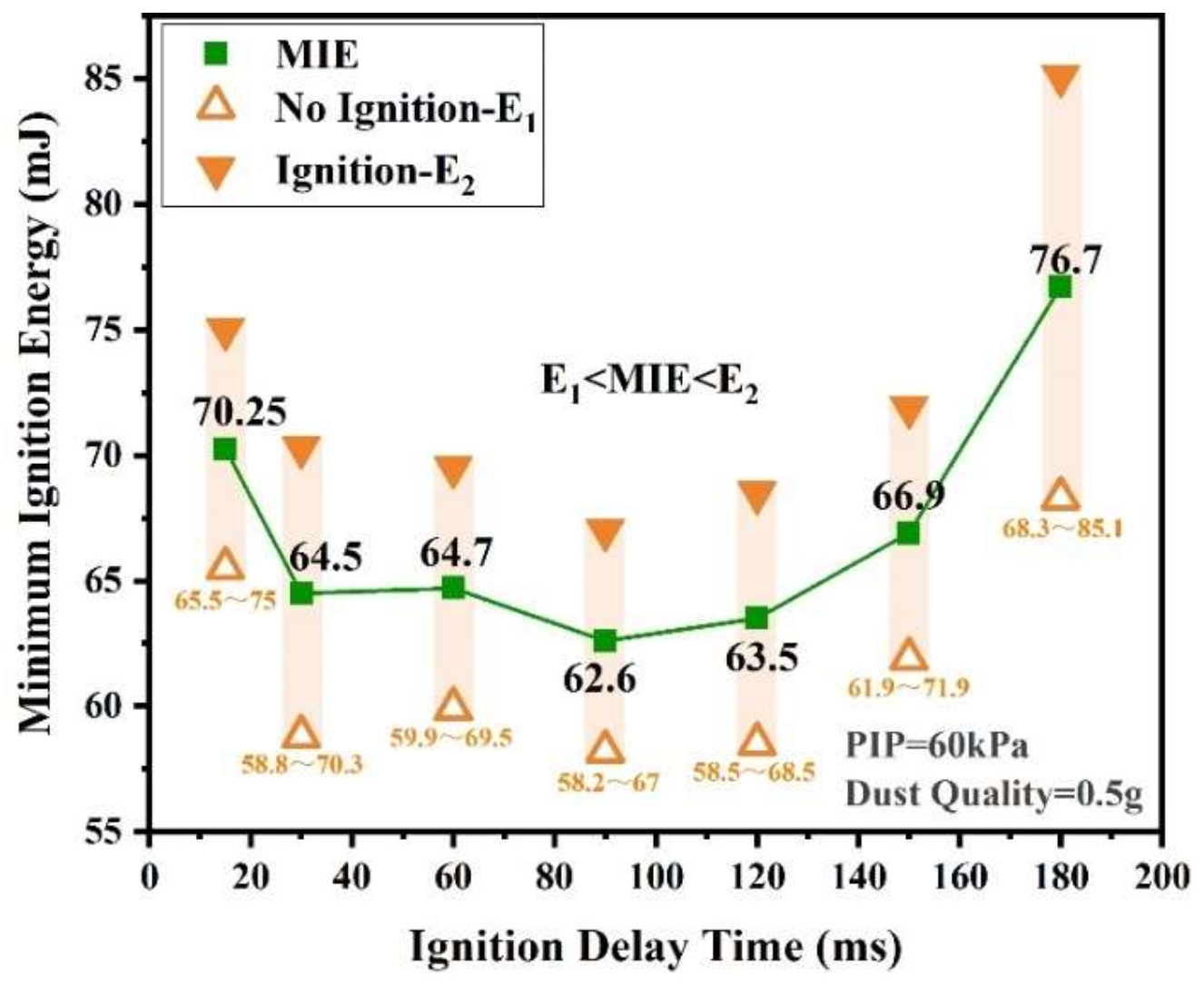
3.1.2. The Relationship between IDT and MIE of Sucrose Dust
3.1.3. The Relationship of PIP and MIE of Sucrose Dust
3.2. Effect of Parameters on MIT of Sucrose Powder Cloud
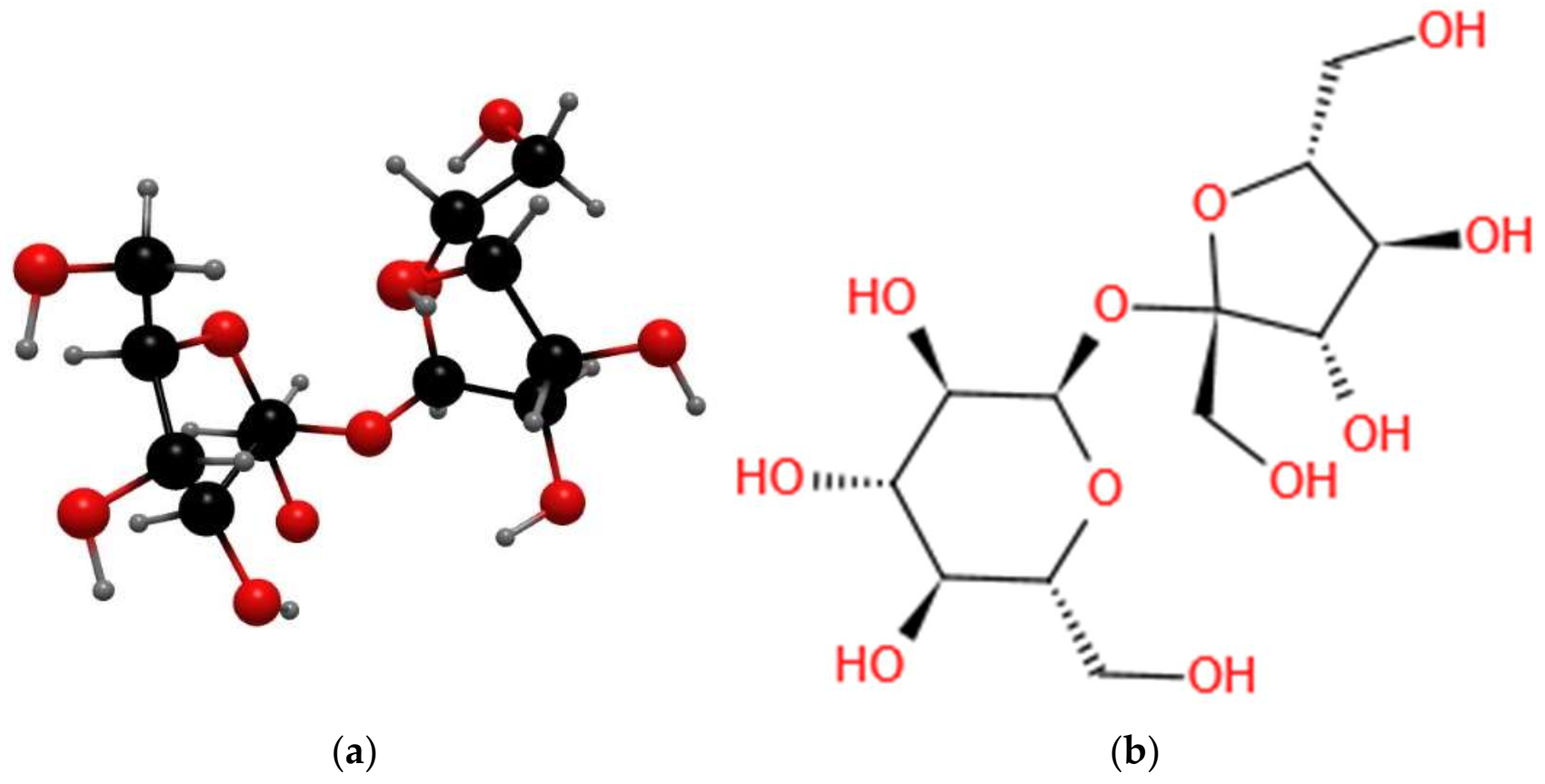
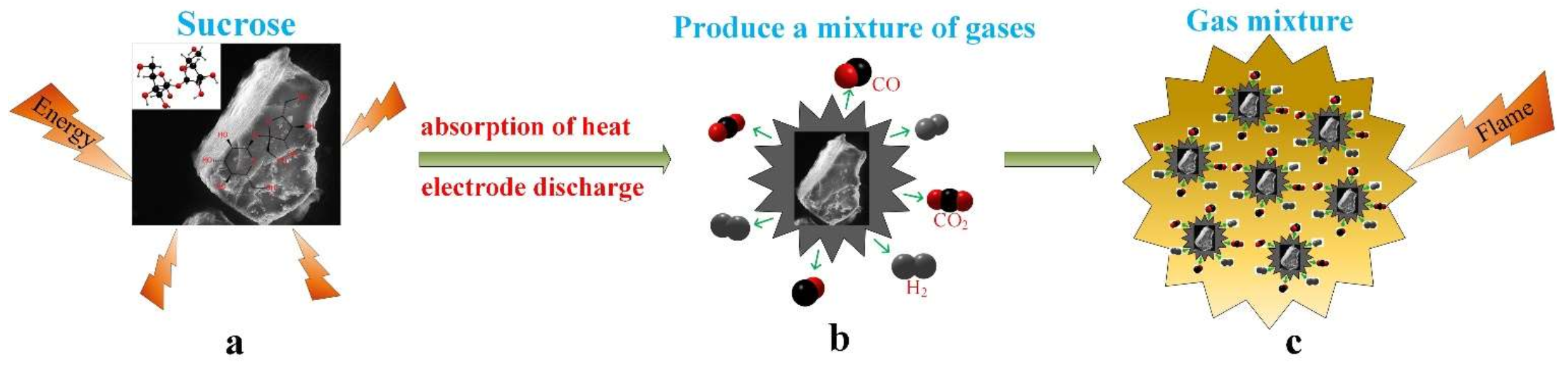
3.3. Theory of Broken Hydroxyl “O–H” Bonds in the Sucrose Dust Molecule
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, W.; Zhang, X.; Zhang, D.; Peng, Q.; Zhang, Q.; Dobashi, R. Flame propagation behaviours in nano-metal dust explosions. Powder Technol. 2017, 321, 154–162. [Google Scholar] [CrossRef]
- Yuan, Z.; Khakzad, N.; Khan, F.; Amyotte, P. Dust explosions: A threat to the process industries. Process Saf. Environ. Protect. 2015, 98, 57–71. [Google Scholar] [CrossRef]
- Adamski, R.; Siuta, D.; Kukfisz, B.; Frydrysiak, M.; Prochoń, M. Integration of Safety Aspects in Modeling of Superheated Steam Flash Drying of Tobacco. Energies 2021, 14, 5927. [Google Scholar] [CrossRef]
- Youssefi, R.; Segers, T.; Norman, F.; Maier, J.; Scheffknecht, G. Experimental Investigations of the Ignitability of Several Coal Dust Qualities. Energies 2021, 14, 6323. [Google Scholar] [CrossRef]
- Vorderbrueggen, J.B. Imperial sugar refinery combustible dust explosion investigation. Process Saf. Prog. 2011, 30, 66–81. [Google Scholar] [CrossRef]
- Malizia, A.; Poggi, L.; Ciparisse, J.-F.; Rossi, R.; Bellecci, C.; Gaudio, P. A Review of Dangerous Dust in Fusion Reactors: From Its Creation to Its Resuspension in Case of LOCA and LOVA. Energies 2016, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Kuracina, R.; Szabová, Z.; Menčík, M. Determination of Explosion Characteristics of Sugar Dust Clouds. Trans. VSB-Tech. Univ. Ostrava Saf. Eng. Ser. 2018, 13, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Berghmans, J.; Degreve, J.; Verplaetsen, F. A model for the minimum ignition energy of dust clouds. Process Saf. Environ. Protect. 2019, 121, 43–49. [Google Scholar] [CrossRef]
- Saletnik, B.; Bajcar, M.; Saletnik, A.; Zaguła, G.; Puchalski, C. Effect of the Pyrolysis Process Applied to Waste Branches Biomass from Fruit Trees on the Calorific Value of the Biochar and Dust Explosivity. Energies 2021, 14, 5898. [Google Scholar] [CrossRef]
- Bagaria, P.; Zhang, J.; Mashuga, C. Effect of dust dispersion on particle breakage and size distribution in the minimum ignition energy apparatus. J. Loss Prev. Process Ind. 2018, 56, 518–523. [Google Scholar] [CrossRef]
- Mishra, D.P.; Azam, S. Experimental investigation on effects of particle size, dust concentration and dust-dispersion-air pressure on minimum ignition temperature and combustion process of coal dust clouds in a G-G furnace. Fuel 2018, 227, 424–433. [Google Scholar] [CrossRef]
- Choi, K.; Kato, T.; Kim, W. Experimental study on the electrostatic characteristics of L-isoleucine powder. Powder Technol. 2019, 347, 125–129. [Google Scholar] [CrossRef]
- Gan, B.; Gao, W.; Jiang, H.; Li, Y.; Zhang, Q.; Bi, M. Flame propagation behaviors and temperature characteristics in polyethylene dust explosions. Powder Technol. 2018, 328, 345–357. [Google Scholar] [CrossRef]
- Bu, Y.; Yuan, C.; Amyotte, P.; Li, C.; Cai, J.; Li, G. Ignition hazard of non-metallic dust clouds exposed to hotspots versus electrical sparks. J. Hazard. Mater. 2019, 365, 895–904. [Google Scholar] [CrossRef]
- Cao, Y.; Su, H.; Ge, L.; Li, Y.; Wang, Y.; Xie, L.; Li, B. Ignition sensitivity and flame propagation of zirconium powder clouds. J. Hazard. Mater. 2019, 365, 413–420. [Google Scholar] [CrossRef]
- Han, H.; Chaudhari, P.; Bagaria, P.; Mashuga, C. Novel method for hybrid gas-dust cloud ignition using a modified standard minimum ignition energy device. J. Loss Prev. Process Ind. 2018, 52, 108–112. [Google Scholar] [CrossRef]
- Song, X.; Su, H.; Xie, L.; Li, B.; Cao, Y.; Wang, Y. Experimental investigations of the ignition delay time, initial ignition energy and lower explosion limit of zirconium powder clouds in a 20 L cylindrical vessel. Process Saf. Environ. Protect. 2020, 134, 429–439. [Google Scholar] [CrossRef]
- Shu, Y.; Li, Z. Experimental Study of the Violence Intensity Parameters of the Explosion of Micron-sized Zinc Powder. Cent. Eur. J. Energ. Mater. 2019, 16, 607–629. [Google Scholar] [CrossRef]
- Sanchirico, R.; Di Sarli, V.; Di Benedetto, A. Volatile point of dust mixtures and hybrid mixtures. J. Loss Prev. Process Ind. 2018, 56, 370–377. [Google Scholar] [CrossRef]
- Sanchirico, R.; Russo, P.; Di Sarli, V.; Di Benedetto, A. On the explosion and flammability behavior of mixtures of combustible dusts. Process Saf. Environ. Protect. 2015, 94, 410–419. [Google Scholar] [CrossRef]
- Sanchirico, R.; Russo, P.; Saliva, A.; Doussot, A.; Di Sarli, V.; Di Benedetto, A. Explosion of lycopodium-nicotinic acid–methane complex hybrid mixtures. J. Loss Prev. Process Ind. 2015, 36, 505–508. [Google Scholar] [CrossRef]
- Addai, E.K.; Gabel, D.; Krause, U. Experimental investigation on the minimum ignition temperature of hybrid mixtures of dusts and gases or solvents. J. Hazard. Mater. 2016, 301, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.D.; Sarli, V.D.; Russo, P. On the determination of the minimum ignition temperature for dust/air mixtures. Chem. Eng. Trans. 2010, 19, 189–194. [Google Scholar]
- Danzi, E.; Bibbona, E.; Di Benedetto, A.; Sanchirico, R.; Di Sarli, V.; Marmo, L. A statistical approach to determine the autoignition temperature of dust clouds. J. Loss Prev. Process Ind. 2018, 56, 181–190. [Google Scholar] [CrossRef]
- Addai, E.K.; Gabel, D.; Krause, U. Models to estimate the minimum ignition temperature of dusts and hybrid mixtures. J. Hazard. Mater. 2016, 304, 73–83. [Google Scholar] [CrossRef]
- Fu, T.; Tsai, Y.-T.; Zhou, Q. Numerical Simulation of Magnesium Dust Dispersion and Explosion in 20 L Apparatus via an Euler–Lagrange Method. Energies 2022, 15, 402. [Google Scholar] [CrossRef]
- Rybak, W.; Moroń, W.; Wach, J. Ignition Studies on High-Vitrinite and High-Inertinite Coals Using TGA/DSC, DTIF, EFR, and 20 L Dust Explosive Chamber. Energies 2021, 14, 3601. [Google Scholar] [CrossRef]
- EN13821; Potentially Explosive Atmosphere. Explosion Prevention and Protection. Determination of Minimum Ignition Energy of Dust/Air Mixtures. BSI: London, UK, 2002.
- BS 5958-1-1991; Code of Practice for Control of Undesirable Static Electricity. General Considerations. BSI: London, UK, 1991.
- Stahmer, K.-W.; Gerhold, M. Study of the explosion reactions of sucrose, activated charcoal, polyethylene and lignite Part 1: Effect of variation in particle surface area upon explosion reaction. J. Loss Prev. Process Ind. 2017, 46, 177–184. [Google Scholar] [CrossRef]
- Ebadat, V.; Prugh, R.W. Case study: Aluminum-dust explosion. Process Saf. Prog. 2007, 26, 324–329. [Google Scholar] [CrossRef]
- Cashdollar, K.L.; Zlochower, I.A. Explosion temperatures and pressures of metals and other elemental dust clouds. J. Loss Prev. Process Ind. 2007, 20, 337–348. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Dust explosions-cases, causes, consequences, and control. J. Hazard. Mater. 2007, 140, 7–44. [Google Scholar] [CrossRef] [PubMed]
- Amyotte, P.R.; Eckhoff, R.K. Dust explosion causation, prevention and mitigation: An overview. J. Chem. Health Saf. 2010, 17, 15–28. [Google Scholar] [CrossRef]
- Yuan, Z.; Khakzad, N.; Khan, F.; Amyotte, P. Domino effect analysis of dust explosions using Bayesian networks. Process Saf. Environ. Protect. 2016, 100, 108–116. [Google Scholar] [CrossRef]
- Montoya, J.; Pecha, B.; Janna, F.C.; Garcia-Perez, M. Micro-explosion of liquid intermediates during the fast pyrolysis of sucrose and organosolv lignin. J. Anal. Appl. Pyrolysis 2016, 122, 106–121. [Google Scholar] [CrossRef] [Green Version]




| MIE (mJ) | Level of Ignition Sensitivity | Precautions |
|---|---|---|
| 500 | Low sensitivity | Earth plant when ignition energy is at or below this level. |
| 100 | Medium sensitivity | Consider earthing personnel when ignition energy is at or below this level. |
| 25 | Medium to high sensitivity | The hazard from electrostatic discharges from dust clouds should be considered, although the majority of ignition is below this level. |
| 10 | High sensitivity | Take above precautions and consider restrictions on the use of high-resistivity materials (plastics). Electrostatic hazard from bulk powders of high resistivity should be considered. |
| 1 | Extreme sensitivity | Precautions should be as for flammable liquids and gases when ignition energy is at or below this level. |
| Dust Quantity (g) | PIP (kPa) | Particles Size (μm) | MIT (°C) |
|---|---|---|---|
| 0.5 | 50 | 48–74 | 390 |
| 38–47 | 370 | ||
| 25–37 | 340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Li, X.; Liang, S.; Zhong, Y.; Yang, L.; Hao, P.; Soar, J. Study of Parameters and Theory of Sucrose Dust Explosion. Energies 2022, 15, 1439. https://doi.org/10.3390/en15041439
Jiang J, Li X, Liang S, Zhong Y, Yang L, Hao P, Soar J. Study of Parameters and Theory of Sucrose Dust Explosion. Energies. 2022; 15(4):1439. https://doi.org/10.3390/en15041439
Chicago/Turabian StyleJiang, Juju, Xiaoquan Li, Siting Liang, Yuankun Zhong, Lei Yang, Peng Hao, and Jeffrey Soar. 2022. "Study of Parameters and Theory of Sucrose Dust Explosion" Energies 15, no. 4: 1439. https://doi.org/10.3390/en15041439
APA StyleJiang, J., Li, X., Liang, S., Zhong, Y., Yang, L., Hao, P., & Soar, J. (2022). Study of Parameters and Theory of Sucrose Dust Explosion. Energies, 15(4), 1439. https://doi.org/10.3390/en15041439










