Characteristics of the Main- and Side-Stream Products of Microwave Assisted Torrefaction of Lignocellulosic Biomass of Different Origination
Abstract
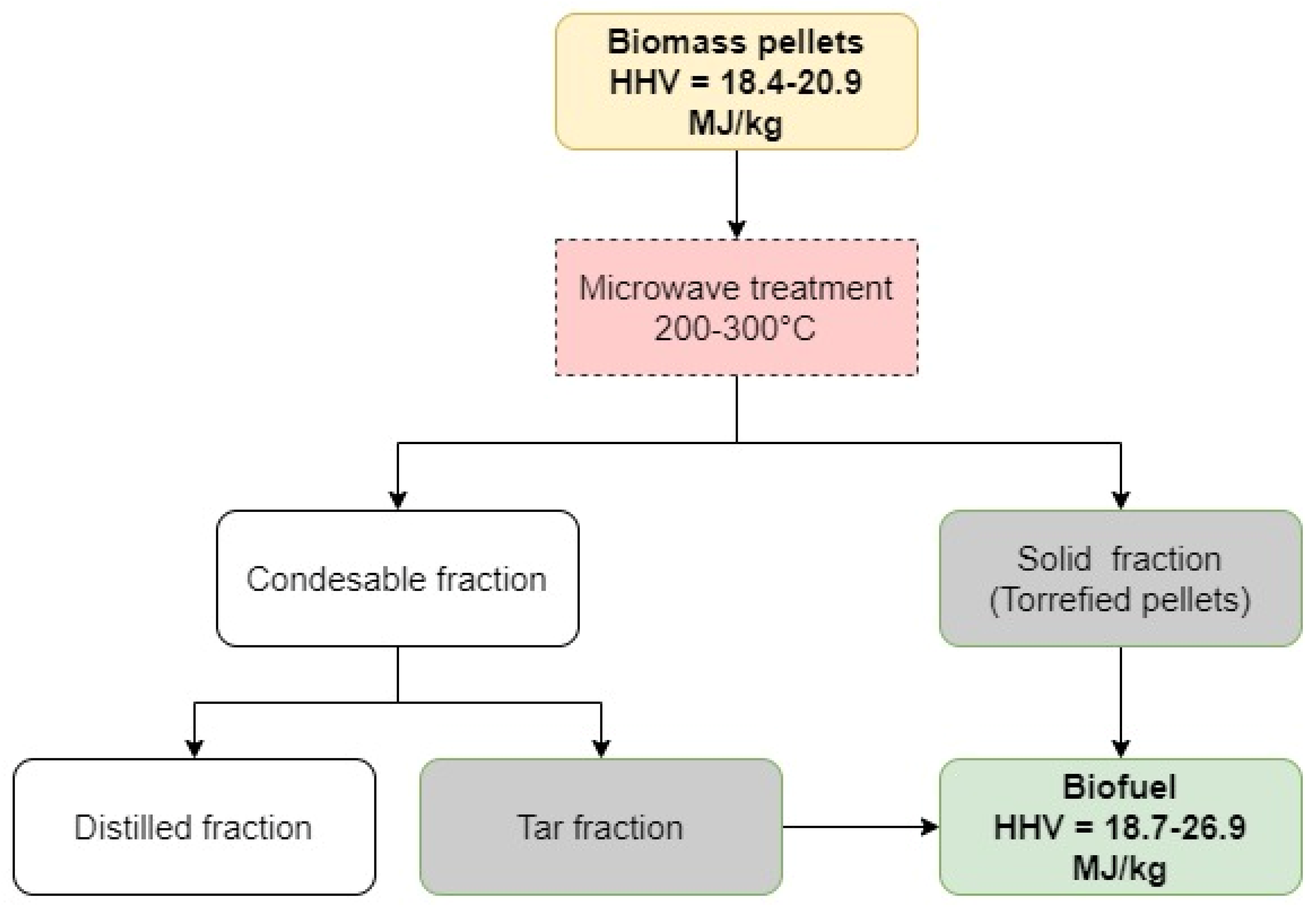
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MW Assisted Torrefaction of Pellets
2.3. Fractionation of Condensable Products
2.4. Analytical Pyrolysis (Py-GC/MS/FID)
2.5. GC/MS/FID
2.6. Potentiometric Titration
2.7. TG/DTG/DSC Analysis
2.8. Elemental Analysis
2.9. Higher Heating Value (HHV)
2.10. Ash Content
3. Results
3.1. Yield of Products of MW Assisted Torrefaction of Plant Biomass of Different Origination
3.2. Separation of Tar from Condensable Fractions and Characteristics of Water Enriched Distillates
3.3. Composition of Solid and Tar Fractions Obtained by MW-Assisted Torrefaction of Different Types of Lignocellulosic Biomass
3.4. Fuel Characteristics of Solid and Tar Fractions Obtained by MW-Assisted Torrefaction of Biomass of Different Origination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Stepping Up Europe’s 2030 Climate Ambition, Investing in a Climate-Neutral Future for the Benefit of Our People; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0562 (accessed on 1 February 2022).
- Calderon, C. Bioenergy Europe Statistical Report; Bioenergy Europe: Brussels, Belgium, 2019; Available online: https://platformduurzamebiobrandstoffen.nl/infotheek/bioenergy-europe-statistical-report-2019/ (accessed on 1 February 2022).
- Jakob, M.; Steckel, J.C.; Jotzo, F.; Sovacool, B.K.; Cornelsen, L.; Chandra, R.; Edenhofer, O.; Holden, C.; Löschel, A.; Nace, T.; et al. The future of coal in a carbon-constrained climate. Nat. Clim. Chang. 2020, 10, 704–707. [Google Scholar] [CrossRef]
- Centre for Research on Energy and Clean Air. Powering Down Coal—COP26’ s Impact on the Global Coal Power. 2020, Volume 590. Available online: https://energyandcleanair.org/wp/wp-content/uploads/2021/11/Glasgow-impact-on-coal.pdf (accessed on 1 February 2022).
- Lang, A.; Bradley, D.; Gauthier, G. Global Bioenergy Statistics, World Bioenergy Association. 2016. Available online: http://www.worldbioenergy.org/uploads/201210%20WBA%20GBS%202020.pdf (accessed on 1 February 2022).
- USDA. USDA Biofuel Annual 2020. In Gain Report; USDA Report E42020-003; USDA: Washington, DC, USA, 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Biofuels%20Annual_The%20Hague_European%20Union_06-29-2020 (accessed on 1 February 2022).
- Nunes, L.J.R.; Matias, J.C.O. Biomass Torrefaction as a Key Driver for the Sustainable Development and Decarbonization of Energy Production. Sustainability 2020, 12, 922. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Batidzirai, B.; Mignot, A.P.R.; Schakel, W.B.; Junginger, H.M.; Faaij, A.P.C. Biomass torrefaction technology: Techno-economic status and future prospects. Energy 2013, 62, 196–214. [Google Scholar] [CrossRef]
- Agar, D.; Wihersaari, M. Bio-coal, torrefied lignocellulosic resources—Key properties for its use in co-firing with fossil coal—Their status. Biomass Bioenergy 2012, 44, 107–111. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef] [Green Version]
- Thrän, D.; Witt, J.; Schaubach, K.; Kiel, J.; Carbo, M.; Maier, J.; Ndibe, C.; Koppejan, J.; Alakangas, E.; Majer, S.; et al. Moving torrefaction towards market introduction: Technical improvements and economic-environmental assessment along the overall torrefaction supply chain through the SECTOR project. Biomass Bioenergy 2016, 89, 184–200. [Google Scholar] [CrossRef] [Green Version]
- Strauss, W. “Black pellets”—A financial analysis of costs and benefits: Can they provide cheaper energy than white pellets? In Globally Respected Consultants in the Wood Pellet Sector; FutureMetrics LLC: New York, NY, USA, 2014; pp. 1–11. [Google Scholar]
- Arpia, A.A.; Chen, W.-H.; Ubando, A.T.; Tabatabaei, M.; Lam, S.S.; Culaba, A.B.; De Luna, M.D.G. Catalytic microwave-assisted torrefaction of sugarcane bagasse with calcium oxide optimized via Taguchi approach: Product characterization and energy analysis. Fuel 2021, 305, 121543. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Akishin, Y.; Zile, E.; Dizhbite, T.; Solodovnik, V.; Telysheva, G. Microwave treatment combined with conventional heating of plant biomass pellets in a rotated reactor as a high rate process for solid biofuel manufacture. Renew. Energy 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Awang Biak, D.R.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Osman, M.S.; Wong, C.C.; Wong, C.S.; Kong, S.H.; Sie, T.S.; Foong, S.Y.; Lam, S.S.; Liew, R.K. Microwave wet torrefaction: A catalytic process to convert waste palm shell into porous biochar. Mater. Sci. Energy Technol. 2020, 3, 742–747. [Google Scholar] [CrossRef]
- Mitani, T. Recent Progress on Microwave Processing of Biomass for Bioenergy Production. J. Jpn. Pet. Inst. 2018, 61, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Lim, C.J.; Bi, X.; Sokhansanj, S.; Melin, S. Determination of effective thermal conductivity and specific heat capacity of wood pellets. Fuel 2013, 103, 347–355. [Google Scholar] [CrossRef]
- Fernandez, Y.; Arenillas, A.; Angel, J. Microwave Heating Applied to Pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; Arenillas, A., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 31. [Google Scholar]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of biomass: A review of production methods for biocoal from cultured and waste lignocellulosic feedstocks. Renew. Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Study on the deacetylation of hemicelluloses during the hydrothermal processing of Eucalyptus wood. Eur. J. Wood Prod. 2001, 59, 53–59. [Google Scholar] [CrossRef]
- Pawar, P.M.A.; Koutaniemi, S.; Tenkanen, M.; Mellerowicz, E.J. Acetylation of woody lignocellulose: Significance and regulation. Front. Plant Sci. 2013, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Sutton, R.; Sposito, G. Molecular Structure in Soil Humic Substances: The New View. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Zhang, Z.; Chang, H.; Jameel, H. Determination of Furfural and Hydroxymethylfurfural Formed From Biomass Under Acidic Conditions. J. Wood Chem. Technol. 2009, 29, 265–276. [Google Scholar] [CrossRef]
- Hänninen, K. Historical and current progress in understanding the origin and structure of humic substances. Chem. Ecol. 2010, 26, 1–11. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives Against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Scott, I.M.; McGarvey, B.D.; Conn, K.; Ferrante, L.; Berruti, F.; Briens, C. Insecticidal and anti-microbial activity of bio-oil derived from fast pyrolysis of lignin, cellulose, and hemicellulose. J. Pest Sci. 2014, 88, 171–179. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Mourant, D.; Gunawan, R.; Lievens, C.; Chaiwat, W.; Gholizadeh, M.; Wu, L.; Li, X.; Li, C.-Z. Polymerization on heating up of bio-oil: A model compound study. AIChE J. 2013, 59, 888–900. [Google Scholar] [CrossRef]
- Kok, M.V.; Özgür, E. Thermal analysis and kinetics of biomass samples. Fuel Process. Technol. 2013, 106, 739–743. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Venderbosch, R.H.; Ardiyanti, A.R.; Wildschut, J.; Oasmaa, A.; Heeres, H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technol. Biotechnol. 2010, 85, 674–686. [Google Scholar] [CrossRef]










| Index | Softwood | Wheat Straw | Peat | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 °C | 250 °C | 300 °C | 200 °C | 250 °C | 300 °C | 200 °C | 250 °C | 300 °C | |
| Total area, (AU) | 0.81 | 3.95 | 7.23 | 3.58 | 10.68 | 15.32 | 0.89 | 5.61 | 5.37 |
| Total carboxylic acids: | |||||||||
| Area (AU) | 0.74 | 3.36 | 4.94 | 3.41 | 9.00 | 11.7 | 0.31 | 2.12 | 1.62 |
| Content (%) * | 91.4 | 85.1 | 68.7 | 95.3 | 84.3 | 76.4 | 34.9 | 37.8 | 30.1 |
| Acetic acid | |||||||||
| Area (AU) | 0.46 | 2.63 | 4.43 | 3.03 | 7.02 | 8.26 | 0.29 | 1.86 | 1.33 |
| Content (%) * | 56.8 | 66.6 | 61.3 | 84.6 | 65.7 | 53.9 | 32.1 | 33.2 | 24.8 |
| Furfural and 5-methyl furfural: | |||||||||
| Area (AU) | |||||||||
| Content (%) * | 0.06 | 0.30 | 0.68 | 0.02 | 0.17 | 0.49 | 0.58 | 3.14 | 3.11 |
| 7.40 | 7.60 | 9.40 | 0.56 | 1.60 | 3.20 | 65.1 | 56.0 | 57.9 | |
| Other organics: | |||||||||
| Area (AU) | 0.02 | 0.29 | 1.58 | 0.16 | 1.51 | 3.09 | - | 0.35 | 0.64 |
| Content (%) | 2.47 | 7.30 | 21.9 | 4.47 | 14.10 | 20.2 | - | 6.22 | 12.0 |
| T, °C | Softwood | |||||||
| Solid fraction | Tar fraction | |||||||
| O/C | H/C | Ash, % | HHV, MJ/kg | O/C | H/C | Ash, % | HHV, MJ/kg | |
| n.t * | 0.64 | 1.46 | 0.32 ± 0.02 | 19.9 ± 0.2 | - | - | - | - |
| 200 °C | 0.63 | 1.44 | 0.33 ± 0.04 | 20.2 ± 0.2 | 0.60 | 1.36 | n.d | 20.7 ± 0.7 |
| 250 °C | 0.56 | 1.34 | 0.36 ± 0.05 | 21.5 ± 0.1 | 0.57 | 1.20 | n.d | 21.0 ± 0.6 |
| 300 °C | 0.41 | 1.14 | 0.41 ± 0.05 | 24.5 ± 0.3 | 0.53 | 1.17 | n.d | 21.7 ± 0.9 |
| Wheat straw | ||||||||
| Solid fraction | Tar fraction | |||||||
| O/C | H/C | Ash, % | HHV, MJ/kg | O/C | H/C | Ash, % | HHV, MJ/kg | |
| n.t * | 0.70 | 1.50 | 3.7 ± 0.2 | 18.4 ± 0.1 | - | - | - | - |
| 200 °C | 0.65 | 1.43 | 3.9 ± 0.3 | 18.7 ± 0.1 | 0.45 | 1.21 | n.d | 23.3 ± 0.2 |
| 250 °C | 0.59 | 1.37 | 6.3 ± 0.5 | 19.6 ± 0.2 | 0.55 | 1.28 | n.d | 21.5 ± 0.2 |
| 300 °C | 0.22 | 0.90 | 10.2 ± 1.1 | 26.0 ± 0.4 | 0.45 | 1.17 | n.d | 23.9 ± 0.6 |
| Peat | ||||||||
| Solid fraction | Tar fraction | |||||||
| O/C | H/C | Ash, % | HHV, MJ/kg | O/C | H/C | Ash, % | HHV, MJ/kg | |
| n.t * | 0.54 | 1.19 | 3.4 ± 0.3 | 20.9 ± 0.2 | - | - | - | |
| 200 °C | 0.47 | 1.12 | 3.6 ± 0.4 | 22.0 ± 0.2 | 0.52 | 0.75 | n.d | 21.4 ± 0.3 |
| 250 °C | 0.31 | 1.03 | 4.7 ± 0.2 | 25.4 ± 0.4 | 0.51 | 1.15 | n.d | 21.8 ± 0.2 |
| 300 °C | 0.24 | 0.88 | 5.2 ± 0.4 | 26.9 ± 0.3 | 0.43 | 1.13 | n.d | 23.7 ± 0.2 |
| MW Treatment, °C | Softwood | |||
| Solid Fraction | Tar Fraction | |||
| Q (kJ/g) | Eff., % | Q (kJ/g) | Eff., % | |
| n.t | 10.1 ± 0.5 | 50.7 | - | - |
| 200 | 10.2 ± 0.6 | 50.5 | 5.5 ± 0.3 | 26.6 |
| 250 | 12.5 ± 0.7 | 58.1 | 5.6 ± 0.2 | 26.7 |
| 300 | 16.6 ± 0.8 | 67.8 | 6.4 ± 0.4 | 29.5 |
| Wheat straw | ||||
| Solid fraction | Tar fraction | |||
| Q (kJ/g) | Eff., % | Q (kJ/g) | Eff., % | |
| n.t | 10.8 ± 0.5 | 58.7 | - | - |
| 200 | 11.0 ± 0.6 | 58.7 | 7.6 ± 0.5 | 32.6 |
| 250 | 13.0 ± 0.8 | 66.3 | 6.2 ± 0.3 | 28.8 |
| 300 | 17.7 ± 1.0 | 68.0 | 6.6 ± 0.3 | 20.7 |
| Peat | ||||
| Solid fraction | Tar fraction | |||
| Q (kJ/g) | Eff., % | Q (kJ/g) | Eff., % | |
| n.t | 14.8 ± 0.7 | 70.8 | - | - |
| 200 | 15.9 ± 0.8 | 72.3 | 9.0 ± 0.5 | 42.0 |
| 250 | 18.5 ± 1.0 | 72.8 | 6.5 ± 0.5 | 29.8 |
| 300 | 20.4 ± 1.2 | 75.8 | 6.9 ± 0.5 | 29.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshanitsa, A.; Jashina, L.; Pals, M.; Ponomarenko, J.; Akishin, Y.; Zake, M. Characteristics of the Main- and Side-Stream Products of Microwave Assisted Torrefaction of Lignocellulosic Biomass of Different Origination. Energies 2022, 15, 1857. https://doi.org/10.3390/en15051857
Arshanitsa A, Jashina L, Pals M, Ponomarenko J, Akishin Y, Zake M. Characteristics of the Main- and Side-Stream Products of Microwave Assisted Torrefaction of Lignocellulosic Biomass of Different Origination. Energies. 2022; 15(5):1857. https://doi.org/10.3390/en15051857
Chicago/Turabian StyleArshanitsa, Alexandr, Lilija Jashina, Matiss Pals, Jevgenija Ponomarenko, Yegor Akishin, and Maja Zake. 2022. "Characteristics of the Main- and Side-Stream Products of Microwave Assisted Torrefaction of Lignocellulosic Biomass of Different Origination" Energies 15, no. 5: 1857. https://doi.org/10.3390/en15051857
APA StyleArshanitsa, A., Jashina, L., Pals, M., Ponomarenko, J., Akishin, Y., & Zake, M. (2022). Characteristics of the Main- and Side-Stream Products of Microwave Assisted Torrefaction of Lignocellulosic Biomass of Different Origination. Energies, 15(5), 1857. https://doi.org/10.3390/en15051857






