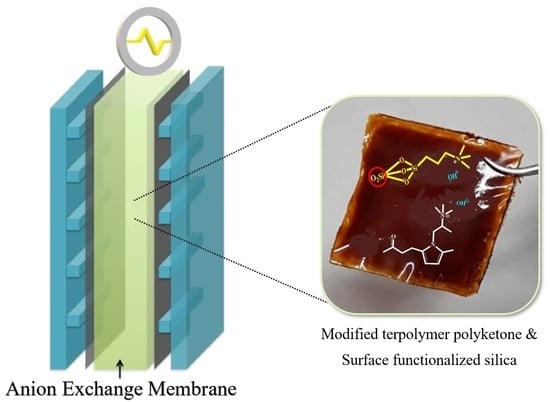
Enhanced OH− Conductivity for Fuel Cells with Anion Exchange Membranes, Based on Modified Terpolymer Polyketone and Surface Functionalized Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
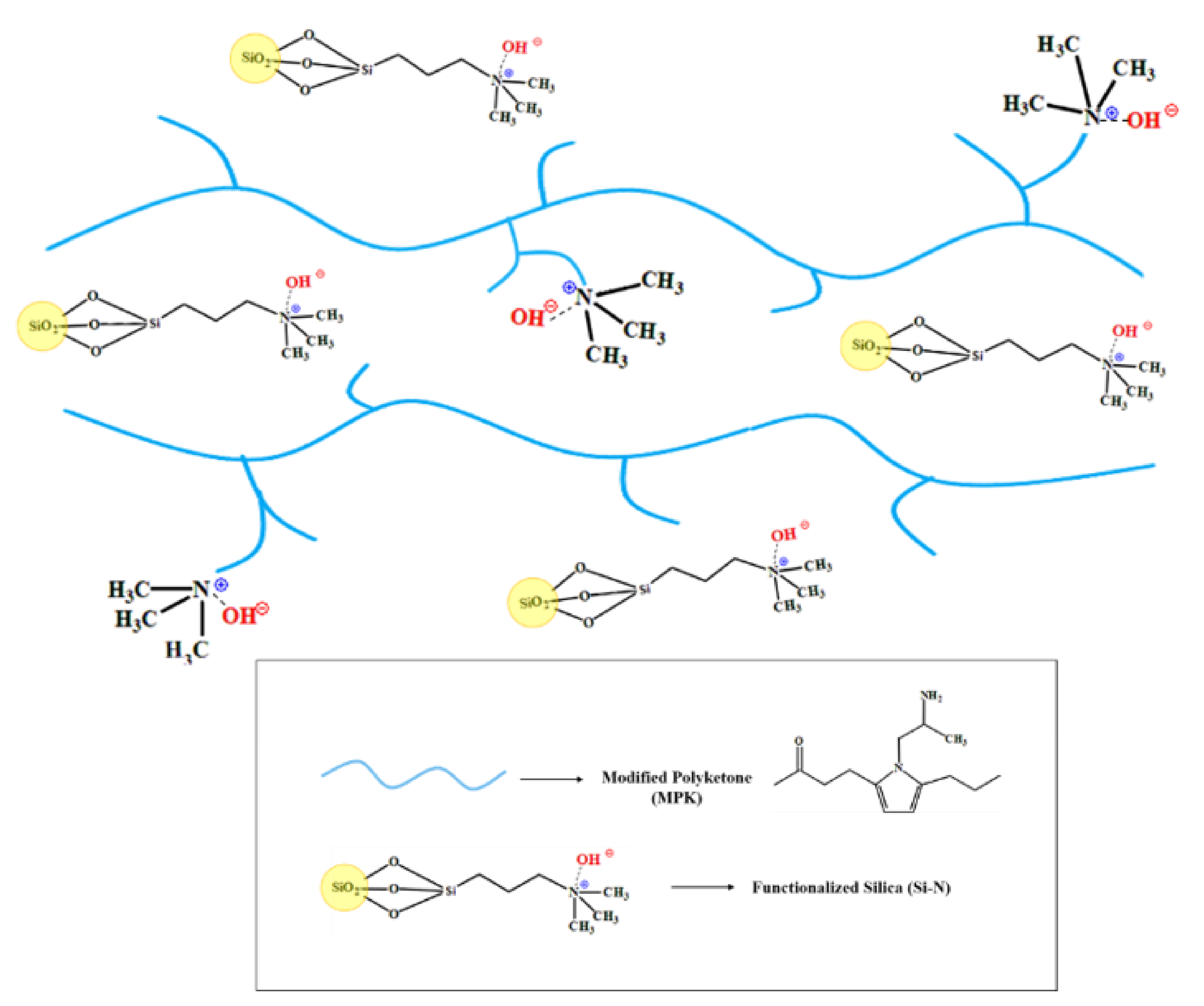
2.2. Preparation of Membranes
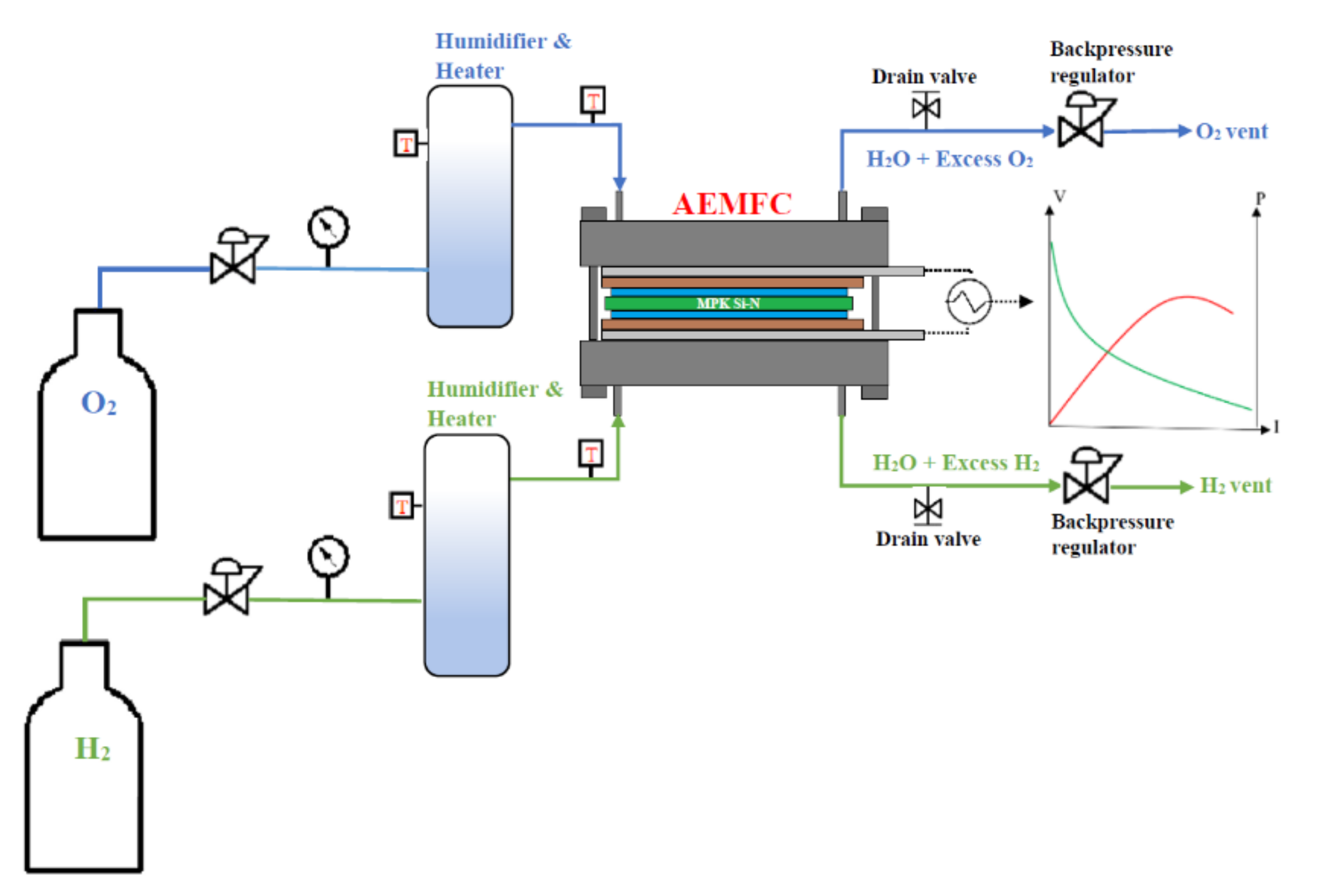
2.3. Measurements and Characterizations
3. Results and Discussion
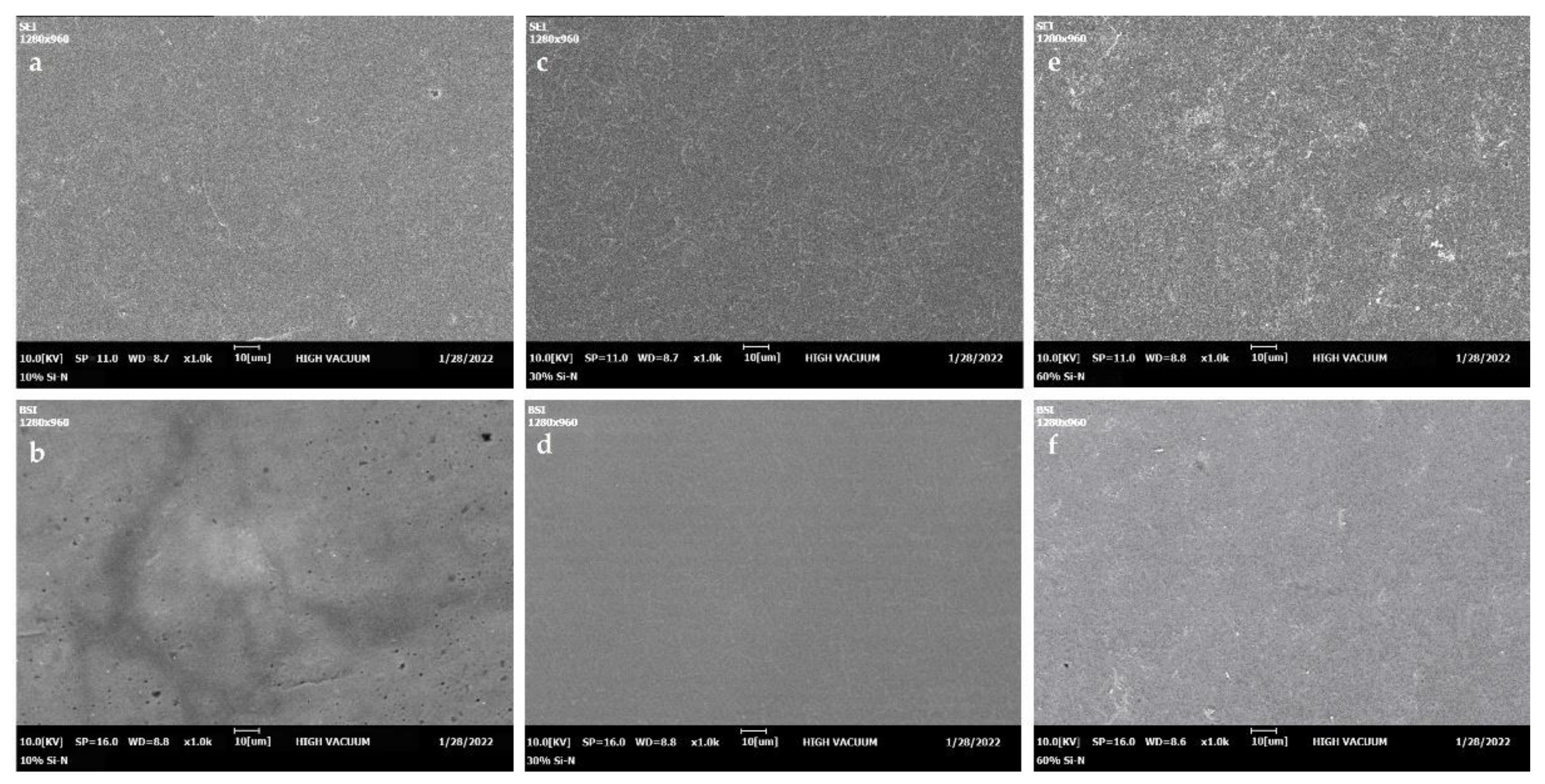
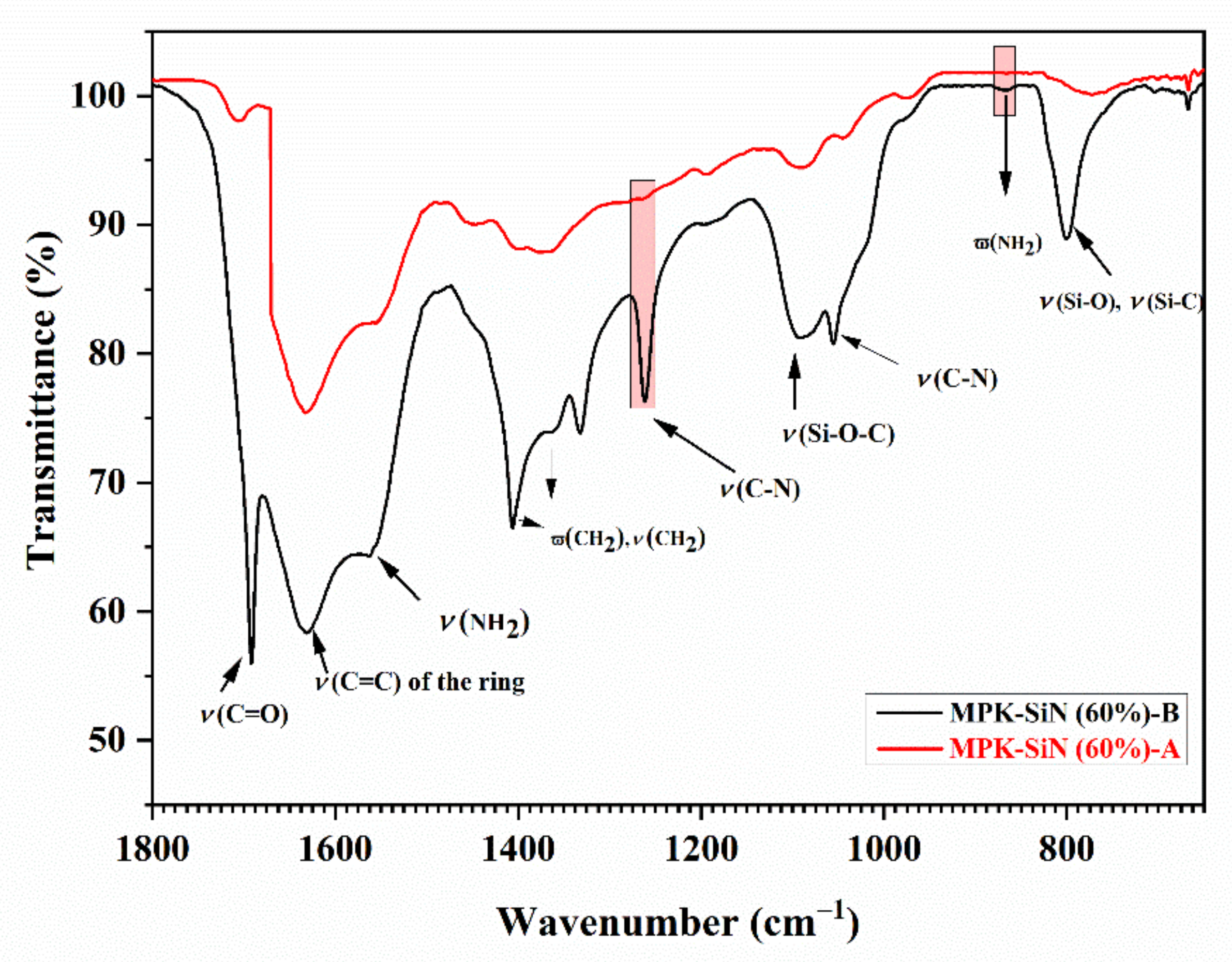
3.1. Conductivity and Morphology
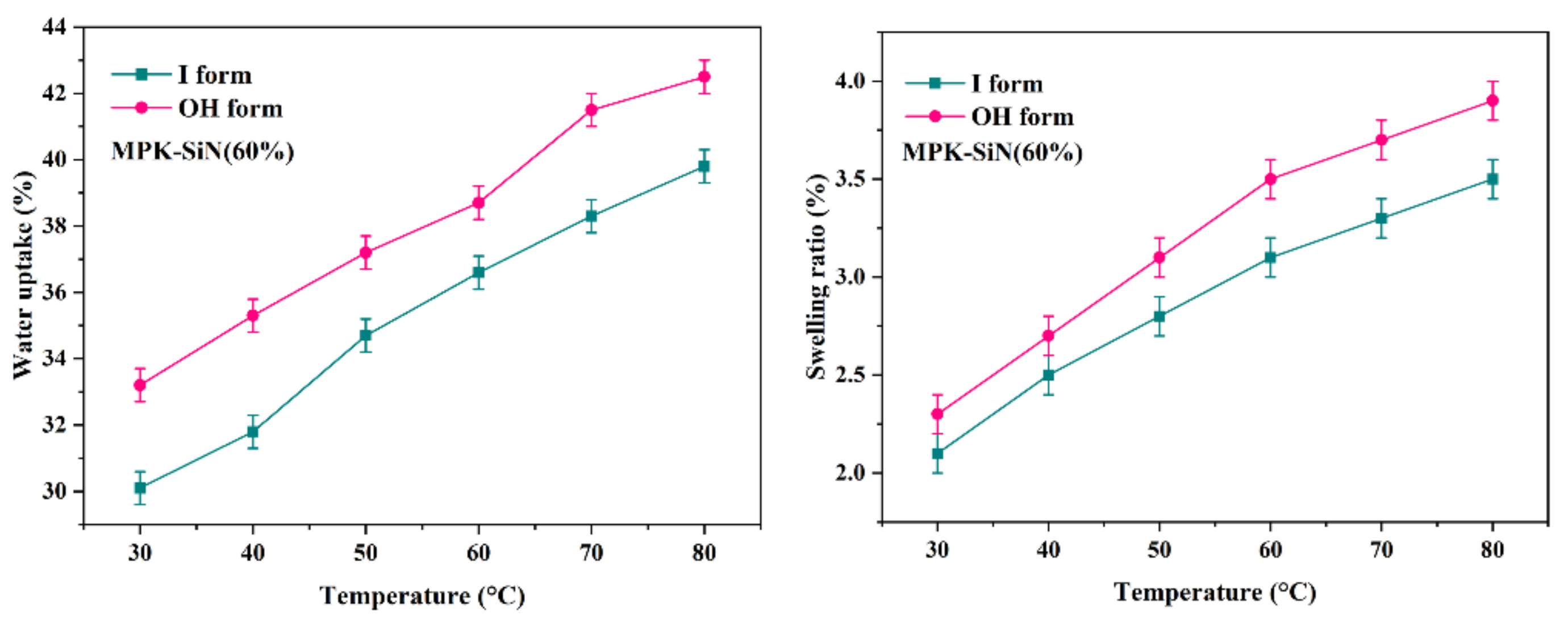
3.2. Water Uptake, IEC, and Swelling Ratio
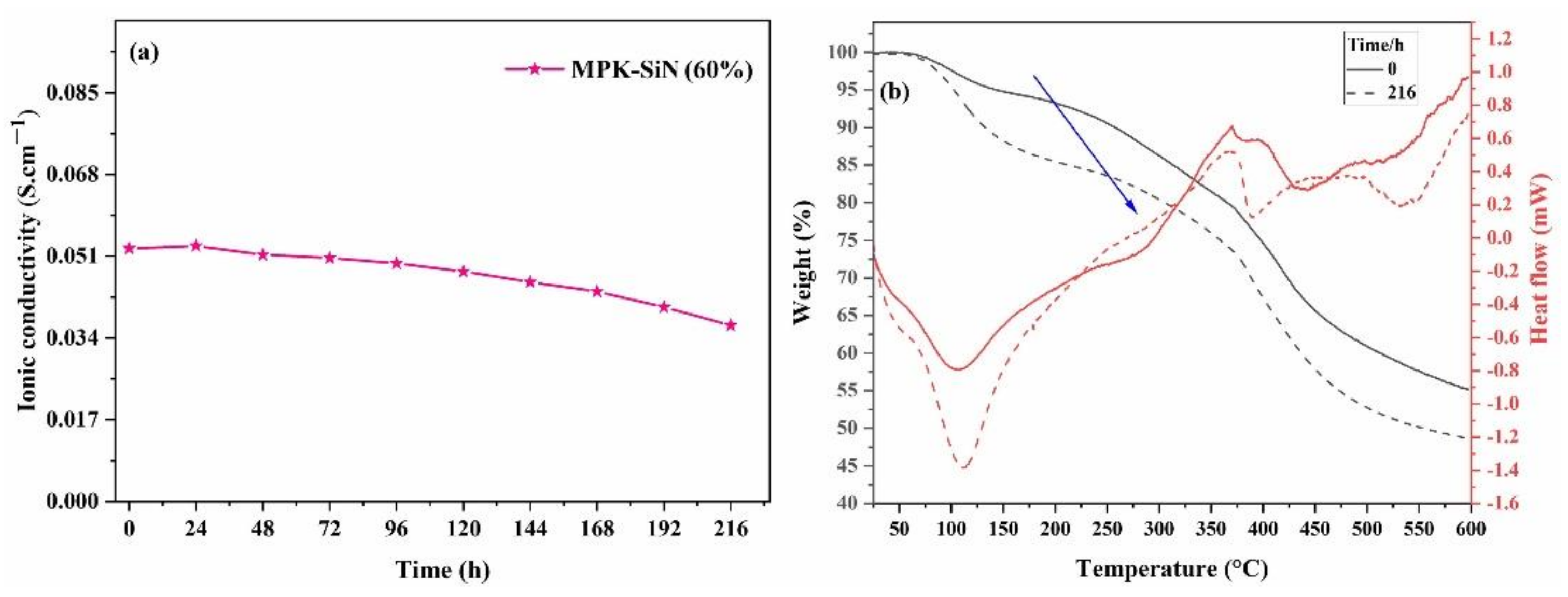
3.3. Alkaline Stability
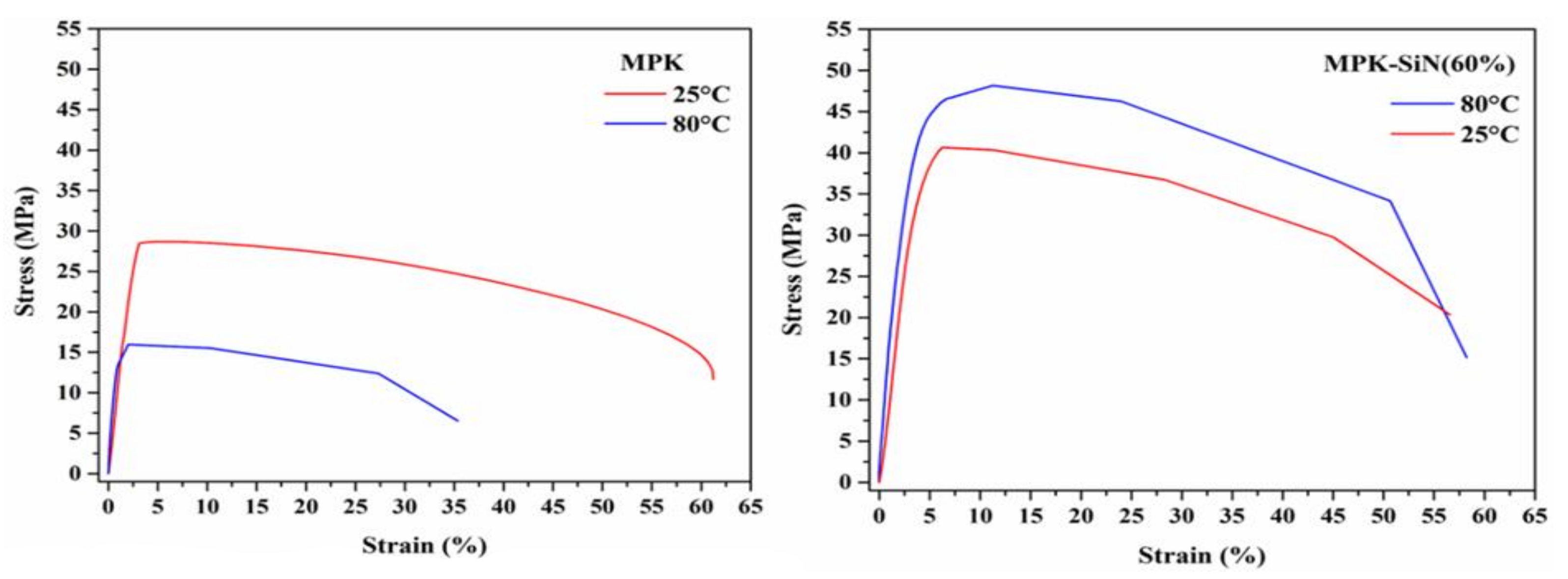
3.4. Mechanical Stability
3.5. Fuel Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a European Strategy for Data—Publications Office of the EU. Available online: https://op.europa.eu/it/publication-detail/-/publication/ac9cd214-53c6-11ea-aece-01aa75ed71a1/language-en (accessed on 6 May 2021).
- Sun, C.; Zhang, H. Review of the development of first-generation redox flow batteries: Iron-chromium system. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Fathabadi, H. Utilizing solar and wind energy in plug-in hybrid electric vehicles. Energy Convers. Manag. 2018, 156, 317–328. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Source 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef] [Green Version]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Source 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Mukherjee, B.; Flor, A.; Scardi, P. Effect of oxygen adsorption and oxidation on the strain state of Pd nanocrystals. Appl. Surf. Sci. 2021, 541, 148508. [Google Scholar] [CrossRef]
- Osmieri, L.; Park, J.; Cullen, D.A.; Zelenay, P.; Myers, D.J.; Neyerlin, K.C. Status and challenges for the application of platinum group metal-free catalysts in proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2021, 25, 100627. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. Prospects for alkaline anion-exchange membranes in low temperature fuel cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Li, Q.; Hu, M.; Xiao, L.; Lu, J.; Zhuang, L. Alkaline polymer electrolyte fuel cells stably working at 80 °C. J. Power Source 2018, 390, 165–167. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Ataollahi, N.; Ahmad, A.; Lee, T.K.; Abdullah, A.R.; Rahman, M.Y.A. Preparation and characterization of PVDF-MG49-NH4CF3SO3 based solid polymer electrolyte. E-Polymers 2014, 14, 115–120. [Google Scholar] [CrossRef]
- Hassanvand, A.; Wei, K.; Talebi, S.; Chen, G.Q.; Kentish, S.E. The role of ion exchange membranes in membrane capacitive deionisation. Membranes 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wu, C.; Yu, F.; Xu, T.; Fu, Y. Free-standing anion-exchange PEO-SiO2 hybrid membranes. J. Memb. Sci. 2008, 307, 28–36. [Google Scholar] [CrossRef]
- Li, X.; Tao, J.; Nie, G.; Wang, L.; Li, L.; Liao, S. Cross-linked multiblock copoly(arylene ether sulfone) ionomer/nano-ZrO2 composite anion exchange membranes for alkaline fuel cells. RSC Adv. 2014, 4, 41398–41410. [Google Scholar] [CrossRef]
- Khoon, L.T.; Fui, M.L.W.; Hassan, N.H.; Su’ait, M.S.; Vedarajan, R.; Matsumi, N.; Bin Kassim, M.; Shyuan, L.K.; Ahmad, A. In situ sol–gel preparation of ZrO2 in nano-composite polymer electrolyte of PVDF-HFP/MG49 for lithium-ion polymer battery. J. Sol-Gel Sci. Technol. 2019, 90, 665–675. [Google Scholar] [CrossRef]
- Nonjola, P.T.; Mathe, M.K.; Modibedi, R.M. Chemical modification of polysulfone: Composite anionic exchange membrane with TiO2 nano-particles. Int. J. Hydrogen Energy 2013, 38, 5115–5121. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite polymers development and application for polymer electrolyte membrane technologies—A review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.; Parrondo, J.; Ramani, V. Composite anion exchange membranes based on quaternized cardo-poly(etherketone) and quaternized inorganic fillers for vanadium redox flow battery applications. Int. J. Hydrogen Energy 2016, 41, 10766–10775. [Google Scholar] [CrossRef] [Green Version]
- Ataollahi, N.; Cappelletto, E.; Vezzù, K.; Di Noto, V.; Cavinato, G.; Callone, E.; Dirè, S.; Scardi, P.; Di Maggio, R. Properties of anion exchange membrane based on polyamine: Effect of functionalized silica particles prepared by sol–gel method. Solid State Ionics 2018, 322, 85–92. [Google Scholar] [CrossRef]
- Ataollahi, N.; Vezzù, K.; Nawn, G.; Pace, G.; Cavinato, G.; Girardi, F.; Scardi, P.; Di Noto, V.; Di Maggio, R. A Polyketone-based Anion Exchange Membrane for Electrochemical Applications: Synthesis and Characterization. Electrochim. Acta 2017, 226, 148–157. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Zhou, L.; Feng, C.P.; Wu, X.T.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Yang, W. Direct modification of polyketone resin for anion exchange membrane of alkaline fuel cells. J. Colloid Interface Sci. 2019, 556, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Nawn, G.; Vezzù, K.; Cavinato, G.; Pace, G.; Bertasi, F.; Pagot, G.; Negro, E.; Di Noto, V. Opening Doors to Future Electrochemical Energy Devices: The Anion-Conducting Polyketone Polyelectrolytes. Adv. Funct. Mater. 2018, 28, 1706522. [Google Scholar] [CrossRef]
- Ataollahi, N.; Girardi, F.; Cappelletto, E.; Vezzù, K.; Di Noto, V.; Scardi, P.; Callone, E.; Di Maggio, R. Chemical modification and structural rearrangements of polyketone-based polymer membrane. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Toncelli, C.; Pino-Pinto, J.P.; Sano, N.; Picchioni, F.; Broekhuis, A.A.; Nishide, H.; Moreno-Villoslada, I. Controlling the aggregation of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin by the use of polycations derived from polyketones bearing charged aromatic groups. Dye Pigment. 2013, 98, 51–63. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.A.; Carlotti, M.; Picchioni, F.; Mattoli, V.; Pucci, A. Electrically-conductive polyketone nanocomposites based on reduced graphene oxide. Polymers 2020, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.J.; Li, W.Y.; Lin, Y.; Li, X.P.; Xiao, X.R.; Fang, S.B. Synthesis and ionic conductivity of a polysiloxane containing quaternary ammonium groups. Polym. Adv. Technol. 2004, 15, 61–64. [Google Scholar] [CrossRef]
- Tuan, C.M.; Tinh, V.D.C.; Kim, D. Anion exchange membranes prepared from quaternized polyepichlorohydrin cross-linked with 1-(3-aminopropyl)imidazole grafted poly(arylene ether ketone) for enhancement of toughness and conductivity. Membranes 2020, 10, 138. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 1999; Volume 5. [Google Scholar]
- Vanýsek, P. Ionic conductivity and diffusion at infinite dilution. CRC Handb. Chem. Phys. 1996, 96, 5–98. [Google Scholar]
- Zawodzinski, T.A.; Springer, T.E.; Davey, J.; Jestel, R.; Lopez, C.; Valerio, J.; Gottesfeld, S. A Comparative Study of Water Uptake By and Transport through Ionomeric Fuel Cell Membranes. J. Electrochem. Soc. 1993, 140, 1981–1985. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.; Zeng, Q.H.; Liu, Q.L. Preparation and characterization of cross-linked quaternized poly(vinyl alcohol) membranes for anion exchange membrane fuel cells. J. Memb. Sci. 2008, 311, 319–325. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.; Gao, S.; Yuan, Y.; Ren, X. Novel imidazole-grafted hybrid anion exchange membranes based on poly(2,6-dimethyl-1,4-phenylene oxide) for fuel cell applications. J. Appl. Polym. Sci. 2018, 135, 46034. [Google Scholar] [CrossRef]
- Gonggo, S.T.; Bundjali, B.; Hariyawati, K.; Arcana, I.M. The influence of nano-silica on properties of sulfonated polystyrene-lignosulfonate membranes as proton exchange membranes for direct methanol fuel cell application. Adv. Polym. Technol. 2018, 37, 1859–1867. [Google Scholar] [CrossRef]
- Miehe, C.; Göktepe, S.; Méndez Diez, J. Finite viscoplasticity of amorphous glassy polymers in the logarithmic strain space. Int. J. Solids Struct. 2009, 46, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Son, T.Y.; Kim, T.-H.; Nam, S.Y. Crosslinked Pore-Filling Anion Exchange Membrane Using the Cylindrical Centrifugal Force for Anion Exchange Membrane Fuel Cell System. Polymers 2020, 12, 2758. [Google Scholar] [CrossRef]
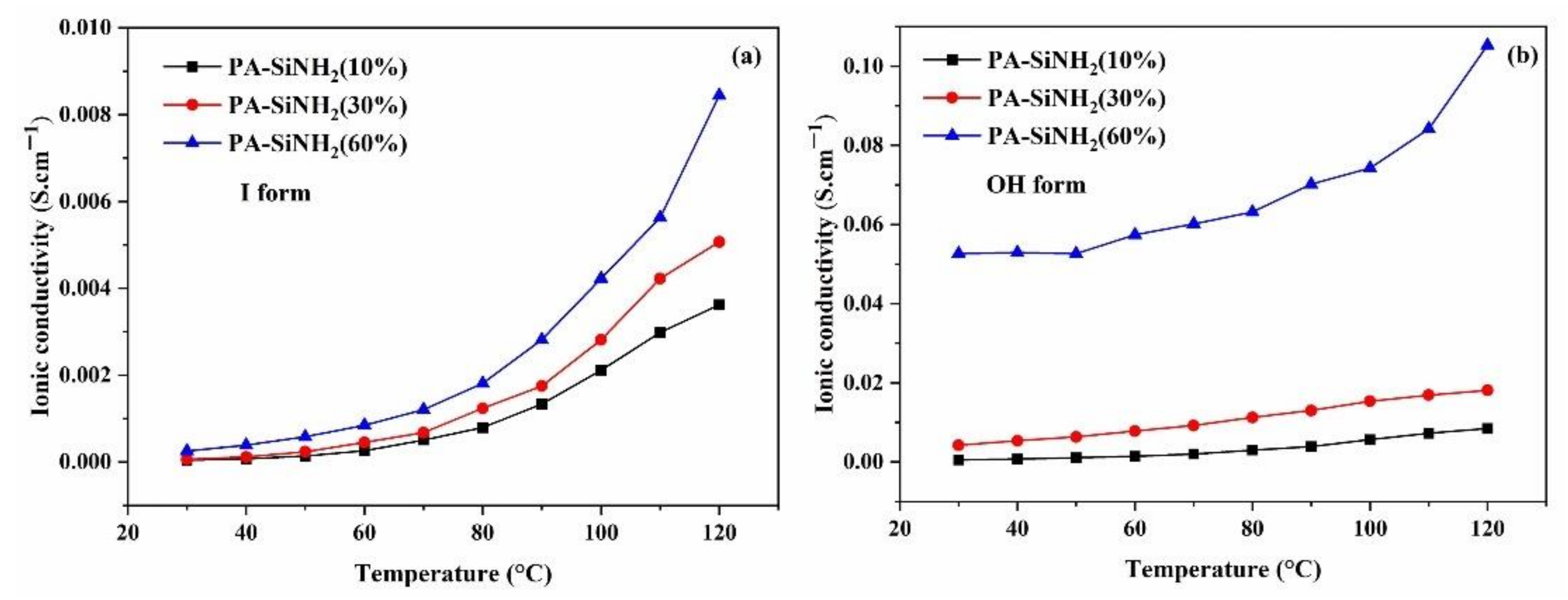

| Conductivity (S·cm−1) at 120 °C | Ea (kJ.mol−1) | |||
|---|---|---|---|---|
| Membranes | I Form | OH Form | I Form | OH Form |
| MPK–SiN (60%) | 8.4 × 10−3 | 1.0 × 10−1 | 38.5 | 6.9 |
| MPK–SiN (30%) | 5.1 × 10−3 | 1.8 × 10−2 | 49.5 | 16.5 |
| MPK–SiN (10%) | 3.6 × 10−3 | 8.4 × 10−3 | 51.5 | 32.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataollahi, N.; Tomasino, E.; Cotini, O.; Di Maggio, R. Enhanced OH− Conductivity for Fuel Cells with Anion Exchange Membranes, Based on Modified Terpolymer Polyketone and Surface Functionalized Silica. Energies 2022, 15, 1953. https://doi.org/10.3390/en15051953
Ataollahi N, Tomasino E, Cotini O, Di Maggio R. Enhanced OH− Conductivity for Fuel Cells with Anion Exchange Membranes, Based on Modified Terpolymer Polyketone and Surface Functionalized Silica. Energies. 2022; 15(5):1953. https://doi.org/10.3390/en15051953
Chicago/Turabian StyleAtaollahi, Narges, Eleonora Tomasino, Oscar Cotini, and Rosa Di Maggio. 2022. "Enhanced OH− Conductivity for Fuel Cells with Anion Exchange Membranes, Based on Modified Terpolymer Polyketone and Surface Functionalized Silica" Energies 15, no. 5: 1953. https://doi.org/10.3390/en15051953
APA StyleAtaollahi, N., Tomasino, E., Cotini, O., & Di Maggio, R. (2022). Enhanced OH− Conductivity for Fuel Cells with Anion Exchange Membranes, Based on Modified Terpolymer Polyketone and Surface Functionalized Silica. Energies, 15(5), 1953. https://doi.org/10.3390/en15051953