Fractional Composition Analysis for Upgrading of Fast Pyrolysis Bio-Oil Produced from Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
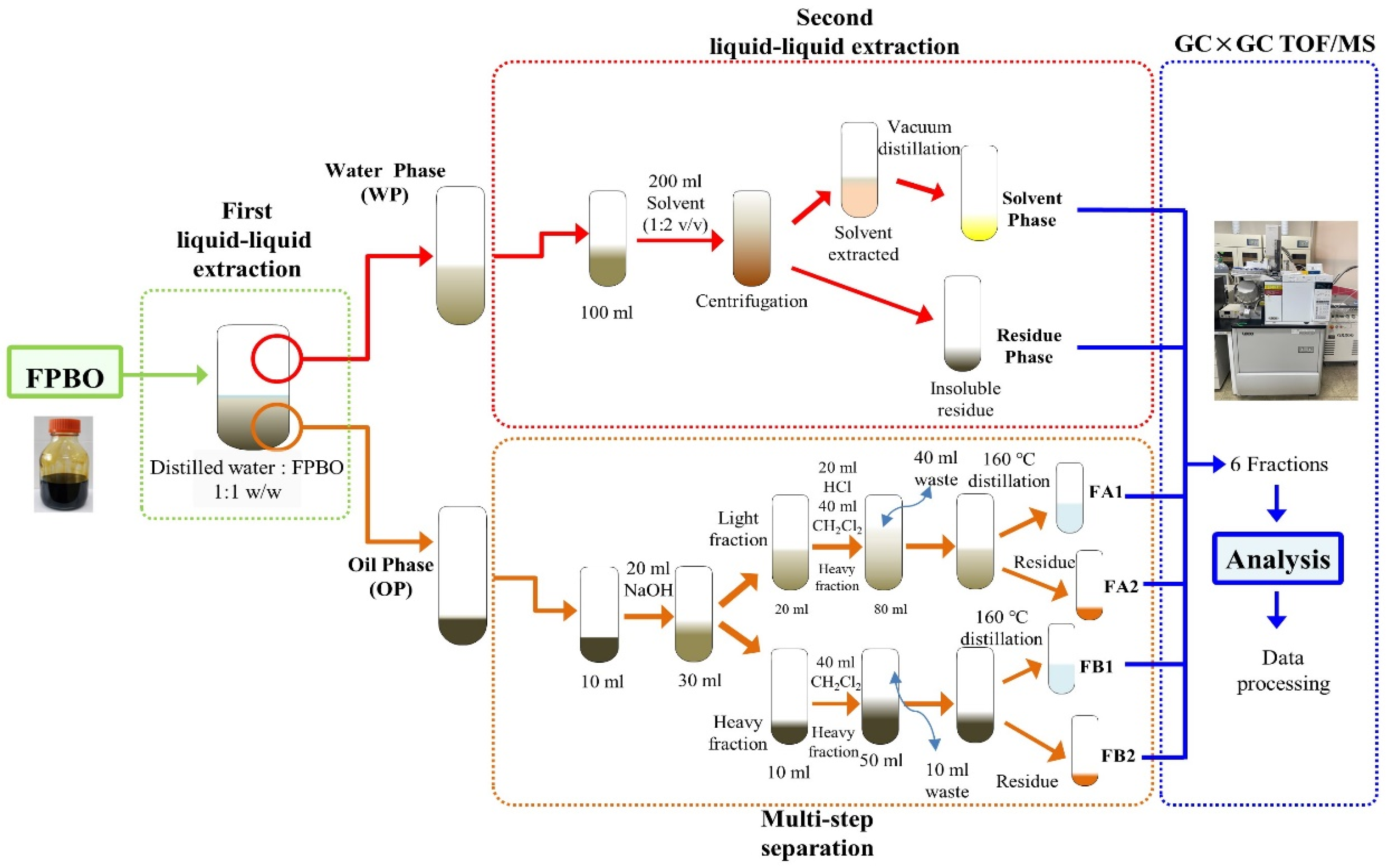
2.2. Liquid-Liquid Extraction
2.3. Multi-Step Separation
2.4. GC×GC TOF/MS for Composition Analysis
3. Results and Discussion
3.1. Properties of FPBO
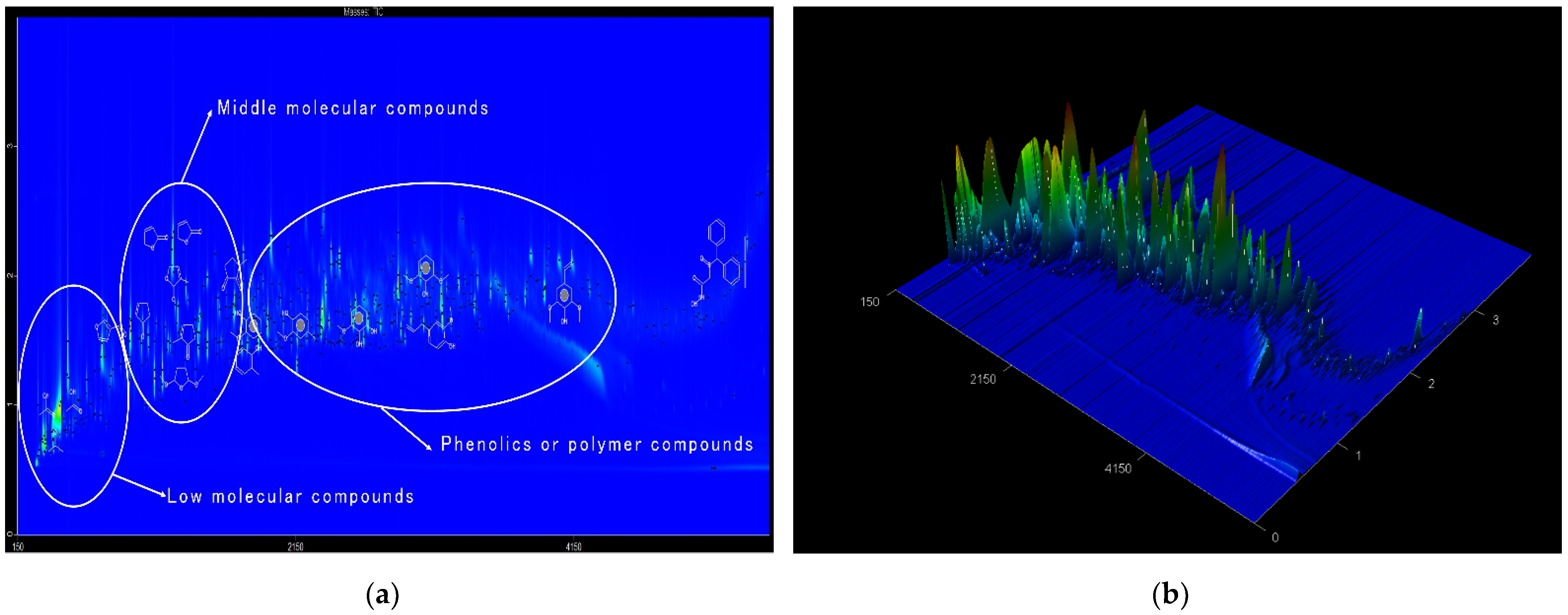
3.2. FPBO Compositions
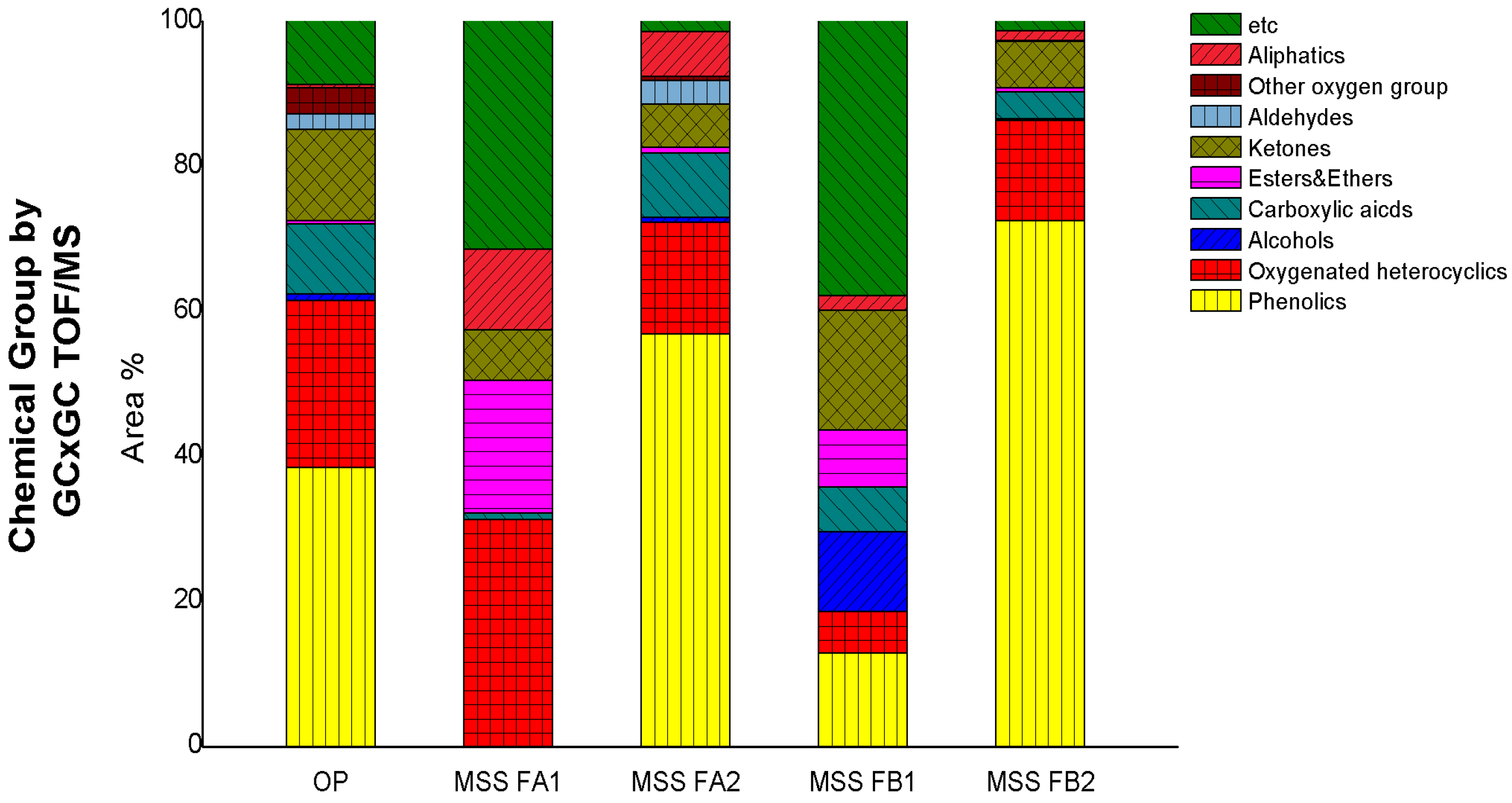
3.3. Compositions from Liquid-Liquid Extraction of WP
3.4. Compositions from Multi-Step Separation of OP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laura, C.; Timur, G. Net Zero by 2050: A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Bridges, A.; Felder, F.A.; McKelvey, K.; Niyogi, I. Uncertainty in energy planning: Estimating the health impacts of air pollution from fossil fuel electricity generation. Energy Res. Soc. Sci. 2015, 6, 74–77. [Google Scholar] [CrossRef]
- Demirbas, A. Future Fuels for Internal Combustion Engines. Energ. Source Part A 2010, 32, 1273–1281. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Sutton, A.D.; Robichaud, D.J. Pyrolysis of Biomass for Aviation Fuel. In Biofuels for Aviation–Feedstocks, Technology and Implementation; Academic Press: Cambridge, MA, USA, 2016; Chapter 8; pp. 191–215. [Google Scholar]
- Bilgili, L. Comparative assessment of alternative marine fuels in life cycle perspective. Renew. Sust. Energy Rev. 2021, 144, 110985. [Google Scholar] [CrossRef]
- Annex 16: Environmental Protection, Volume IV–Carbon Offsetting and Reduction Scheme for International Aviation; International Civil Aviation Organization: Montreal, QC, Canada, 2020.
- Deshavath, N.N.; Veeranki, V.D.; Goud, V.V. Lignocellulosic Feedstocks for the Production of Bioethanol: Availability, Structure, and Composition. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar]
- Wang, H.; Yang, B.; Zhang, Q.; Zhu, W. Catalytic routes for the conversion of lignocellulosic biomass to aviation fuel range hydrocarbons. Renew. Sust. Energy Rev. 2020, 120, 109612. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Dri, L.L.; Hector, G.; Moon, R.J.; Zavattieri, P.D. Anisotropy of the elastic properties of crystalline cellulose I β from first principles density functional theory with Van der Waals Interactions. Cellulose 2013, 20, 2703–2718. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, Z.; Lu, Q.; Yang, Y. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood. Energy Convers. Manag. 2012, 57, 49–59. [Google Scholar] [CrossRef]
- Basu, P. Tar production and Destruction. In Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010; Chapter 4; pp. 117–166. [Google Scholar]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Source 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.K.; Oh, C.H.; Park, J.W.; Kwon, E.E. Production of bio-oil from fast pyrolysis of biomass using a pilot-scale circulating fluidized bed reactor and its characterization. J. Environ. Manag. 2019, 234, 138–144. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on condensing system for biomass pyrolysis process. Fuel Process. Technol. 2018, 180, 1–13. [Google Scholar] [CrossRef]
- Kim, I.; Dwiatmoko, A.A.; Choi, J.W.; Suh, D.J.; Jae, J.; Ha, J.M.; Kim, J.K. Upgrading of sawdust pyrolysis oil to hydrocarbon fuels using tungstate-zirconia-supported Ru catalysts with less formation of cokes. J. Ind. Eng. Chem. 2017, 56, 74–81. [Google Scholar] [CrossRef]
- Vitasari, C.R.; Meindersma, G.W.; De Haan, A.B. Glycolaldehyde co-extraction during the reactive extraction of acetic acid with tri-n-octylamine/2-ethyl-1-hexanol from a wood-based pyrolysis oil-derived aqueous phase. Sep. Purif. Technol. 2012, 95, 39–43. [Google Scholar] [CrossRef]
- Vitasari, C.R.; Meindersma, G.W.; De Haan, A.B. Water extraction of pyrolysis oil: The first step for the recovery of renewable chemicals. Bioresour. Technol. 2011, 102, 7204–7210. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenerg. 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Ribeiro, L.A.B.; Martins, R.C.; Mesa-Pérez, J.M.; Bizzo, W.A. Study of bio-oil properties and ageing through fractionation and ternary mixtures with the heavy fraction as the main component. Energy 2019, 169, 344–355. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Cai, Q.; Wang, X.; Jin, H.; Luo, Z. Multi-step separation of monophenols and pyrolytic lignins from the water-insoluble phase of bio-oil. Sep. Purif. Technol. 2014, 122, 248–255. [Google Scholar] [CrossRef]
- Pierce, K.M.; Hope, J.L.; Hoggard, J.C. A principal component analysis based method to discover chemical differences in comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GC × GC-TOFMS) separations of metabolites in plant samples. Talanta 2006, 70, 797–804. [Google Scholar] [CrossRef]
- EN 16900:2017; Fast Pyrolysis Bio-oils for Industrial Boilers-Requirement and Test Methods. European Standard: Pilsen, Czech Republic, 2017.
- Wei, Y.; Lei, H.; Wang, L.; Zhu, L.; Zhang, X.; Liu, Y.; Chen, S.; Ahring, B. Liquid-liquid extraction of biomass pyrolysis bio-oil. Energy Fuel 2014, 2, 1207–1212. [Google Scholar] [CrossRef]
- Tao, J.; Li, C.; Li, J.; Yan, B.; Chen, G.; Cheng, Z.; Li, W.; Lin, F.; Hou, L. Multi-step separation of different chemical groups from the heavy fraction in biomass fast pyrolysis oil. Fuel Process. Technol. 2020, 202, 106366. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, D.; Pan, S.; Wang, Z.; Liu, Q.; Liu, B. Compositional characterization of neutral fractions in <300 °C distillates of six shale oils using extrography followed by GC-TOF/MS analysis. Fuel 2018, 224, 610–618. [Google Scholar]
- ASTM D975-21; Standard Specification for Diesel Fuel. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ASTM D1655-21b; Standard Specification for Aviation Turbine Fuels. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Boateng, A.A. Condensed-phase pyrolysis oil upgrading. In Pyrolysis of Biomass for Fuels and Chemicals; Academic Press: Cambridge, MA, USA, 2020; Chapter 5; pp. 119–147. [Google Scholar]
- Lakshmanan, C.M.; Hoelscher, H.E. Production of levoglucosan by pyrolysis of carbohydrates pyrolysis in hot inert gas stream. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 57–59. [Google Scholar]
- Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Severa, G.; Kumar, G.; Troung, M.; Young, G.; Cooney, M.J. Simultaneous extraction and separation of phorbol esters and bio-oil form Jatropha biomass using ionic liquid-methanol co-solvents. Sep. Purif. Technol. 2013, 116, 265–270. [Google Scholar] [CrossRef]
- Park, L.K.E.; Ren, S.; Yiacoumi, S.; Ye, X.P.; Borole, A.P.; Tsouris, C. Separation of switchgrass bio-oil by water/organic solvent addition and pH adjustment. Energy Fuel. 2016, 33, 2164–2173. [Google Scholar] [CrossRef]
- Shafaghat, H.; Jae, J.; Park, Y.K. Effect of the two-stage process comprised of ether extraction and supercritical hydrodeoxygenation on pyrolysis oil upgrading. Chem. Eng. J. 2021, 404, 126531. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.S.; Liu, Y.; Wang, Q. Properties and mechanisms of different guanidine flame retardant wood pulp paper. J. Anal. Appl. Pyrolysis 2017, 128, 224–231. [Google Scholar] [CrossRef]



| Process | Hydrocarbon Contents (w/w %) | ||
|---|---|---|---|
| FPBO | 100.00 | ||
| Water phase | 41.59 | ||
| Liquid-liquid extraction chloroform solvent phase | 3.32 | ||
| Liquid-liquid extraction chloroform residue phase | 38.27 | ||
| Liquid-liquid extraction ether solvent phase | 3.27 | ||
| Liquid-liquid extraction ether residue phase | 38.32 | ||
| Oil phase | 58.41 | ||
| Multi-step separation FA1 | 13.25 | ||
| Multi-step separation FA2 | 4.10 | ||
| Multi-step separation FB1 | 31.37 | ||
| Multi-step separation FB2 | 9.69 | ||
| Property | Unit | EN 16900 (Boiler) | ASTM D975 (Diesel) | ASTM D975 (Diesel) | FPBO |
|---|---|---|---|---|---|
| Net calorific value | MJ/Kg | Min 14.0 | - | Min 42.8 | 17.4 |
| Water content | m/m % | Max 30 | Max 0.05 | Max 0.006 | 29.5 |
| pH | Min 2.0 | - | - | 2.72 | |
| Density, 15 °C | Kg/m3 | Max 1 300 | 820 to 860 | 775–840 | 1177 |
| Pour point | °C | Max -9 | −35 to −15 | - | −25.0 |
| Nitrogen content | (m/m %) | Report | - | - | 0.113 |
| Kinematic viscosity 40 °C | mm2/s | Max 50 | 2.0 to 4.5 | Max 8 (−20 °C) | 18.07 |
| Sulfur content | m/m % | Max 0.05 | Max 0.0015 | Max 0.2 | 0.43 |
| Solid content | m/m % | Max 0.5 | - | - | 0.26 |
| Ash content | m/m % | Max 0.05 | Max 0.01 | - | 0.10 |
| Na | m/m % | Max 0.02 | - | - | 0.01 |
| K | m/m % | Max 0.02 | - | - | 0.01 |
| Ca | m/m % | Max 0.02 | - | - | 0.01 |
| No. | Compounds | Rx (%) |
|---|---|---|
| 1 | Acetic Acid | 14.28 |
| 2 | Levoglucosan | 13.97 |
| 3 | 3-furaldehyde | 1.75 |
| 4 | Phenol | 1.69 |
| 5 | 1,2-benzendiol | 1.64 |
| 6 | 2-propanone, 1-hydroxy- | 1.48 |
| 7 | Hydroquinone | 1.38 |
| 8 | 5-hydro-methyl dihydrofuran-2-one | 1.36 |
| 9 | 2(5H)-furanone | 1.33 |
| 10 | Pentanoic Acid, 4-oxo- | 1.17 |
| 11 | Anhydro Sugar | 1.16 |
| 12 | Syringyl Acetone | 1.05 |
| 13 | 1-hydroxy-2-butanone | 1.03 |
| 14 | 2(5H)-furanone | 0.92 |
| 15 | Phenol, 4-propyl- | 0.81 |
| 16 | Isometric Dihydro-methyl-furanone | 0.77 |
| 17 | 1,4:3,6-dianhydro-a-d-glucopyranose | 0.73 |
| 18 | 2-hexyldecanoic Acid | 0.73 |
| 19 | Ethenone,1-(4-hydroxy-3,5-dimethoxyphenyl)- | 0.71 |
| 20 | 1,3-di-o-acetyl-a-a-d-ribopyransoe | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.; Park, J.-Y.; Lee, J.W.; Oh, C.-H.; Kim, J.-K.; Yoon, J. Fractional Composition Analysis for Upgrading of Fast Pyrolysis Bio-Oil Produced from Sawdust. Energies 2022, 15, 2054. https://doi.org/10.3390/en15062054
Jeon H, Park J-Y, Lee JW, Oh C-H, Kim J-K, Yoon J. Fractional Composition Analysis for Upgrading of Fast Pyrolysis Bio-Oil Produced from Sawdust. Energies. 2022; 15(6):2054. https://doi.org/10.3390/en15062054
Chicago/Turabian StyleJeon, Hwayeon, Jo-Yong Park, Jae Woo Lee, Chang-Ho Oh, Jae-Kon Kim, and Jaeyoung Yoon. 2022. "Fractional Composition Analysis for Upgrading of Fast Pyrolysis Bio-Oil Produced from Sawdust" Energies 15, no. 6: 2054. https://doi.org/10.3390/en15062054
APA StyleJeon, H., Park, J.-Y., Lee, J. W., Oh, C.-H., Kim, J.-K., & Yoon, J. (2022). Fractional Composition Analysis for Upgrading of Fast Pyrolysis Bio-Oil Produced from Sawdust. Energies, 15(6), 2054. https://doi.org/10.3390/en15062054







