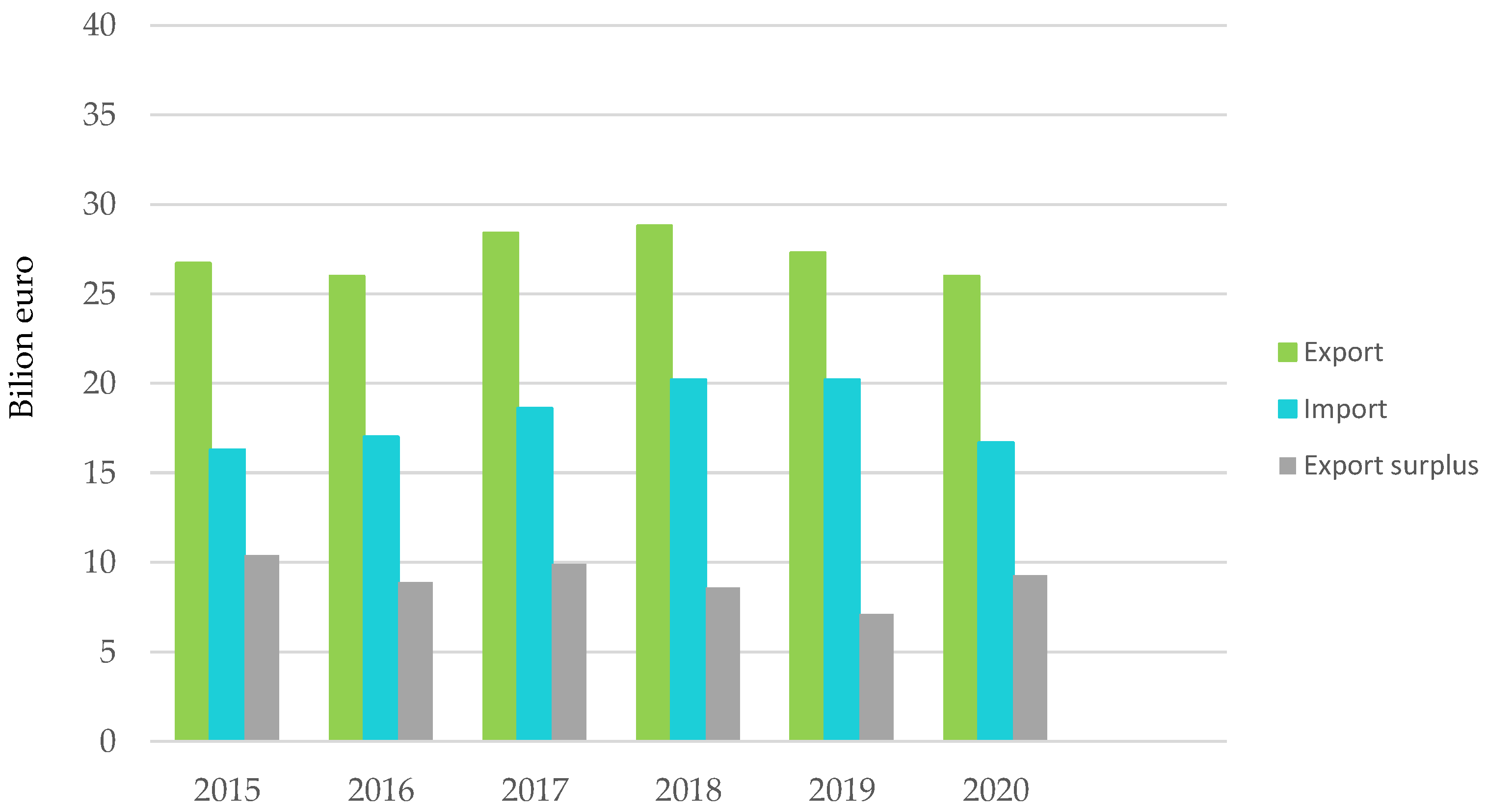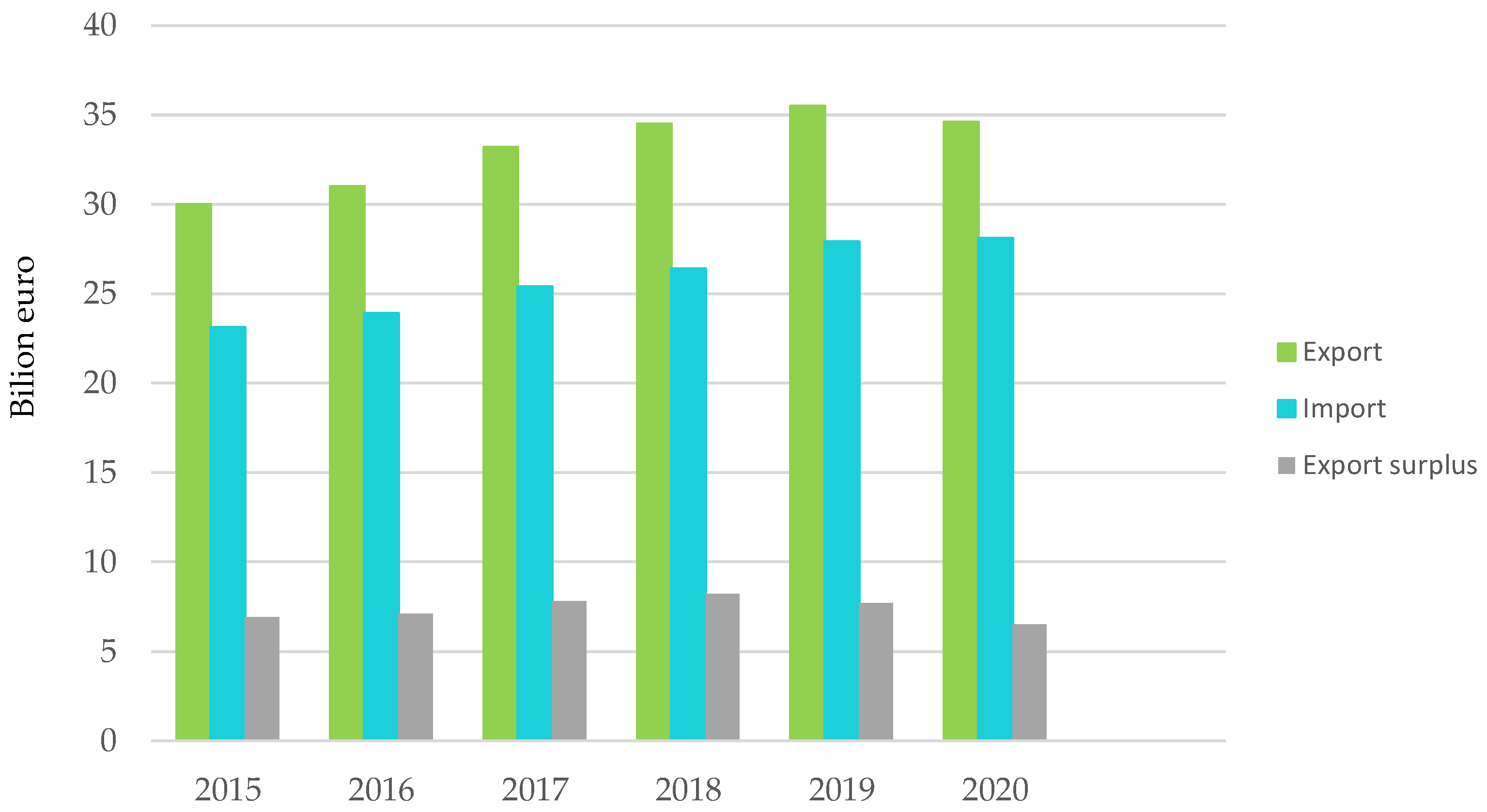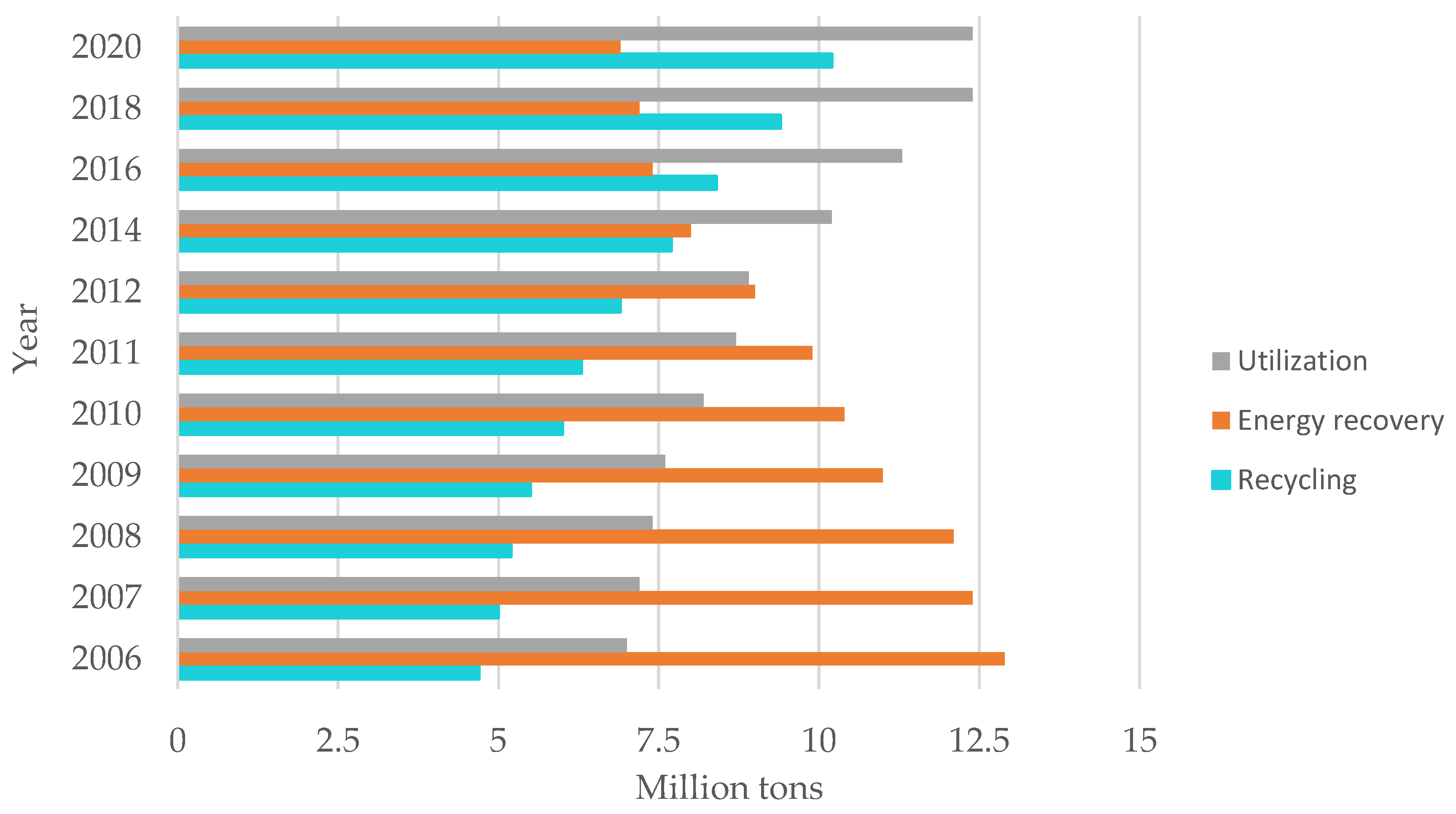Abstract
The civilization development requires improvement of technologies and satisfaction of people’s needs on the one side, but on the other one it is directly connected with the increasing production of waste. In this paper, the authors dealt with the second of these aspects, reviewing the recycling of plastic waste, which can be processed without changing its chemical structure (mechanical recycling), and with changing its chemical structure (chemical recycling, of which thermal recycling). Mechanical recycling involves shredding the waste in order to obtain recyclate or regranulate that meets specific quality requirements. Chemical recycling consists of the degradation of the material into low-molecular compounds, and it can take place in the processes of hydrolysis, glycolysis, methanolysis by means of chemical solvents, and during thermal processes of hydrocracking, gasification, pyrolysis, combustion, enabling the recovery of gaseous and liquid hydrocarbons foundings in application as a fuel in the energy and cement-lime industry and enabling the recovery of thermal energy contained in plastics. The paper focuses on thermal methods of plastics recycling that become more important due to legal regulations limiting the landfilling of waste. The authors also took up the properties of plastics and their production in European conditions.
Keywords:
plastics; waste; thermal properties; management; recycling; pyrolysis; gasification; combustion 1. Introduction
Plastics are derivatives of the natural organic materials, inter alia, they are generated in the crude oil processing. They are made of large particles—polymers, which are made up of smaller particles—monomers. The most popular plastics (taking into account their exemplary use) include: polyethylene terephthalate—PET (oven meal trays, soft drink bottles), polyethylene—PE (automotive and food industry), polypropylene—PP (medicine bottles, ketchup bottles), polystyrene—PS (plastic cutlery, coffee cups), poly-vinyl chloride—PVC (mineral water bottles, shampoo bottles), high density polyethylene—HDPE (toys, washing-up bottles), low density polyethylene—LDPE (bags for food) [1,2].
After fulfilling their useful function, plastics usually end up in landfills. As the vast majority of them are not biodegradable, they are an increasingly serious problem, especially in the areas with high urbanization rates. The biodegradation process of plastics is very slow. Depending on the material used, plastic products decompose in the ground for up to several hundred years.
The plastics sector ensures that plastics must deliver benefits, with the minimal impact on environmental [3].
More and more restrictive legal regulations in European countries limit the possibility of storing plastic waste, forcing more reasonable plastics management and deployment different solutions for neutralization of plastics waste and recycling [4,5,6].
The plastics recycling is a very important not only because of the high waste landfills costs, but also the possibility of the recovery of energy from plastics and the fact that recycled products are cheaper than virginal products. [2]. Section 2 of this paper is devoted to the subject of recycling, with particular emphasis on thermal recycling. It should be emphasized that the composition and properties of plastics (Table 1 and Table 2) [7,8,9] enable their use in the thermal recycling process.

Table 1.
Selected thermal properties of plastics. Made based on [7].

Table 2.
Proximate and ultimate analyses of different materials. Made based on [8,9]. a* analytical (air-dry) state of the material.
For example, comparison of the PA6 polyamide properties with hard coal, biomass, coal sludge, and sewage sludge, can indicate that PA6 polyamide is distinguished by a high content of volatile parts, carbon element, nitrogen element and high calorific value.
1.1. Production of Plastics in the EU
The plastics industry in Europe (the EU 27 + 3 countries) includes producers, converters, recyclers of plastics, and manufacturers of equipment and machinery for processing of plastics. The trade balance in 2020 amounted to nearly EUR 16 billion [3].
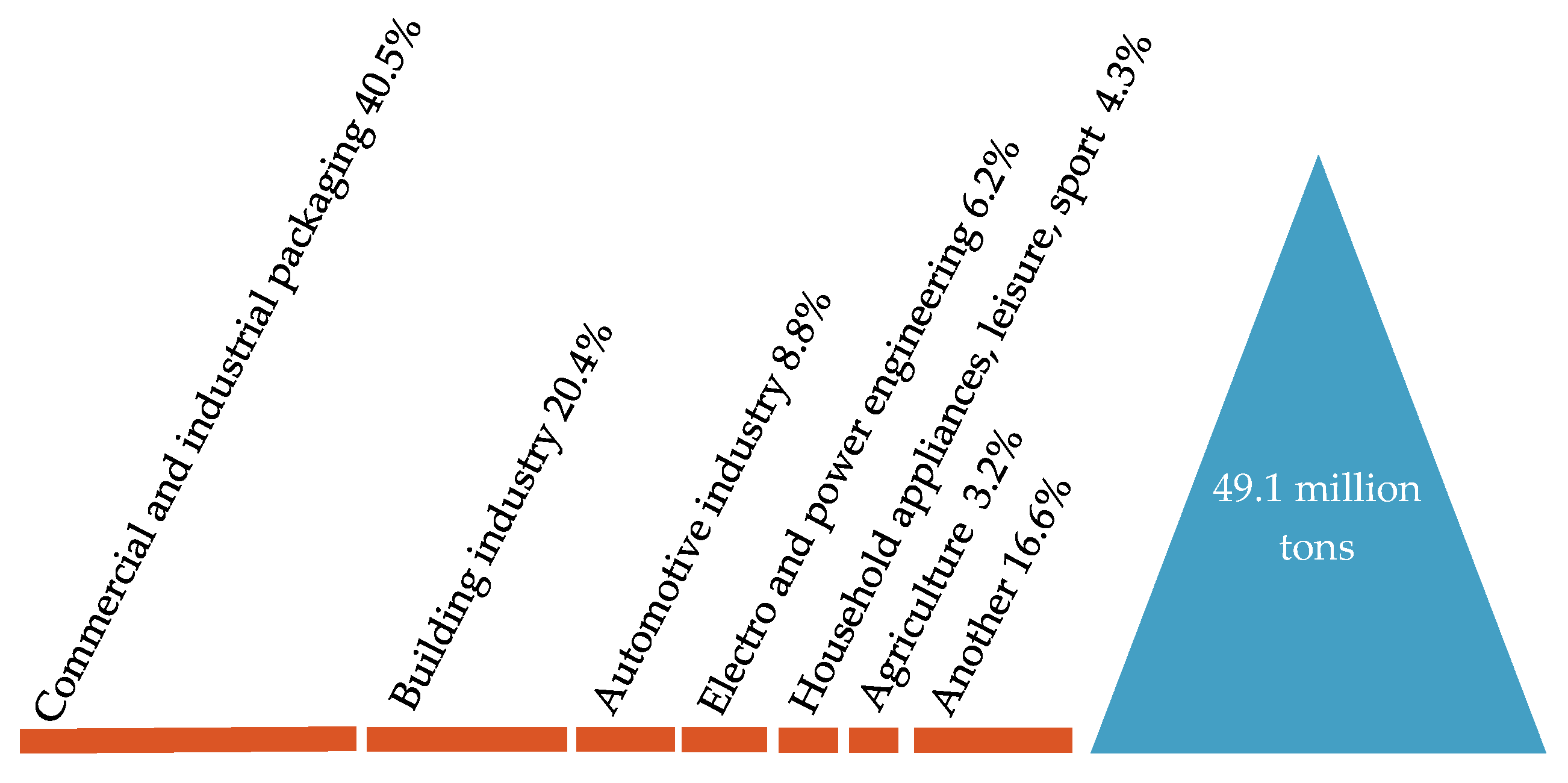
In Europe, the packaging sector uses 39.7% of all plastics. The second largest sector of using plastics is the construction industries, whose share in the total consumption of plastics is 19.8%. The automotive industry is in third place with a share of 10.1%. Applications of plastics in the electrical and electronics industries account for 6.2% of overall plastics consumption, the household appliances for 4.1% and agriculture for 3.4%, relatively. In other application areas, including household appliances and equipment, furniture or medical devices, together, 21.7% of plastics are consumed [3,10,11].
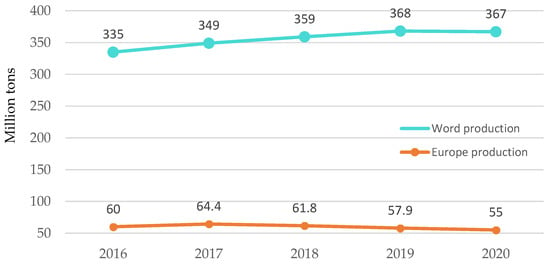
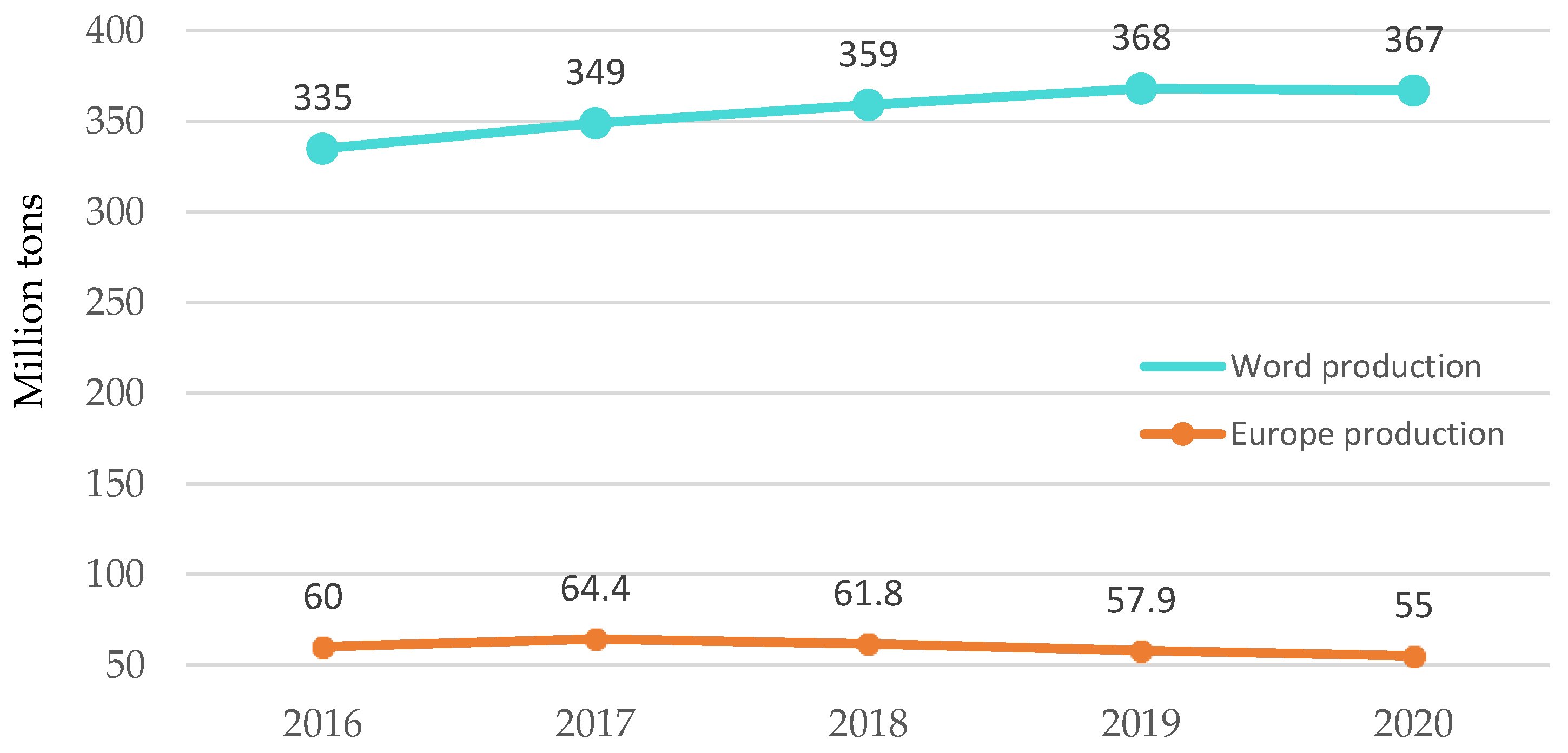
Figure 1.
Worldwide and European development of plastics production. Made based on [3].
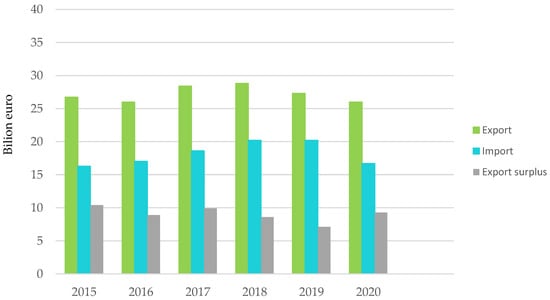
Figure 2.
Plastics production and trade balance with non-EU27 countries. Made based on [3].
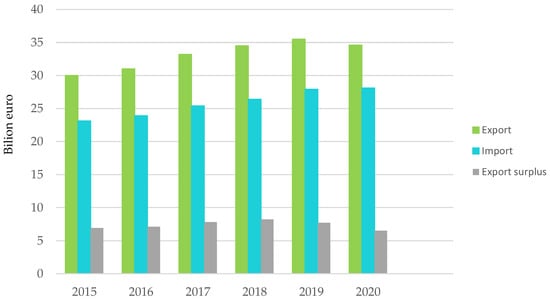
Figure 3.
Plastics processing and trade balance with non-EU27 countries. Made based on [3].
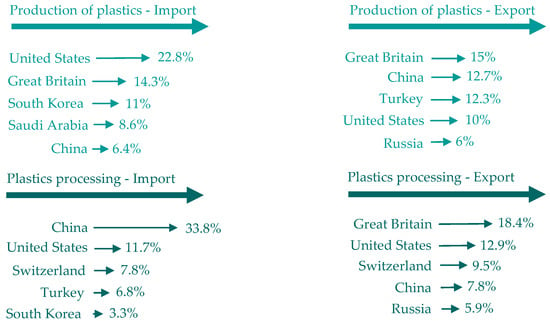
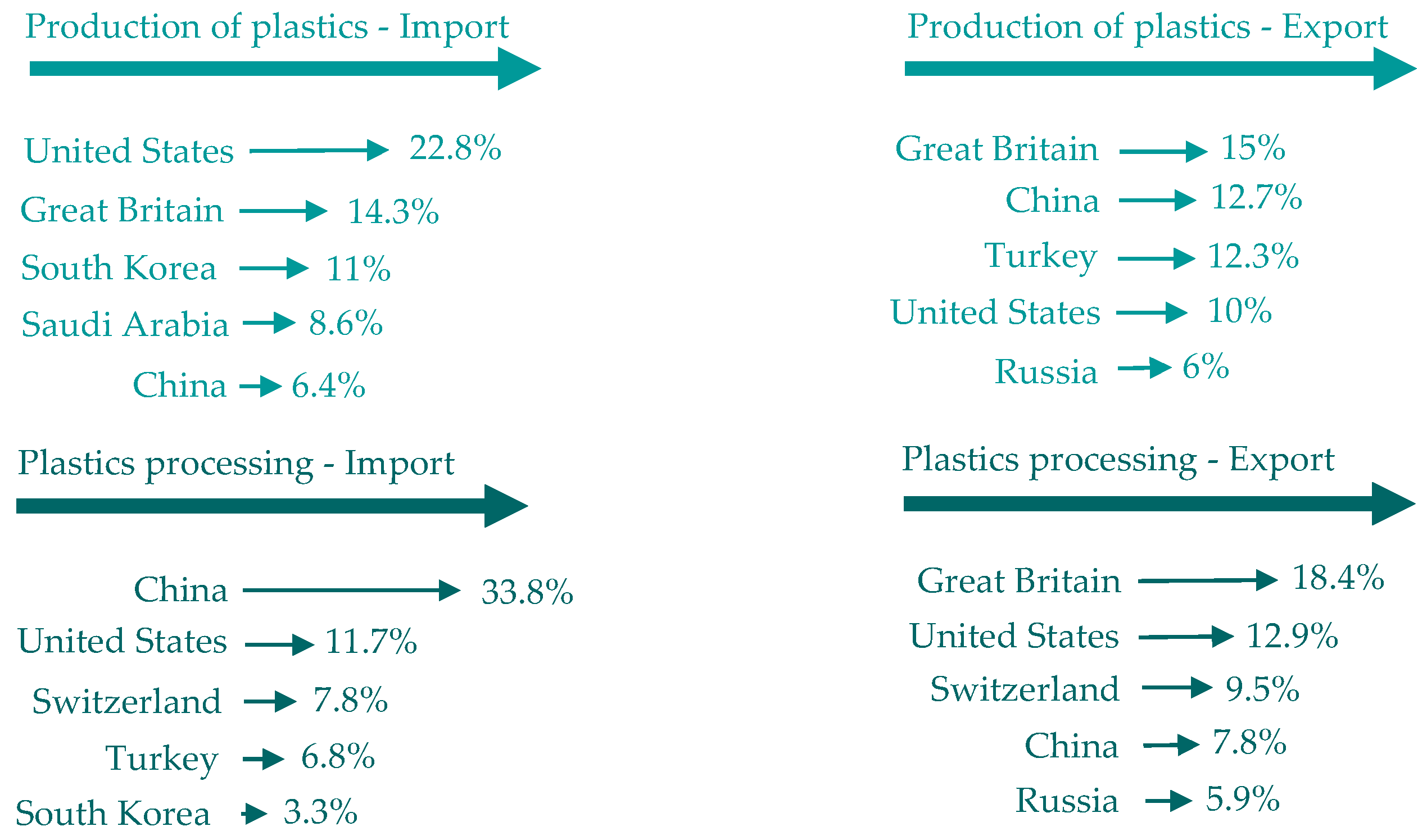
Figure 4.
Trade outside the EU in terms of sales value. Made based on [3].
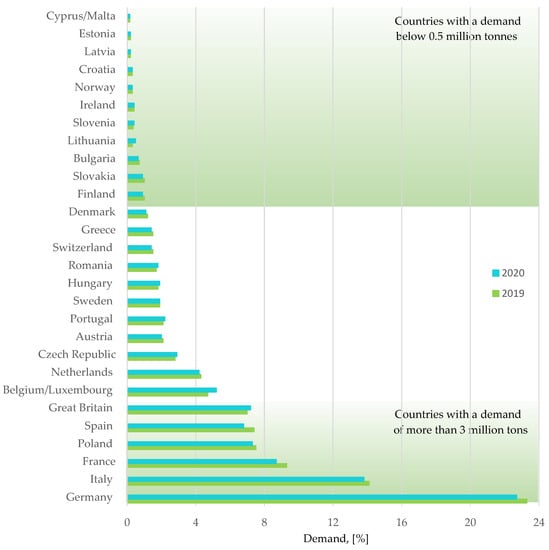
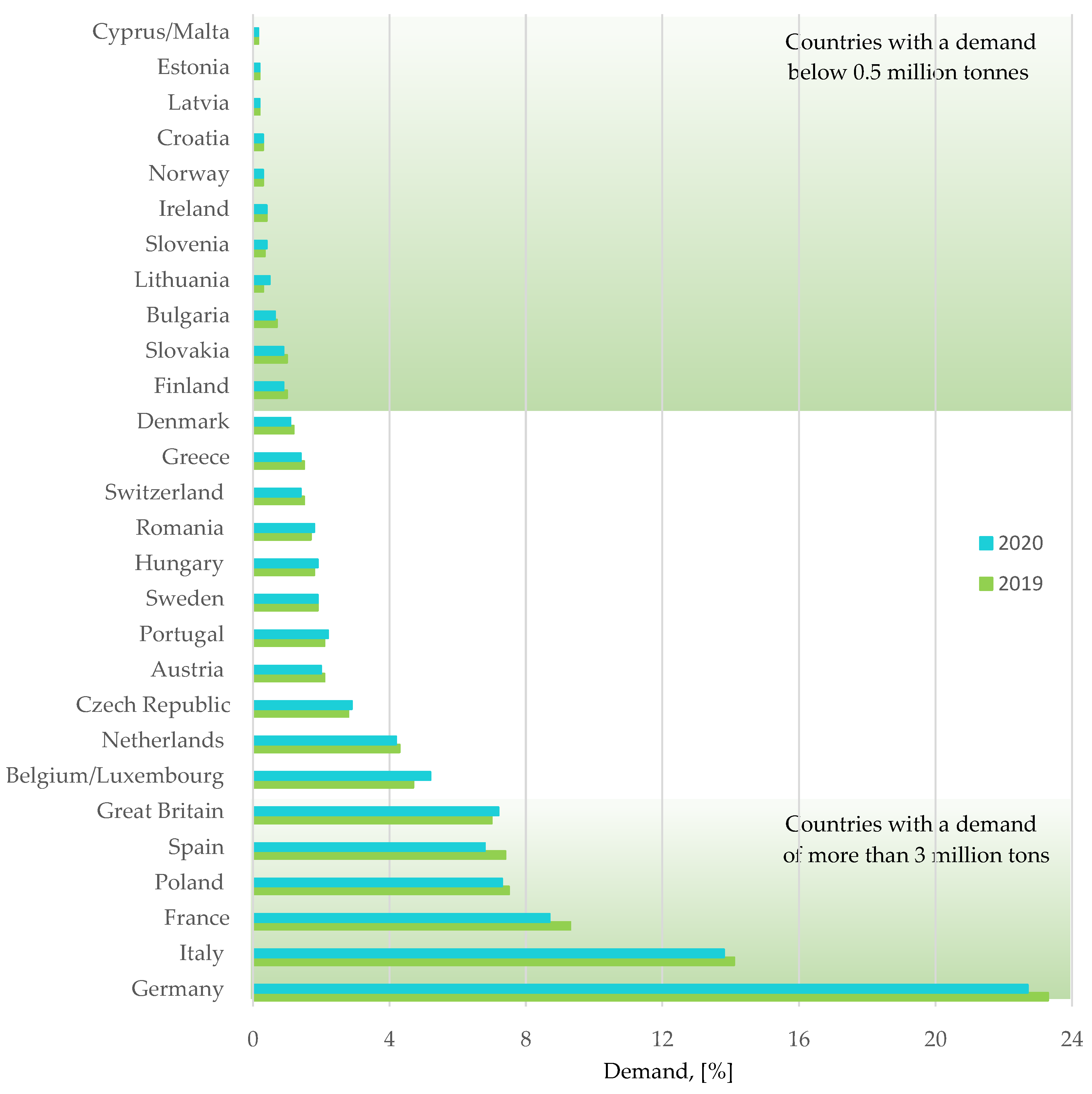
Figure 5.
Plastics demand by country. Made based on [3].
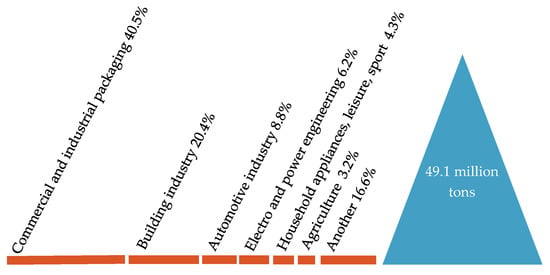
Figure 6.
Demand for plastics in the EU27 + 3 by application segments. Made based on [3].
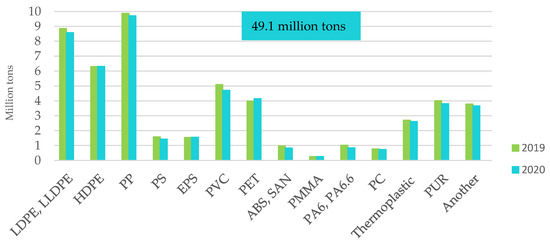
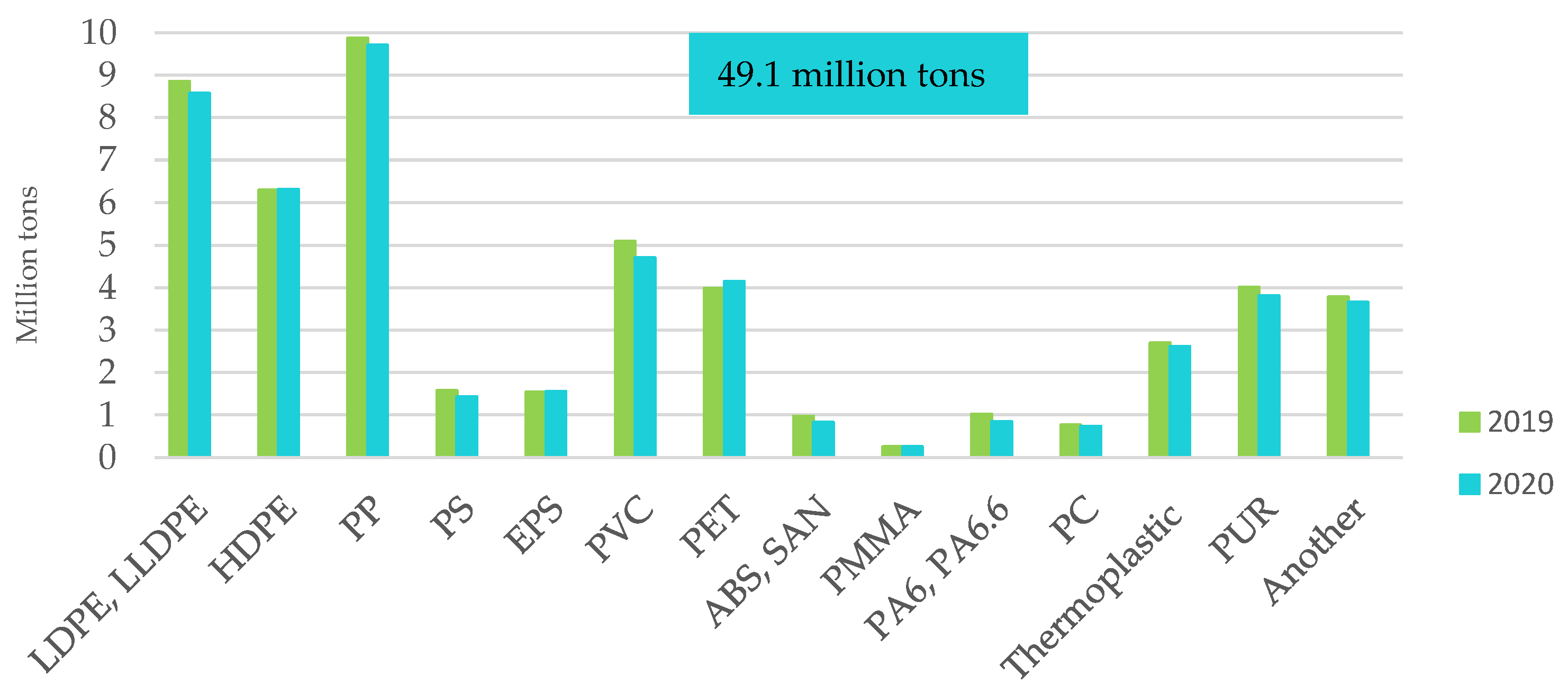
Figure 7.
Demand for plastics in the EU27 + 3 by type of polymer in 2020. Made based on [3].
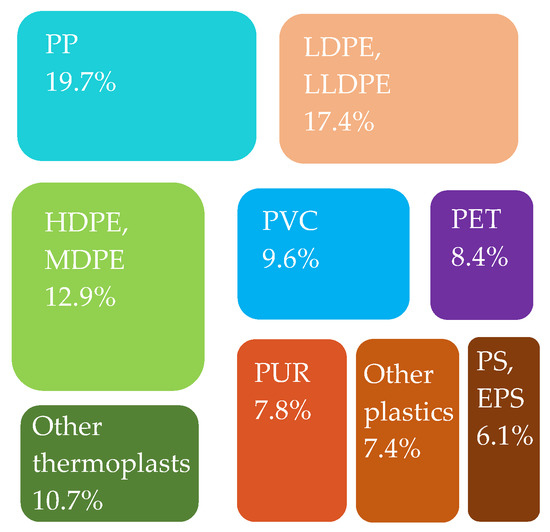
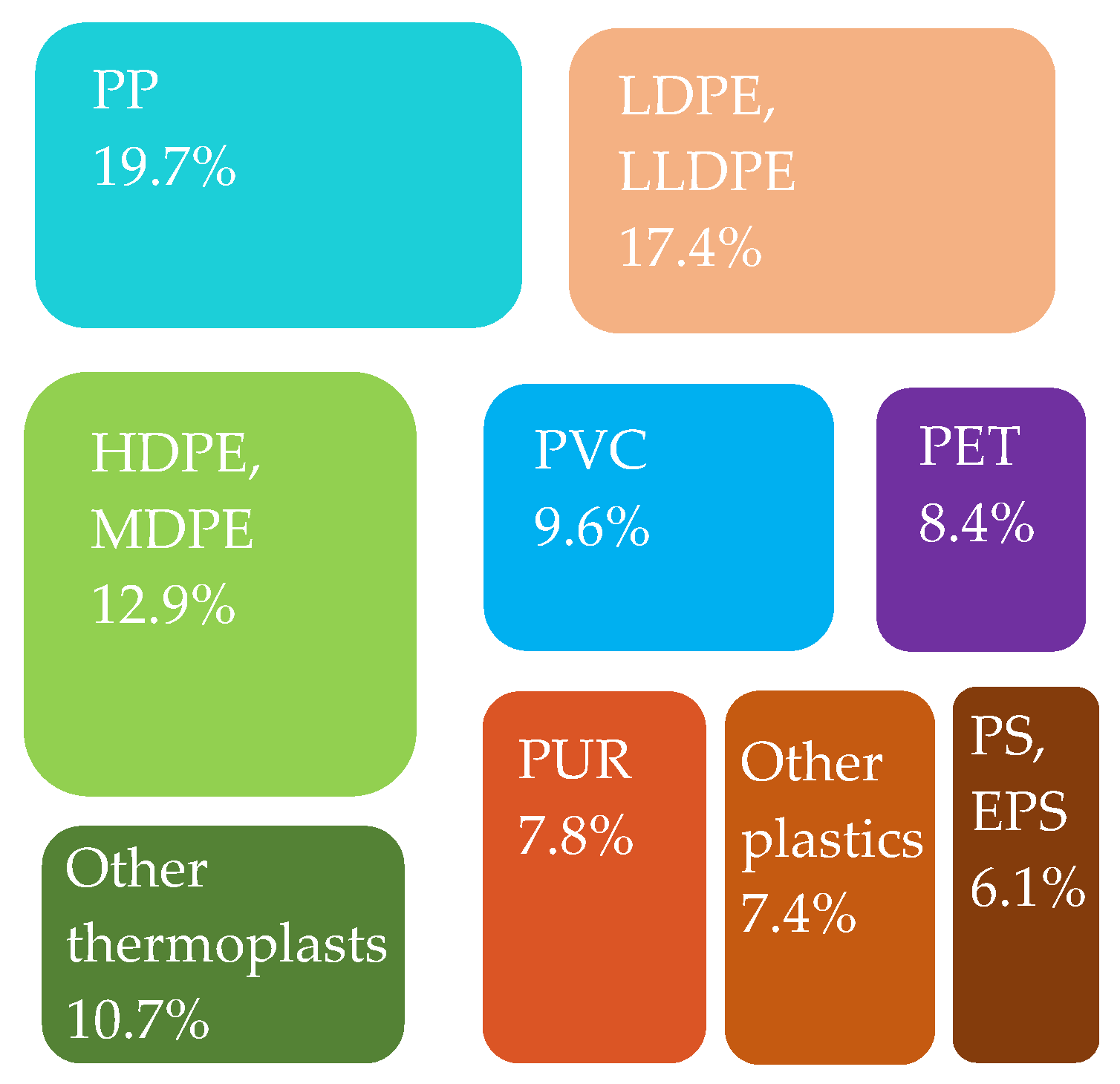
Figure 8.
Plastics converters’ demand in the EU27 + 3 by polymer type in 2020. Made based on [3].
- The global and European development in the plastics production;
- The plastics production and the trade balance with non-EU 27 countries;
- The processing of plastics and the trade balance with non-EU 27 countries;
- The trade outside the EU in terms of sales value (in 2020, the main trading partners of the EU 27 were the US, UK and China);
- The demand for plastics by country;
- The plastics demand in the EU 27 + 3 by application, in 2020;
- The demand for plastics in the EU 27 + 3 by polymer type, in 2020;
- The plastics converters’ demand in the EU 27 + 3 by polymer type, in 2020 [3].
Plastic waste is a threat to the natural environment. Mismanaged plastics can enter the rivers, seas, oceans, and soil. This is extremely important due to the decomposition time of plastic waste (for example, a foil bag decomposes for almost 450 years, and a PET bottle, for a thousand years) [11].
1.2. Management of Plastics in the EU
In European countries 39.7% of all plastics is used in the sector of packaging. Then, in terms of size, the sectors of using plastics are the construction and industry of construction (19.8%), the industry of automotive (10.1%), the electrical and electronic industry (6.2%), the household appliances sector (4.1%), the agricultural sector (3.4%), and for example: household appliances and products, furniture, medical devices (21.7%) [3,10].
In recent years, the amount of plastic waste recycled has increased, with simultaneous decrease in the amount of waste going to landfills and the maintaining the level of waste destined for energy recovery. For example, in 2020, nearly 10.2 million tons of waste of plastic was recycled and stored [3].
The fight against plastic in the European Union has been intensified since 2018. March 2019 brought the approval of The Single-Use Plastics Directive by the European Parliament. The waste package means new obligations for member states in the field of waste management and disposal, it also introduces new levels of recycling:
- 55% by 2025;
- 60% by 2030;
- 65% by 2035.
The Plastics Directive in the EU is a series of regulations that are phasing out non-recyclable plastic from production. The most important assumptions are:
- From 2021—a ban on the placing of plastic single-use products on the market, for example food containers and polystyrene cups;
- From 2025—all plastic bottles must be produced from recycled material, with a minimum of 25%;
- From 2030—all plastic bottles must be made from recycled material, with a minimum of 30%;
- By 2025—the collection and recycling of single-use plastic drinking bottles will reach of 77%;
- By 2029—the collection and recycling of single-use plastic drinking bottles will reach of 90%;
- From 2025—plastic caps and lids must be permanently attached to bottles and containers (then they will be allowed to be placed on the market) [12].
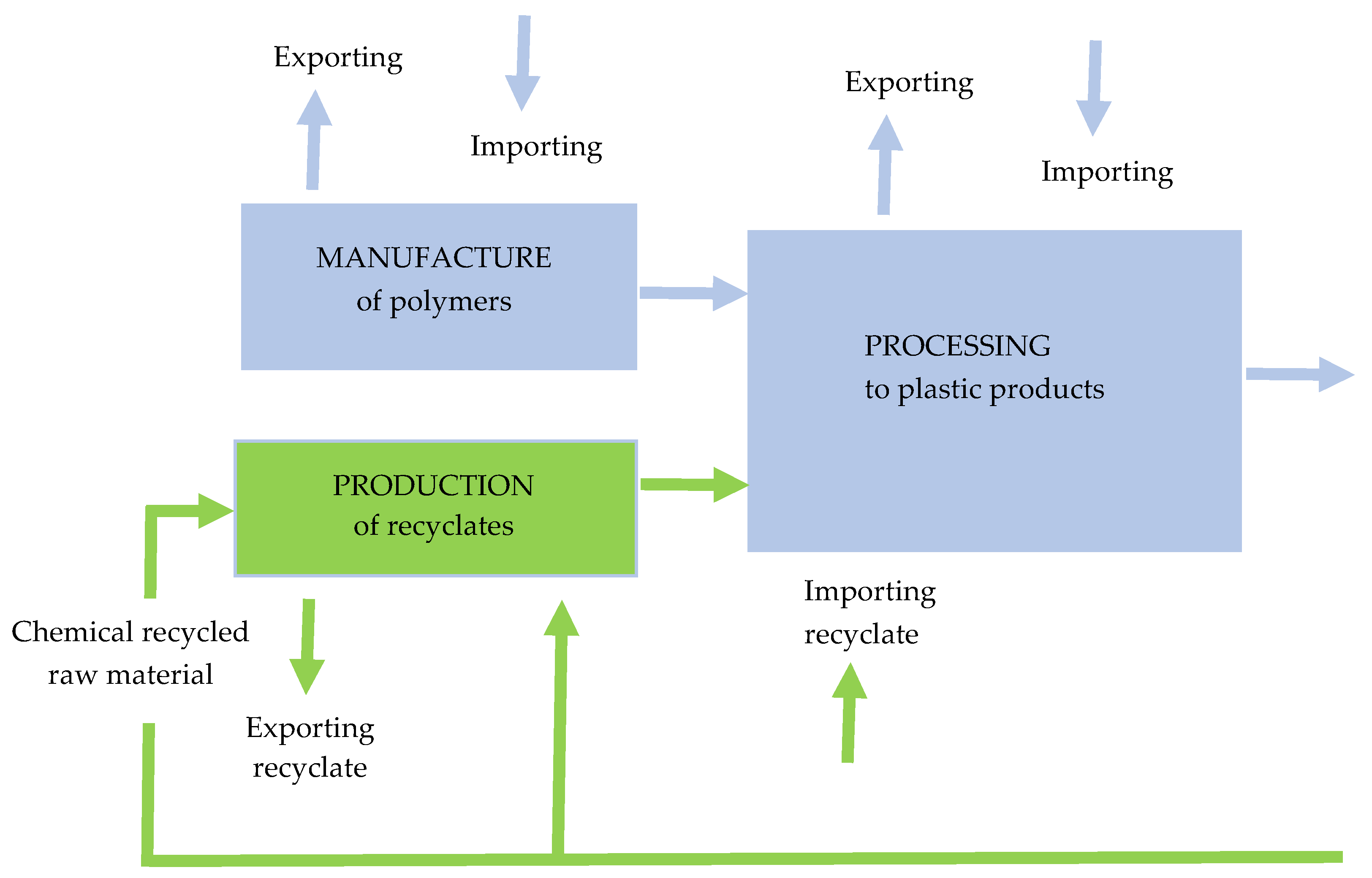
Figure 9 illustrates the circularity of plastics (production, conversion, recycling, with aspects of exports and imports) [13].
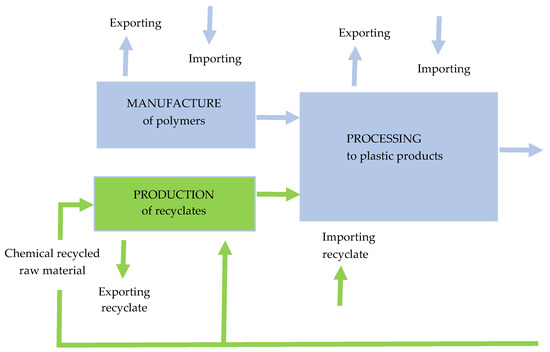
Figure 9.
The circularity of plastics. Made based on [13].
The sources of plastics for recycling are from household, agricultural, municipal, industrial waste, and the waste of restaurants, hotels, and shops [2].
For example, from 2009 to 2019, the quantity of packaging plastic waste generated (per one inhabitant in the EU) increased by about 24% [14].
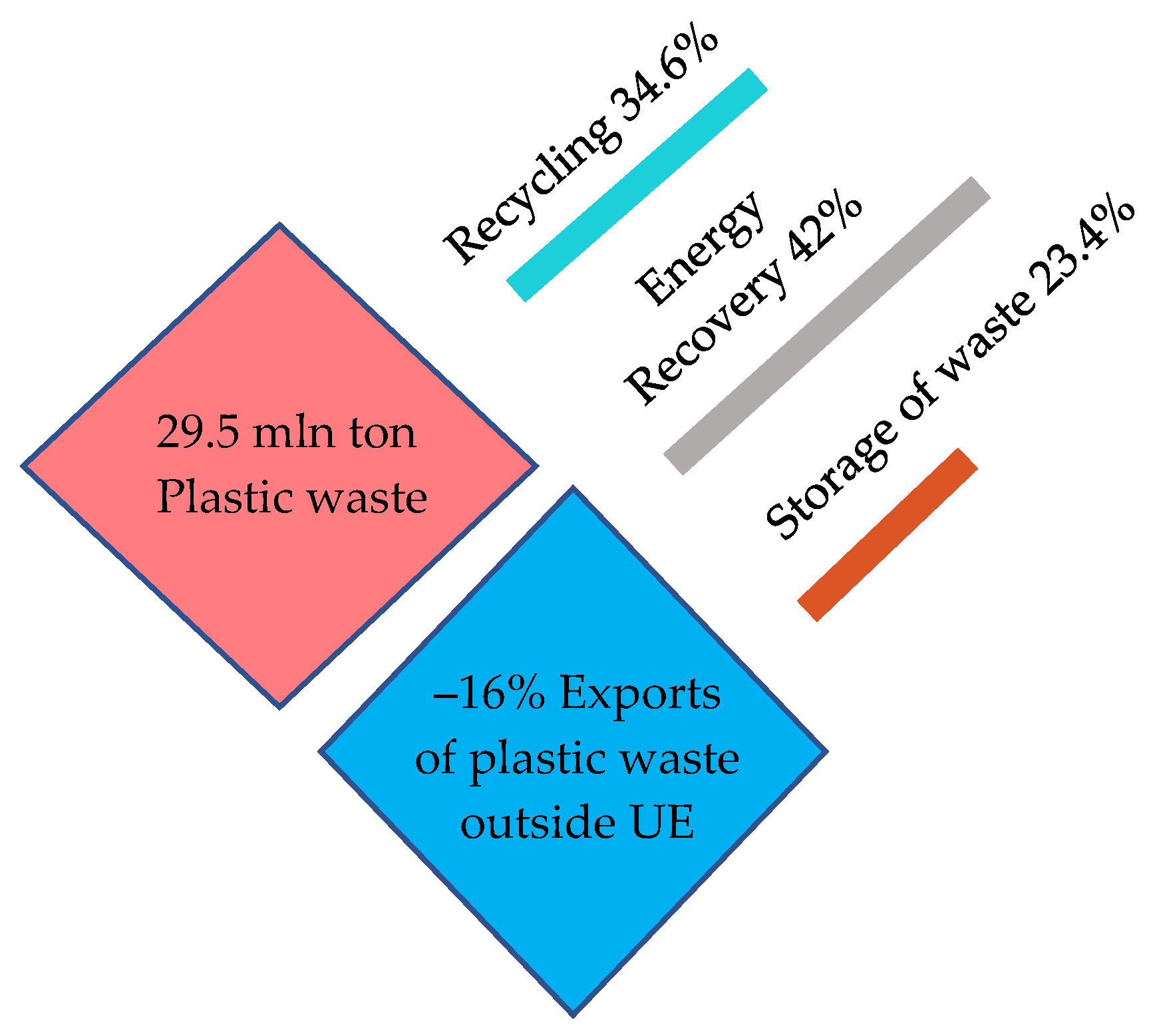
In 2020, more than 1/3 of plastic waste was recycled (inside or outside the EU27 + 3), 40% of plastic waste was energy recovered, and over 23% of plastic waste was landfilled.
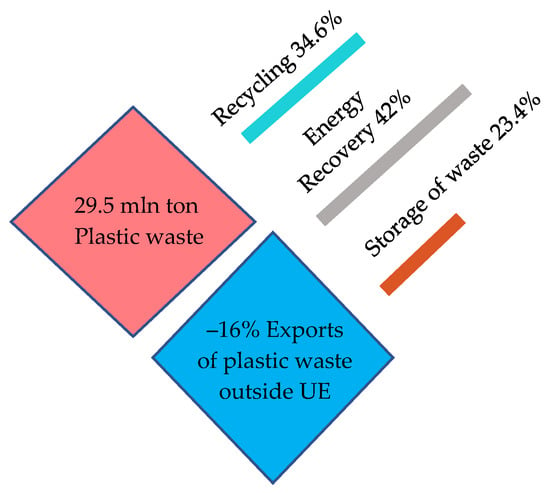
Figure 10.
Waste management in 2020. Made based on [3].
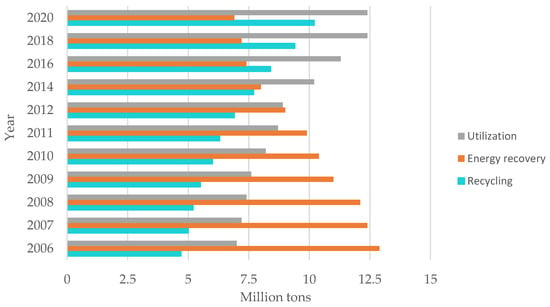
Figure 11.
Waste management in 2020. Made based on [3].
2. Recycling of Plastics
Plastics can be recycled in two ways:
- Without changing the chemical structure—the so-called material (mechanical) recycling, consisting of shredding the waste in order to obtain regranulate or recyclate that meets specific quality requirements. In material recycling, the thermoplastic properties of processed materials are used (most often, bottles and packaging films are subject to mechanical recycling);
- With a change in the chemical structure—the so-called chemical (raw material) recycling, consisting of the material degradation to low-molecular compounds or to the initial compounds and their derivatives.
Raw material recycling may take place in the following processes:
- Glycolysis, hydrolysis, methanolysis (when chemical solvents are used);
- Pyrolysis (thermal decomposition without the use of oxygen), gasification (decomposition to gaseous products), hydrocracking (decomposition in the presence of hydrogen), combustion, and co-incineration—when the change takes place under the influence of temperature.
The above-mentioned processes allow the recovery of liquid and gaseous hydrocarbons, which are used as fuel in the energy and cement-lime industry and as a raw material for the production of further products. An example of raw material recycling is, among others, the processing of PET bottles into unsaturated polyester resins, which are a component of paints and varnishes [1].
Together with the sustainable development strategy, which aims to slow down the depletion of raw materials and energy resources and irreversible changes in the natural environment, the following are necessary:
- Increasing the renewable sources share in the acquisition of raw materials and energy carriers, not only in order to save the existing non-renewable sources and reduce environmental damage, but also to supplement the increasingly unfavorable energy balance in the world;
- Striving for the multiple use of materials and energy;
- Saving raw materials and energy, also due to the currently unrealistic reduction in production and the production of long-lasting goods [10,15,16].
- Fractional composition of waste is:
- The proportion of mass-use polymers (PE, PP, PS, PVC, PET) in polymer waste is dominant—approx. 71%;
- Polyolefins and PET, with a short life cycle (max. 2 years) in the economy, constitute of over 50% of total waste.
Mixed fractions, which contribute to approx. 11% of polymer waste, can be considered as poorly useful or not suitable for secondary trading or mechanical recycling.
However, they are a good material for energy recovery, the pyrolysis process, and the gasification process [6,15,16,17,18,19,20,21,22,23].
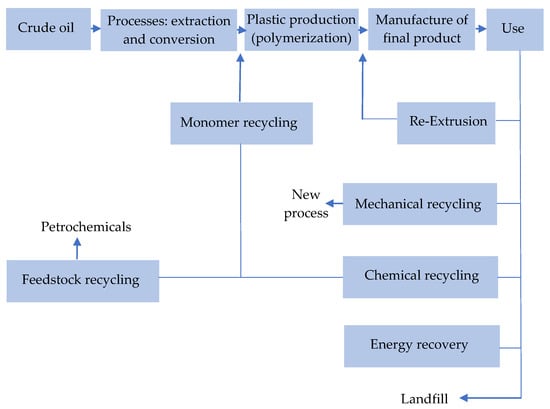
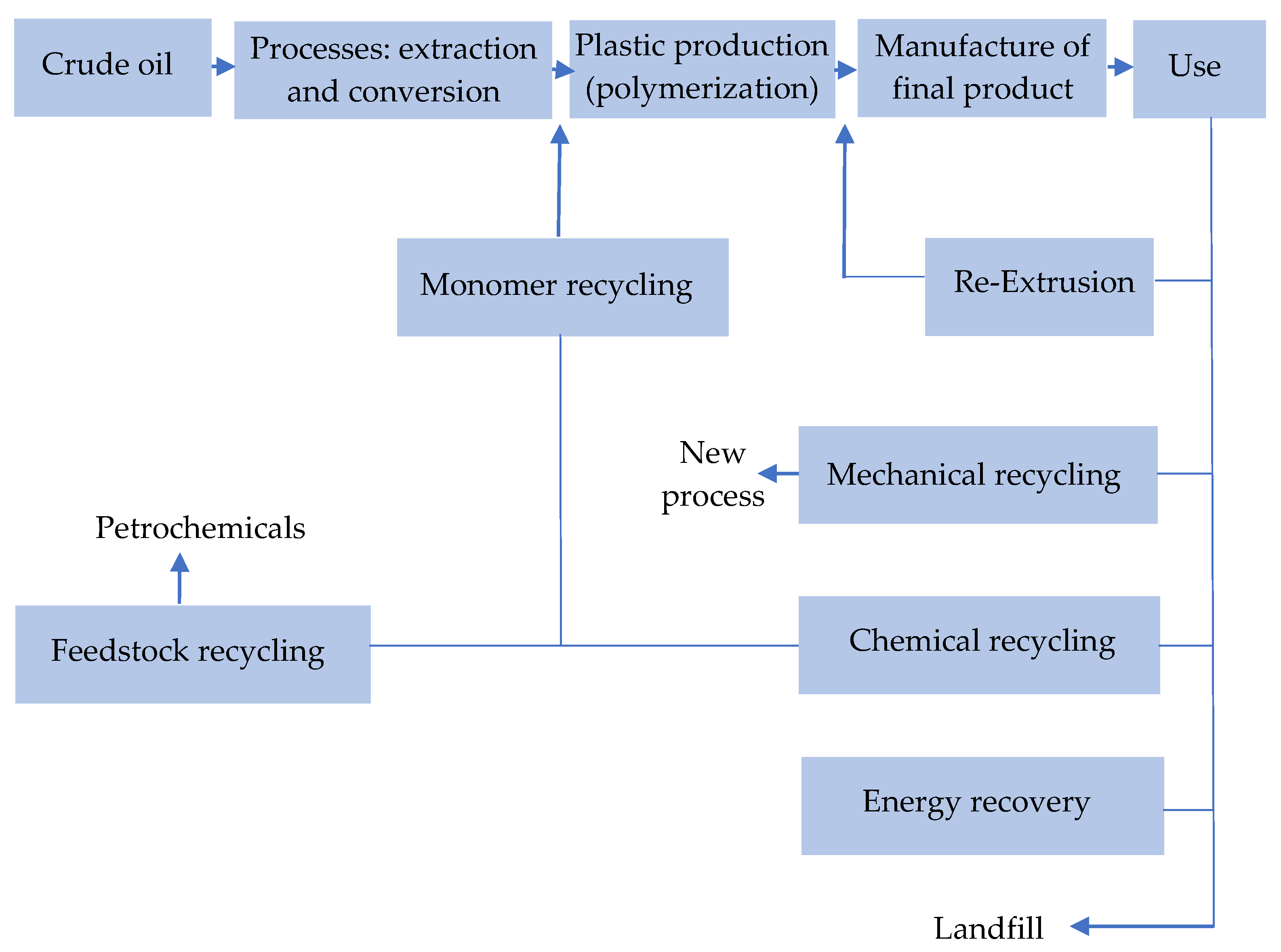
Figure 12.
Schematic of recycling methods. Made based on [24,25].
The choice of the recycling plastics optimal method depends on contamination degree of the material and the size of the waste stream.
Chemical recycling (including hydrolysis) is often applied for plastics of a quality that can be reused in the production of polyesters, polyamides, polyurethanes, and polycarbonates. Due to the use of steam, the hydrolysis enables the decomposition of polycondensation polymers and their reuse. Plastic bottles (PET), PVC window profiles, PVC pipes, polystyrene (EPS), agricultural films, polyethylene (HDPE) bottles, car bumpers are mainly intended for mechanical recycling. In the recycling process, by creating mixtures, introducing additives and various types of innovations, these wastes are refined in order to increase the recyclate strength, stiffness, and viscosity. Plastics with a high degree of pollution are incinerated. In this way, the thermal energy contained in the plastics is recovered. Factors hindering the process of recycling plastics:
- Hard-to-remove elements made of other materials: labels, etc.;
- Solvent-based adhesives used for sticking paper labels and other such elements.
As a result of plastics recycling, there are, among others: bottles for household chemicals, PVC window frames, garbage bags, car accessories, furniture, garden furniture, city benches, buckets, canisters, containers, pots, road bollards, fences, noise reduction screens, foils, thermal insulation boards, elastic threads, toys, and pens [1].
During primary recycling, the plastic waste that has similar features to the original products is used. These issues were discussed, among others, in the papers [25,26,27].
2.1. Mechanical Recycling
Mechanical recycling is the secondary recycling. It is the process of plastic waste recovering for the re-use in products manufacture (for example window and door profiles), with the mechanical means. Mechanical recycling can be performed for single-polymer plastic, for example PP and PE, because the more contaminated and complex the waste is, the more difficult mechanical recycling is. [28,29,30].
The issue of mechanical recycling of plastics was also undertaken in a number of papers, including [25,31,32,33,34,35,36,37].
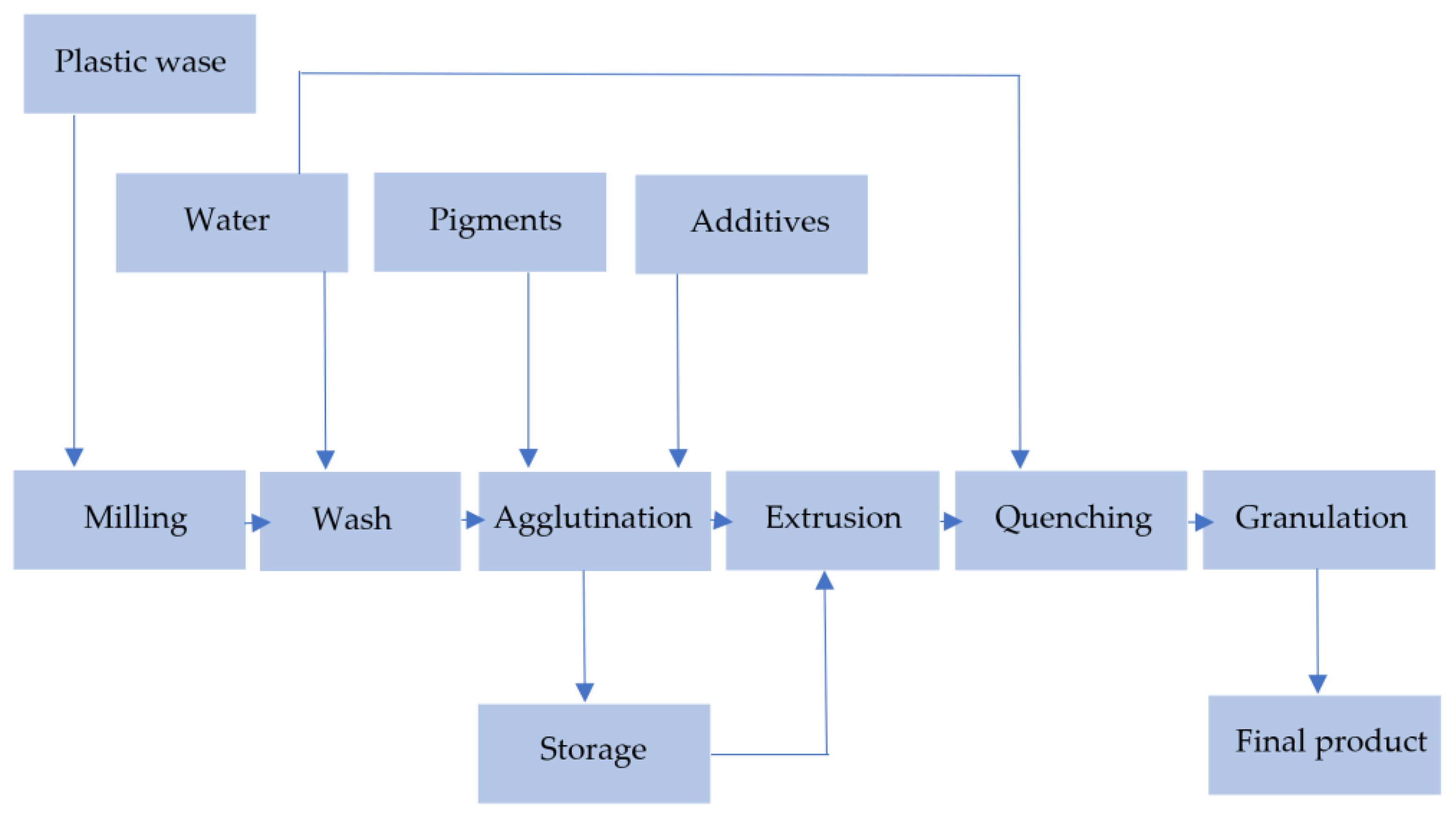
The mechanical recycling of plastics involves the following steps:
- Cutting and shredding;
- Contaminant separation;
- Floating;
- Milling;
- Water washing, chemical washing, drying;
- Agglutination;
- Extrusion;
- Pelletizing, quenching, and selling as a final product [2,37].
Figure 13 presents the most general scheme, described by [37].
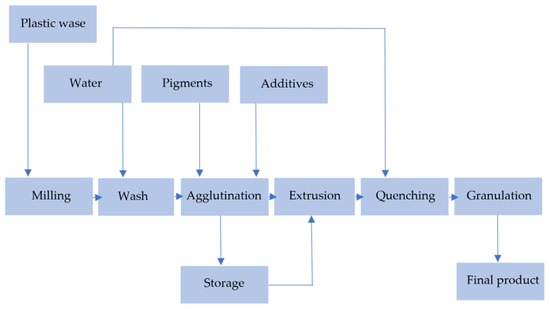
Figure 13.
Steps of mechanical recycling. Made based on [31,37].
2.2. Chemical Recycling: Thermal Utilization of Plastic Waste
Chemical recycling is a process in which a plastic polymer is split into monomers [2,28].
Chemical recycling is more expensive than mechanical recycling. The chemical recycling methods of plastics are: cracking, depolymerization, pyrolysis, gasification (the production of alternative fuels from plastic waste), and combustion—for example, in waste incinerators. The heat of combusted plastic waste can be used for production of electricity or steam [2,24,25,28,31,38,39,40,41,42,43,44,45,46].
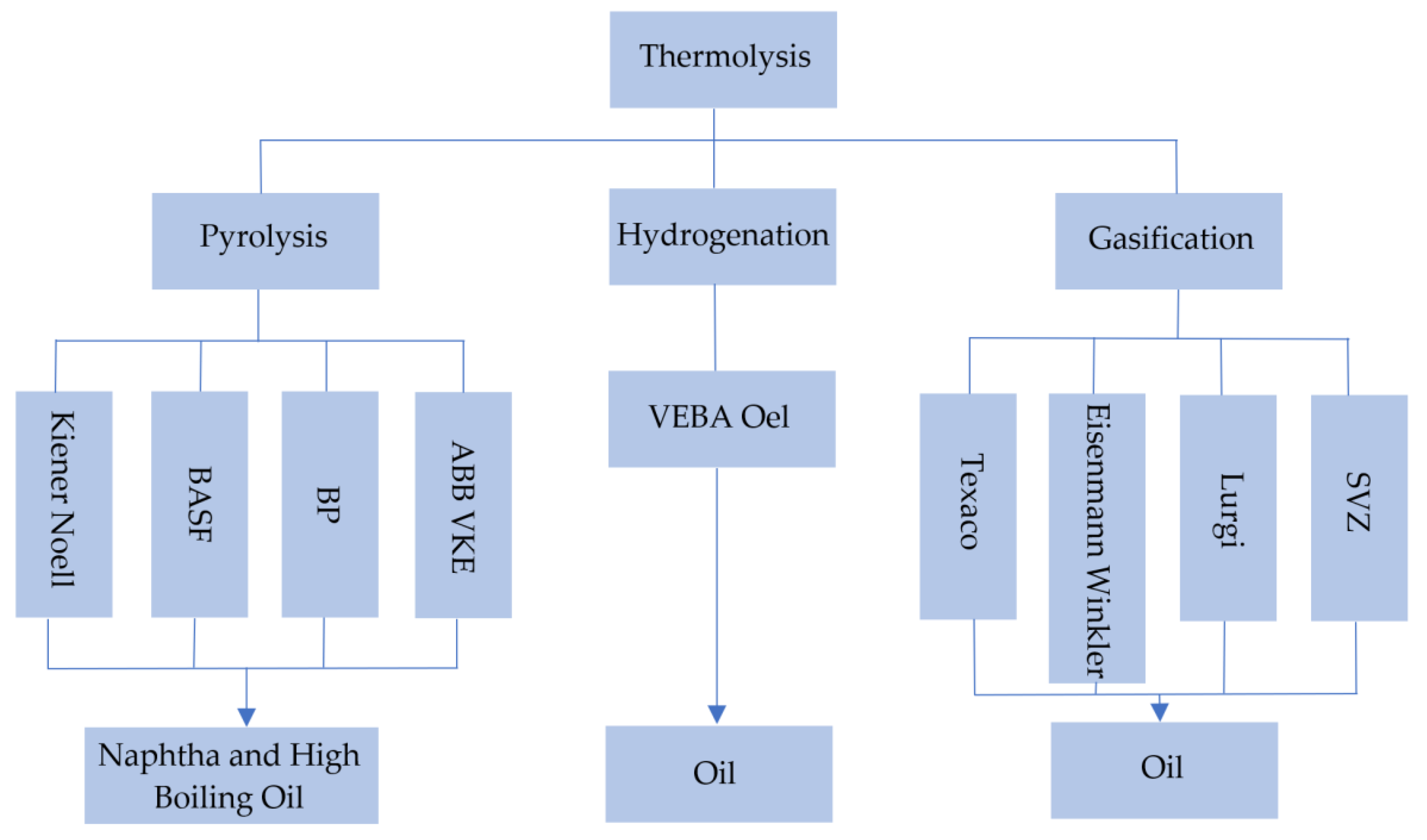
Figure 14 shows thermolysis scheme [28].
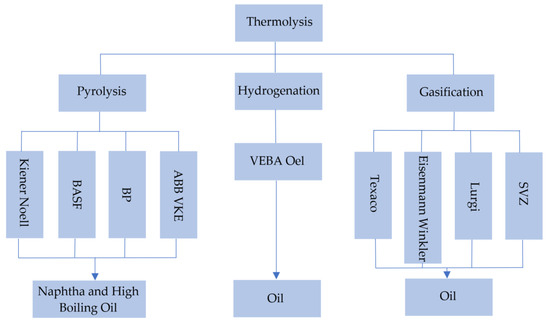
Figure 14.
Types of thermolysis. Made based on [24,28].
The subject of thermal utilization of plastic waste is extremely important in relation to:
- The need of waste disposal that cannot be reprocessed;
- More and more restrictive environmental legal regulations related to the storage of this type of waste;
- Energy recovery possibilities.
Polymer waste is characterized by a high calorific value (30 MJ/kg), in comparison to the calorific value of hard coal (30 MJ/kg), diesel oil (43 MJ/kg), or gasoline (46 MJ/kg). The high calorific value of “garbage” polymer waste is determined by the dominant polyolefins, characterized by a high H: C ratio (calorific value of pure polyethylene is 43 MJ/kg). Direct incineration of polymer waste in typical grate furnaces causes some technical difficulties—veneering the walls of chambers and grates in furnaces is also expensive, because the economical balance of incineration must include the costs of separation of polymeric materials from the total municipal waste stream. The incineration of polymer waste together with municipal waste is much easier, and the presence of the former makes it possible to incinerate municipal waste without introducing additional fuel. It is worth adding that polymer materials are much more “ecological” fuel than some mineral fuels containing heavy metals and even radioactive elements that remain in the ash. Energy recovery from municipal waste containing polymeric materials is most often carried out in waste incineration plants, but also in cement kilns, energy installations, and in metallurgical processes. A large part of the plastic fraction already separated in waste segregation installations is also used for energy recovery [3,10,11,15,16].
Energy recovery from mixed plastic waste in waste technologies has many opponents, too. One of the main arguments against it is the formation of CO2 and a large number of toxic compounds in these processes, such as chlorine, hydrogen chloride, phosgene, benzene and its derivatives, hydrogen cyanide and ammonia, formic acid, formaldehyde, phenol, as well as polychlorinated dioxins and dibenzofurans. Such risk is posed by the high content of halogens and nitrogen in the composition of some polymers. The sources of the greatest emission hazards in combustion processes are PVC and condensation polymers (PUR, polyamides and phenyl-formaldehyde resin). While taking the highest degree of caution when selecting the combustion technology of the aforementioned polymers and resins, the use of technology is extremely important in cleaning flue gases from the above-mentioned hazardous substances and other volatile organic compounds [3,10,11,15,16].
2.2.1. Cracking
Cracking is a technological process used to transform heavy crude oil into gasoline and oils. Cracking is the initiation of a controlled decomposition of long aliphatic hydrocarbons contained in heavy fractions, such as mazout and oil fractions, obtained in the refining of crude oil, into compounds with shorter carbon chains, such as those found in gasoline and diesel fuel. The chemical reactions taking place during cracking boil down to the breaking of single chemical carbon–carbon bonds, with the formation of free radicals. As a result of the reactions, the short-chain aliphatic hydrocarbons, and methane, LPG, unsaturated hydrocarbons and coke are produced. Cracking can be initiated thermally, catalytically or by radiation (using ionizing radiation). Thermal-catalytic cracking takes place at about 400 °C, in the presence of a catalyst (metal-activated aluminum silicates), under pressure p = 0.2–0.5 MPa. A variation of cracking is catalytic hydrocracking that is conducted at 250–450 °C, in the presence of hydrogen at a pressure of 3–15 MPa. Cracking of polymer waste to light hydrocarbon fractions can be conducted as thermal cracking, catalytic cracking (pyrolysis) and hydrocracking. All these operations reflect the processing of heavy fractions of crude oil. Process solutions in this area are created on the basis of very advanced refining and petrochemical solutions, both in the field of equipment solutions and the catalysts used [47,48,49].
2.2.2. Depolymerization
Depolymerization is a chemical recovery method. Condensation polymers (polyamides, polyesters, polyethers, PET) can be depolymerized, as a result of processes opposite to polycondensation, leading to the starting carboxylic acids: diols or diamines. Common depolymerization reactions are hydrolysis, alcoholysis, glycolysis and acidolysis. In addition to the starting materials, these processes produce oligomers with functional groups, suitable for the production of alkyl and polyester resins as well as polyurethanes or plasticizers for polymers [50,51,52].
2.2.3. Pyrolysis
Pyrolysis of plastics is the thermal decomposition of, for example, polyethylene terephthalate, polystyrene, polymethylmethacrylate and certain polyamides, into monomers, in the oxygen absence, in the temperature of 400–980 °C. The products of pyrolysis are: a fuel gas (CO, CO2, H2, CnHm), liquid—oils, and solid residue—char—black carbon. The higher the temperature of process, the lower the liquid/gas ratio [2,41,42,43,46].
The plastics pyrolysis is a promising technique of waste utilization. The authors presented the studies of polyolefins and polystyrene pyrolysis under autogenic atmosphere, at 450–700 °C. At 450 °C, polyolefins were converted to ~90–97 wt% pyrolysis oil. The polystyrene was converted to ~9 wt% of char, ~89 wt% of pyrolysis oil, and ~2 wt% of gas. The authors stated that, the yields of pyrolysis char and methane increase with the increase of temperature. At the temperature of 700 °C, the yields of pyrolysis chars were ~45 (for polyolefins) and ~74 wt% (for polystyrene), and ~1 wt% of pyrolysis oil [44].
The use of solid catalysts (silica-alumina, zeolites), during the reforming and catalytic cracking, affect better converts polyolefins into liquid fuel. This process gives lighter fractions compared to thermal cracking [24].
According to [53], the elemental compositions of majority of plastics waste and their HHV are comparable to diesel and gasoline, with a difference of sulfur content [53,54,55,56,57,58,59].
The pyrolysis conditions (no-oxygen atmosphere) lead to lower CO and CO2 emission [60,61,62,63].
A long residence time of plastics in the high temperature is connected with higher production of hydrocarbons. A short residence time of plastics in the high temperature causes that the volatiles matter minimize production of liquid, methane, and char [17,20,53,64,65,66,67].
Inclusions to pyrolysis the cold plasma allows the conversion of plastics waste into ethylene, hydrogen, methane, and recover chemicals [18].
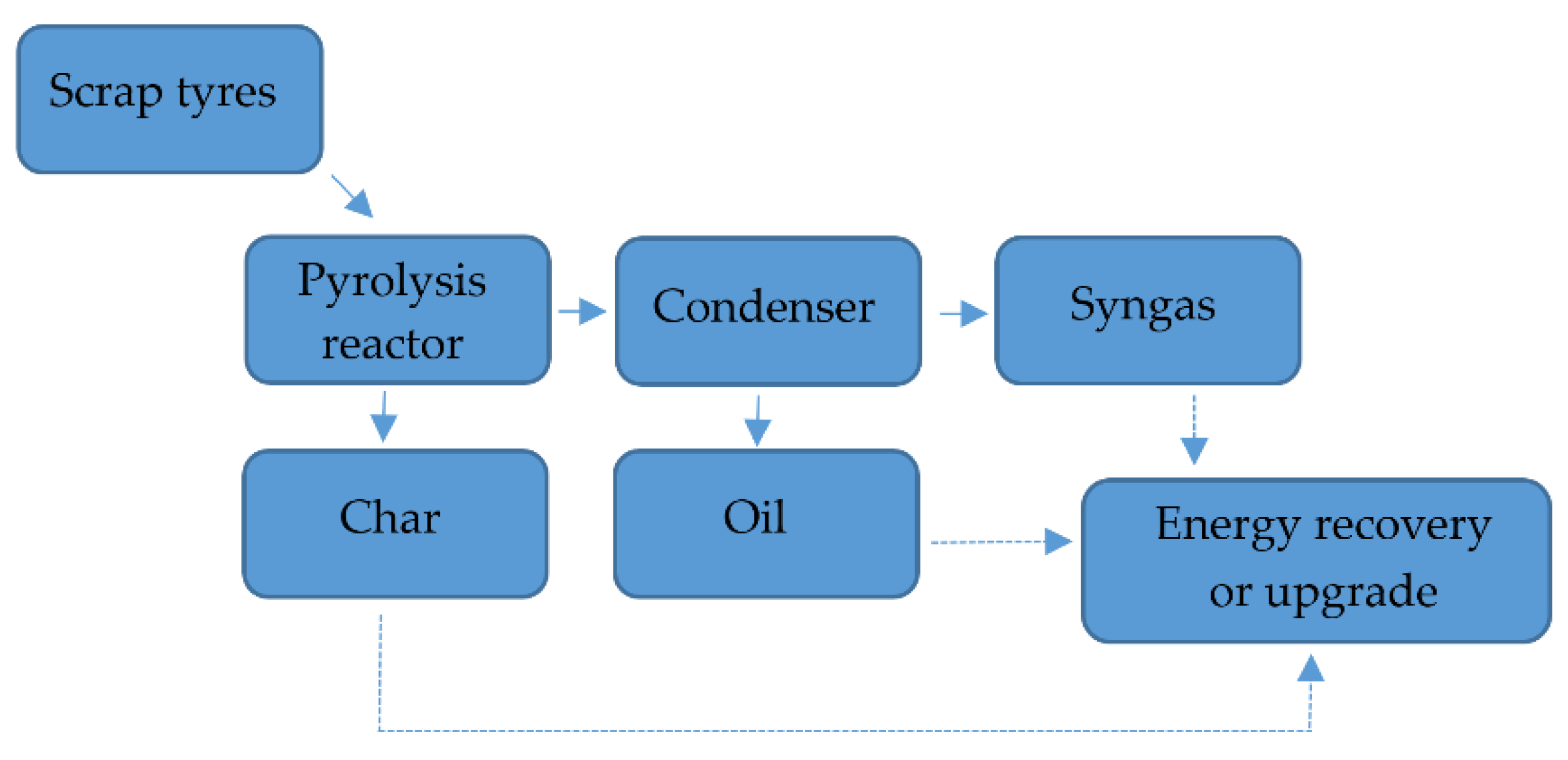
The authors of the paper [68] considered waste tires pyrolysis, at 500–600 °C. The products of the process were combustible gases (e.g., CH4), non-combustible gases (e.g., CO2), a solid fraction (e.g., ash, metals, aggregates) and the liquid fraction (pyrolytic oil). The higher temperature of pyrolysis means production of more gases, and the lower temperature of process provides more of the liquid fraction.
Figure 15 presents a pyrolysis process [68].
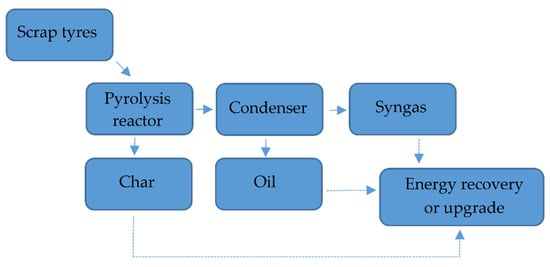
Figure 15.
Scheme of pyrolysis Made based on [68].
Pyrolytic substance is a mixture of hydrocarbons, consisting of naphthalenes, alkylated benzenes, phenanthrenes, alkenes from C8 to C15, n-alkanes from C11 to C24, and sulfur [68].
In the article [69], the authors considered the different pyrolytic reactors, from the point of view of waste tire pyrolytic recycling. They described the fixed-bed, the movable-bed reactors, and pneumatic, mechanical reactors, and reactors with moving of charge under gravity.
The authors of the paper [70] provided explorations on the effects of various catalysts on the pyrolysis performance, co-pyrolysis of biomass, and plastics and explorations on the effects of reactor designs and process parameters on performance process.
In the paper [71], the authors provided the chemical recycling of plastic waste in a fluidized bed condition. This technology is characterized by a high degree of heat transfer, which is an important solution for recycling this type of waste.
The increase in the creation of crude oil with lower hydrocarbon can be obtained by appropriate selection of catalysts, and co-pyrolysis of plastics waste with shale oil or coal improves crude oil quality [72].
The authors of this paper [73] presented TG-FTIR tests during pyrolysis of PE, PS, and PVC waste. They suggested that the process of PS and PE has one stage, but PVC has two stages. HCl is the main product of pyrolysis of PVC.
In the paper [74] the authors presented pyrolysis of different plastic waste, at 300–700 °C. HDPE produced the highest oil yield, and PET and PP produced the lowest yield. PP, LDPE, HDPE, and PS pyrolysis oils were characterized by higher calorific values than some coal and wood.
In the paper [75], the authors presented the influence of mixing plastics waste with mahua seeds for fuel properties of the obtained products, during co-pyrolysis process. The tests results revealed improvement in hydrocarbons in these products, compared to the products of plastics.
2.2.4. Gasification
Gasification is a process of organic substance (in material) thermal conversion to a combustible gas, in the presence of an oxidant. The main purpose of gasification is to obtain synthesis gas with a high hydrogen content, which is both an energy carrier and a universal resource for chemical synthesis. The synthesis gas contains hydrocarbons, too. The quality and quantity of the obtained gas depend on the composition of the polymers and conditions of the thermal process [2,46]. In the papers [2,46] the authors presented three technologies of gasification process: fluidized bed, fixed bed, and entrained flow.
In the paper [24,76,77,78,79], the authors provided the thermal processes of polymeric materials with other raw materials, as a result of which biomass and waste are developed. The gasification of plastic waste is mostly conducted at 600–800 °C [24].
The product of process is syngas (carbon monoxide, hydrogen, gaseous hydrocarbons). This gas can be a substitute for natural gas or a feedstock for the production of chemicals (in the chemical industry) [39,80,81,82]. Gasification transforms waste to the technically usable raw materials and energy [83].
The important technological aspect is co-gasification of plastics waste with other materials and fuels. The authors of papers [83,84] studied co-gasification of PE, coal, and pine in the fluidized bed conditions. For example, co-gasification of polymers with biomass has led to an increased creation of hydrogen, and to a reduced creation of CO during the process [83].
In paper [85] the authors presented gasification of PVC in steam, in a bubbling fluidized bed. The authors of paper [86] made co-gasified of PE, paper, wood, and municipal solid waste, at 400–800 °C, in a fluidized bed. They determined that to reach a LHV of about 10,000 kJ/Nm3, plastic should be gasified in excess of 500 °C.
In paper [87], the authors stipulated that the increase of temperature and oxygen content in atmosphere, during plastics gasification, led to higher quantities of multi-ring aromatic hydrocarbons in products, and to higher n-paraffin/n-olefin ratio.
The long residence time of volatiles matter in the reactors during gasification and the rise of process temperatures decrease tar formation and increase char production [24,37,88].
The increase of temperature above 500 °C leads to reduction of tar content in the gas product during gasification of plastics waste and mixtures of PSW, biomass and coal [83,85,89,90,91].
The larger olefins and paraffins produced during plastics decomposition are cracked to lighter hydrocarbons, CO, H2, CH4, and CO2, at temperatures above 800 °C [92].
During gasification of PSW, in steam and CO2, high heating rates create a more reactive char [93,94,95,96].
The high temperatures of gasification lead to the increase in the gas yield, the H2 concentration [84], and LHV [97].
2.2.5. Combustion
Combustion and co-combustion of fuels, including waste, is the subject of many scientific papers, including [8,98,99,100,101,102,103]. For example, the authors of the paper [8] presented combustion of polypropylene, polyethylene, polyvinyl chloride, and polystyrene, in reference to fuels: peat, pine wood, bituminous coal, in the atmospheric fluidized bed, and the pressurized fluidized bed. They stated that PE, PP, and PS burned such as oil, and their samples combusted completely during the pyrolysis stage, leaving no char. Only PVC produced a carbonaceous residue. Its times of heating, devolatilization, and char combustion were similar to peat and wood, and shorter than other plastics.
Combustion of scrap tires in incinerators allows to recover of 27,900–37,200 kJ/kg energy [46].
The major components of plastic extracts, during plastics combustion in open fires, are inter alia, the plasticizer di-2-ethylhexyl phthalate, even-carbon-chain n-alkanes, and the components of smoke are chiefly n-alkanes, phthalates, and terephthalic acid [104].
In paper [105] the authors emphasized that reaching net zero greenhouse gas emissions by 2050–2070 and the decarbonizing global industry are necessary to limit global warming to 2 °C. It is very important in development of technologies of iron and steel, cement, chemicals and plastics industries.
In paper [106] the authors took up the issue of plastics waste utilization in the metallurgical industry. In this technology the plastics waste is used as an additive to coal in the coking process or as a substitute for coke in the blast furnace process.
In paper [107] the authors suggested that municipal solid waste combustion in the circulating fluidized bed combustor is a very good technology of waste-to-energy processing due to the specificity of the fluidized bed process. The problem, however, is the depositing of emerging particulate matters on heat transfer surface of boiler, and releasing them to the atmosphere. It is very important to use suitable technology separation of particulate matters.
The aim of the paper [108] was the studies of plastic and biomass combustion in the fluidized bed. The polymers melted and merged with particles of sand. It was connected with the intensive release of pyrolytic gases. The dominating stage of polymers combustion was the flame combustion (about 85% of the total time of combustion). The biomass combusted slower, and it released less of combustible gases. The flame combustion of biomass was about 27% of whole process time, and the flameless burning was about 73%.
Authors of paper [109] suggested that the use of plastics waste in cement plants brings ecological and economical benefits, which result from the conditions they provide rotary furnaces.
The results of the paper [110] showed that the physical characteristics of landfill plastics are similar to diesel oil, and their thermal transformation is more ecological than diesel fuel.
In the paper [111], the authors showed the values of combustion heats for forty-nine commercial polymers.
The authors of paper [112] presented an experimental study connected with particulate emissions during polymers combustion. PS produces more fine particulates than PE, PP, PVC and PMMA. The conducting of the combustion process with excess of oxygen significantly reduces the particulate emissions from PP, PS, PE, and slightly from PVC and PMMA.
3. Summary
The year of 2020, initially triggered by the COVID-19 pandemic, followed with the sharp decline in production in plastics sector in Europe, where there has been observed an increase in plastics production, analogously associated with growth of their waste [10]. Recycling of plastics protect the environment from pollution of stored plastic, allows the plastic waste to be reprocessed into new products, and allows energy recovery. The legal conditions introduced aiming at the elimination of waste storage favor the development of the recycling industry.
The paper discusses the following types of plastic recycling:
- Mechanical one is the most widely used. First, the waste is sorted out according to the type of material, then cleaned, washed, and dried, and then it is moved to the shredding device. The resulting recyclate (regranulate) is suitable for reprocessing. The chemical properties and the composition of recyclate remain unchanged. It can be enriched with various additives in order to improve the quality of products generated in the recycling process;
- Chemical consists in subjecting the material to actions that lead to chemical reactions that change its structure and properties. The substances obtained as a result of this process (gases or liquid hydrocarbons) can be reused as raw materials for the synthesized polymers or the production of other materials;
- Thermal is a method of using plastic as a fuel or raw material for the production of heat or energy. It is applicable for high-calorific fractions of plastic waste that are not suitable for other recycling. This method is used more and more in Europe, it is environmentally safe and efficient.
The European Union, striving to improve the level of plastic waste recovery, introduces new regulations, including a tax on plastic, and the Plastics Directive. Its aim is to reduce the pollution of the oceans, seas and soil with plastic waste as much as possible. Consumables made of plastic, such as plates, cutlery, food containers and polystyrene cups, are gradually withdrawn from the market. Producers of drinks in cartons were forbidden to attach plastic straws to them [113].
The European Union is striving to achieve a circular economy model. This is due to the devastating impact of waste on the natural environment and the declining availability of raw materials for the primary production of products and packaging. Its aim is to recycle and reuse waste as much as possible for the production of new goods and packaging. It is necessary to minimize both waste production and the use of virginal raw materials. It is important to develop new ways to use existing secondary materials, by:
- Recycling and use of artificial intelligence (AI), machine learning and robotics (artificial intelligence allows you to speed up the process of secondary waste segregation and increase the level of recovery also from mixed fractions. In the future, advanced AI technologies can separate even small pollutants);
- Startups in waste sorting;
- New technologies for cleansing dyes, adhesives, and fats;
- Biodegradation technologies (the use of bacteria and living organisms to plastics degrade) [114,115];
- New applications of secondary raw materials (technologies enabling the mass use of recycled plastic in construction and furniture);
- In the future, the focus should be on an ecosystem capable of replacing disposable products and packaging with reusable solutions [114].
The authors of paper [116] presented the potential feedstocks for thermal plastics conversion, the pre-treatments used for plastic-containing wastes, prior to thermal conversion processes, the effect of catalyst in thermal processes, the technologies of thermal conversion of plastic and products obtained during the processes, emphasizing the need to a further development of plastics recycling technology.
It is important to optimize parameters of plastics thermal conversion, for example by choice of: catalysts, ratio of plastic to catalyst, reactor design, or process temperature. The fluidized bed technology is the promising technology in thermal plastics waste treatment [73].
It is important to constantly recognize and implement energy recovery technologies from plastics waste.
The converting plastics to fuel can replace fossil fuel which leads to lower greenhouse gas emissions [117].
It is very important to implement co-combustion of plastics with other materials and fuels, for example with biomass. For example, the thermal process of biomass/polyethylene (10%) does not show increase in pollutant emissions and these blends lead to the improvement of physiochemical properties of pellets and briquettes [118].
It is important to develop further the efficiency of fuels production of methods from plastics and their mixtures with other materials.
The growing demand for fuels and the need to neutralize plastic waste, provide for necessary further recognition and continuation of the research on the issues of plastics management.
Author Contributions
Conceptualization, A.K.-K. and A.G.; formal analysis, A.K.-K. and A.G.; investigation, A.K.-K. and A.G.; resources, A.G. and A.K.-K.; visualization, A.K.-K. and A.G.; supervision, A.K.-K. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Everything about Recycling. Available online: https://www.oostdam.pl/recykling-tworzyw-sztucznych/ (accessed on 17 March 2015).
- Plastics Recycling. Available online: http://www.substech.com/dokuwiki/doku.php?id=plastics_recycling#energy_recovery (accessed on 13 November 2018).
- Tworzywa—Fakty2021. Available online: https://plasticseurope.org/pl/wp-content/uploads/sites/7/2022/01/tworzywa-fakty2021.pdf (accessed on 7 January 2022).
- Dodbiba, G.; Fujita, T. Progress in separating plastic materials for recycling. Phys. Sep. Sci. Eng. 2004, 13, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Plastic Waste Management Institute. Available online: https://www.pwmi.or.jp/ei/plastic_recycling_2019.pdf (accessed on 2 January 2020).
- Adelodun, A.A. Plastic recovery and utilization: From ocean pollution to green economy. Front. Environ. Sci. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Professional Plastics. Available online: https://www.professionalplastics.com (accessed on 2 January 2021).
- Kijo-Kleczkowska, A.; Gnatowski, A.; Szumera, M.; Kwiatkowski, D. Application of thermal analysis methods in researches of polyamide, coal fuels and their composites. Pol. J. Chem. Technol. 2020, 22, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Kijo-Kleczkowska, A.; Łącz, A.; Szumera, M.; Sroda, K. Comparative analysis of sewage sludge and other fuels and their mixes, made on the basis of thermogravimetry and mass spectrometry. Rynek Energii 2016, 2, 111–120. [Google Scholar]
- Plastics—The Facts 2017. Available online: https://plasticseurope.org/pl/wp-content/uploads/sites/7/2021/11/2017-Plastics-the-facts.pdf (accessed on 7 November 2017).
- Interia Biznes. Available online: https://biznes.interia.pl/gospodarka/news-plastik-staje-sie-wiekszym-problemem-dla-srodowiska-niz-wegi,nId,5808270 (accessed on 6 February 2018).
- WWF Poland. Available online: https://www.wwf.pl/aktualnosci/plastik-not-fantastic (accessed on 30 December 2020).
- Plastics—The Facts 2020. Available online: https://plasticseurope.org/wp-content/uploads/2021/09/Plastics_the_facts-WEB-2020_versionJun21_final.pdf (accessed on 7 September 2021).
- Eurostat. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/ddn-20211027-2 (accessed on 27 October 2021).
- Bledzki, A.K.; Jeziorska, R.; Kijenski, J. Recovery and Recycling of Polymeric Materials; Scientific Publisher PWN SA: Warsaw, Poland, 2011. [Google Scholar]
- Datta, J.; Trzebiatowska, P.; Kasprzyk, P. Selected Issues of Recycling of Plastics and Rubber; Gdansk University of Technology: Gdansk, Poland, 2018. [Google Scholar]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Awasthi, A.; Shivashankar, M.; Majumder, S. Plastic solid waste utilization technologies: A review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022024. [Google Scholar] [CrossRef]
- Almeida, D.; Marques, M.D.F. Thermal and catalytic pyrolysis of plastic waste. Polimeros 2016, 1, 44–51. [Google Scholar] [CrossRef]
- Hidayah, N.; Syafrudin. A review on landfill management in the utilization of plastic waste as an alternative fuel. E3S Web Conf. 2018, 31, 05013. [Google Scholar] [CrossRef] [Green Version]
- Olufemi, A.; Olagboye, S. Thermal conversion of waste plastics into fuel oil. Int. J. Petrochem. Sci. Eng. 2017, 2, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Punkkinen, H.; Oasmaa, A.; Luntama, J.L.; Nieminen, M.; Laine-Ylijoki, J. Thermal conversion of plasticcontaining waste: A review. VTT Res. Rep. 2017, D4-1-22, 1–77. [Google Scholar]
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of plastic waste as waste-to-energy or waste-to-syngas recovery route. Nat. Sci. 2013, 05, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M. Establishing an integrated databank for plastic manufacturers and converters in Kuwait. Waste Manag. 2009, 29, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C. Intelligent Recycling (Presentation); Department of Engineering, Institute for Manufacturing, University of Cambridge: Cambridge, UK, 2008. [Google Scholar]
- Mastellone, M.L. Thermal Treatments of Plastic Wastes by Means of Fluidized Bed Reactors. Ph.D. Thesis, Department of Chemical Engineering, Second University of Naples, Caserta, Italy, 1999. [Google Scholar]
- Basfar, A.A.; Idriss, K.M. Ali Natural weathering test for films of various formulations of low density polyethylene (LDPE) and linear low density polyethylene (LLDPE). Polym. Degrad. Stab. 2006, 3, 437–443. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Influence of natural and accelerated weathering on various formulations of linear low density polyethylene (LLDPE) films. Mater. Des. 2009, 30, 1729–1736. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Kowalska, E.; Wielgosz, Z.; Pelka, J. Use of post-life waste and production waste in thermoplastic polymer compositions. Polym. Polym. Compos. 2002, 10, 83–92. [Google Scholar] [CrossRef]
- Strapasson, R.; Amico, S.C.; Pereira, M.F.R.; Sydenstricker, T.H.D. Tensile and impact behaviour of polypropylene/low density polyethylene blends. Polym. Test. 2005, 4, 468–473. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Q.; Yao, F.; Xu, Y. Preparation and properties of recycled HDPE/natural fibre composites. Compos. Part A Appl. Sci. Manuf. 2007, 7, 1664–1674. [Google Scholar] [CrossRef]
- Meran, C.; Öztürk, O.; Yüksel, M. Examination of the possibility of recycling and utilizing recycled polyethylene and polypropylene. Mater. Des. 2007, 29, 701–705. [Google Scholar] [CrossRef]
- Brachet, P.; Høydal, L.T.; Hinrichsen, E.L.; Melum, F. Modification of mechanical properties of recycled polypropylene from post-consumer containers. Waste Manag. 2008, 28, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Baeyens, J.; Brems, A.; Dewil, R. Recovery and recycling of post-consumer waste materials. Part 2. Target wastes (glass beverage bottles, plastics, scrap metal and steel cans, end-of-life tyres, batteries and household hazardous waste). Int. J. Sustain. Eng. 2010, 3, 232–245. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Ahrenfeldt, J. Characterisation of Biomass Producer Gas as Fuel for Stationary Gas Engines in Combined Heat and Power Production. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2007. [Google Scholar]
- Yoshioka, T.; Grause, G.; Eger, C.; Kaminsky, W.; Okuwaki, A. Pyrolysis of poly(ethylene terephthalate) in a fluidised bed plant. Polym. Degrad. Stab. 2004, 86, 499–504. [Google Scholar] [CrossRef]
- Smolders, K.; Baeyens, J. Thermal degradation of PMMA in fluidised beds. Waste Manag. 2004, 24, 849–857. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Beerlandt, J.; Dewil, R. Thermogravimetric pyrolysis of waste polyethylene-terephthalate and polystyrene: A critical assessment of kinetics modelling. Resour. Conserv. Recycl. 2011, 55, 772–781. [Google Scholar] [CrossRef]
- Zhou, X.-L.; He, P.-J.; Peng, W.; Lü, F.; Shao, L.-M.; Zhang, H. From plastics to methane and carbon spheres: The evolution of pyrolysis products during pyrolysis under autogenic atmosphere. J. Anal. Appl. Pyrolysis 2022, 161, 105421. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.; Miguel, G.S.; Escola, J.M.; Rodríguez, J. Catalytic activity of zeolitic and mesostructured catalysts in the cracking of pure and waste polyolefins. J. Anal. Appl. Pyrolysis 2007, 78, 153–161. [Google Scholar] [CrossRef]
- Combustion, Pyrolysis and Gasification of Scrap Tires. Available online: http://www.substech.com/dokuwiki/doku.php?id=combustion_pyrolysis_and_gasification_of_scrap_tires#pyrolysis (accessed on 27 July 2013).
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A review. J. Environ. Chem. Eng. 2022, 10, 106867. [Google Scholar] [CrossRef]
- Yan, G.; Jing, X.; Wen, H.; Xiang, S. Thermal cracking of virgin and waste plastics of PP and LDPE in a semibatch reactor under atmospheric pressure. Energy Fuels 2015, 29, 2289–2298. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645. [Google Scholar] [CrossRef]
- Miao, Y.; von Jouanne, A.; Yokochi, A. Current technologies in depolymerization process and the road ahead. Polymers 2021, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; von Wolff, N.; Rauch, M.; Zou, Y.-Q.; Shmul, G.; Ben-David, Y.; Leitus, G.; Avram, L.; Milstein, D. Hydrogenative depolymerization of nylons. J. Am. Chem. Soc. 2020, 142, 14267–14275. [Google Scholar] [CrossRef] [PubMed]
- Kamber, N.E.; Tsujii, Y.; Keets, K.; Waymouth, R.M.; Pratt, R.C.; Nyce, G.W.; Hedrick, J.L. The depolymerization of poly(ethylene terephthalate) (PET) Using N-heterocyclic carbenes from ionic liquids. J. Chem. Educ. 2010, 87, 519–521. [Google Scholar] [CrossRef]
- Yansaneh, O.Y.; Zein, S.H. Recent advances on waste plastic thermal pyrolysis: A critical overview. Processes 2022, 10, 332. [Google Scholar] [CrossRef]
- Jung, S.-H.; Cho, M.-H.; Kang, B.-S.; Kim, J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Zannikos, F.; Kalligeros, S.; Anastopoulos, G.; Lois, E. Converting biomass and waste plastic to solid fuel briquettes. J. Renew. Energy 2012, 2013, 360368. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Yang, W. Effect of heat transfer model on the prediction of refuse-derived fuel pyrolysis process. Fuel 2015, 142, 46–57. [Google Scholar] [CrossRef]
- Park, H.J.; Park, Y.-K.; Dong, J.-I.; Kim, J.-S.; Jeon, J.-K.; Kim, S.-S.; Kim, J.; Song, B.; Park, J.; Lee, K.-J. Pyrolysis characteristics of Oriental white oak: Kinetic study and fast pyrolysis in a fluidized bed with an improved reaction system. Fuel Process. Technol. 2009, 90, 186–195. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; Abomohra, A.E.-F.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Encinar, J.; González, J.F.G. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Process. Technol. 2008, 89, 678–686. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef] [PubMed]
- McKay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Qinglan, H.; Chang, W.; Dingqiang, L.; Yao, W.; Dan, L.; Guiju, L. Production of hydrogen-rich gas from plant biomass by catalytic pyrolysis at low temperature. Int. J. Hydrogen Energy 2010, 35, 8884–8890. [Google Scholar] [CrossRef]
- Gao, F. Pyrolysis of Waste Plastics into Fuels. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2011. [Google Scholar]
- Ayhan, D. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Mastellone, M.; Perugini, F.; Ponte, M.; Arena, U. Fluidized bed pyrolysis of a recycled polyethylene. Polym. Degrad. Stab. 2002, 76, 479–487. [Google Scholar] [CrossRef]
- The Conversation. Available online: https://theconversation.com/how-we-can-turn-plastic-waste-into-green-energy-104072 (accessed on 1 October 2018).
- Ruwona, W.; Danha, G.; Muzenda, E. A Review on material and energy recovery from waste tyres. Procedia Manuf. 2019, 35, 216–222. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic waste recycling via pyrolysis: A bibliometric survey and literature review. J. Anal. Appl. Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 8, 100023. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Wang, P.; Zhong, L.; Ma, W.; Chen, G. Study on the pyrolysis behaviors of mixed waste plastics. Renew. Energy 2021, 173, 662–674. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Ahmetli, G.; Yel, E. A Comparative study on waste plastics pyrolysis liquid products quantity and energy recovery potential. Energy Procedia 2017, 118, 221–226. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Co-pyrolysis of waste biomass and waste plastics (polystyrene and waste nitrile gloves) into renewable fuel and value-added chemicals. Carbon Resour. Convers. 2020, 3, 145–155. [Google Scholar] [CrossRef]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of plastic waste for synthesis gas production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Antelava, A.; Jablonska, N.; Constantinou, A.; Manos, G.; Salaudeen, S.A.; Dutta, A.; Al-Salem, S.M. Energy potential of plastic waste valorization: A short comparative assessment of pyrolysis versus gasification. Energy Fuels 2021, 35, 3558–3571. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory. Available online: https://www.netl.doe.gov/research/Coal/energy-systems/gasification/gasifipedia/intro-to-gasification (accessed on 8 February 2022).
- Arena, U.; Mastellone, M.L. Fluidized bed pyrolysis of plastic wastes. In Feedstock Recycling and Pyrolysis of Plastic Wastes: Converting Waste Plastics into Diesel and Other Fuels; Scheirs, J., Kaminsky, W., Eds.; John Wiley and Sons: New York, NY, USA, 2006. [Google Scholar]
- Scheirs, J. Polymer Recycling; John Wiley and Sons: New York, NY, USA, 1998. [Google Scholar]
- Vermeulen, I.; Van Caneghem, J.; Block, C.; Baeyens, J.; Vandecasteele, C. Automotive shredder residue (ASR): Reviewing its productions from end-of-life vehicles (ELVs) and its recycling, energy and chemicals valorization. J. Hazard. Mater. 2011, 190, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; Franco, C.; Andre, R.; Miranda, M.; Gulyurtlu, I.; Cabrita, I. Co-gasification study of biomass mixed with plastic wastes. Fuel 2002, 81, 291–297. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; André, R.N.; Tavares, C.; Dias, M.; Gulyurtlu, I.; Cabrita, I. Effect of experimental conditions on co-gasification of coal, biomass and plastics wastes with air/steam mixtures in a fluidized bed system. Fuel 2003, 82, 1967–1976. [Google Scholar] [CrossRef]
- Slapak, M.J.P.; Kasteren, J.M.N.V.; Drinkenburg, A.A.H. Design of a process fors team gasification of PVC waste. Res. Conserv. Recycl. 2000, 30, 81–93. [Google Scholar] [CrossRef]
- Xiao, G.; Ni, M.-J.; Chi, Y.; Jin, B.-S.; Xiao, R.; Zhong, Z.-P.; Huang, Y.-J. Gasification characteristics of MSW and an ANN prediction model. Waste Manag. 2009, 29, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Al-Asadi, M.; Miskolczi, N.; Eller, Z. Pyrolysis-gasification of wastes plastics for syngas production using metal modified zeolite catalysts under different ratio of nitrogen/oxygen. J. Clean. Prod. 2020, 271, 122186. [Google Scholar] [CrossRef]
- Cozzani, V.; Nicolella, C.; Rovatti, M.; Tognotti, L. Influence of gas-phase reactions on the product yields obtained in the pyrolysis of polyethylene. Ind. Eng. Chem. Res. 1997, 36, 342–348. [Google Scholar] [CrossRef]
- Stiles, H.; Kandiyoti, R. Secondary reactions of flash pyrolysis tars measured in a fluidized bed pyrolysis reactor with some novel design features. Fuel 1989, 68, 275–282. [Google Scholar] [CrossRef]
- Miscolczi, N.; Bartha, L.; Deak, G.; Jover, B. Thermal degradation of municipal solid waste for production of fuel-like hydrocarbons. Polym. Degrad. Stab. 2004, 86, 357–366. [Google Scholar] [CrossRef]
- Ciliz, N.K.; Ekinci, E.; Snape, C. Pyrolysis of virgin and waste polypropylene and its mixtures with waste polyethylene and polystyrene. Waste Manag. 2004, 24, 173–181. [Google Scholar] [CrossRef]
- Ponzio, A.; Kalisz, S.; Blasiak, W. Effect of operating conditions on tar and gas composition in high temperature air/steam gasification (HTAG) of plastic containing waste. Fuel Process. Technol. 2006, 87, 223–233. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process☆. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Marquez-Montesinos, F.; Cordero, T.; Rodríguez-Mirasol, J.; Rodríguez, J. CO2 and steam gasification of a grapefruit skin char. Fuel 2002, 81, 423–429. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjöström, K.; Björnbom, E. Rapid high-temperature pyrolysis of biomass in a free-fall reactor. Fuel 1996, 75, 545–550. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjöström, K.; Björnbom, E. Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Narvaez, A.; Orio, A.; Aznar, M.P.; Corella, J. Biomass gasification with air in an atmospheric bubbling fluidized bed: Effect of six operational parameters. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, Y.; Zhong, W.; Yu, A. Co-firing of coal and biomass in oxy-fuel fluidized bed for CO2 capture: A review of recent advances. Chin. J. Chem. Eng. 2019, 27, 2261–2272. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C. Conversion of char-N into NOx and N2O during combustion of biomass char. Fuel 2019, 242, 389–397. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A.; Kijo-Kleczkowska, A. Pollutant emissions during Oxy-Fuel combustion of biomass in a bench scale CFB combustor. Energies 2022, 15, 706. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Experimental research of sewage sludge with coal and biomass co-combustion, in pellet form. Waste Manag. 2016, 53, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Zevenhoven, R.; Karlsson, M.; Hupa, M.; Frankenhaeuser, M. Combustion and gasification properties of plastics particles. J. Air Waste Manag. Assoc. 1997, 47, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoneit, B.R.T.; Medeiros, P.M.; Didyk, B.M. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ. Sci. Technol. 2005, 39, 6961–6970. [Google Scholar] [CrossRef] [PubMed]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R.; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and policies to decarbonize global industry: Review and assessment of mitigation drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Kuznia, M. Methods of utilization of plastic waste in the metallurgical proces. Works IMZ AGH Univ. Sci. Technol. 2010, 3, 23–29. [Google Scholar]
- Zhou, Z.; Qiu, X.; Wang, Y.; Duan, Y.; Li, L.; Lin, H.; Luo, Y.; Sun, Z.; Duan, L. Particulate matter formation during shoe manufacturing waste combustion in a full-scale CFB boiler. Fuel Process. Technol. 2021, 221, 106914. [Google Scholar] [CrossRef]
- Żukowski, W.; Jankowski, D.; Baron, J.; Wrona, J. Combustion dynamics of polymer wastes in a bubbling fluidized bed. J. Clean. Prod. 2021, 320, 128807. [Google Scholar] [CrossRef]
- Chatziaras, N.; Psomopoulos, C.S.; Themelis, N.J. Use of waste derived fuels in cement industry: A review. Manag. Environ. Qual. Int. J. 2016, 27, 178–193. [Google Scholar] [CrossRef]
- Janyalertadun, A.; Santaweesuk, C.; Sanongraj, S. Fuel production, performance, and emission of a CI engine using waste plastics oil. World J. Eng. 2017, 14, 114–120. [Google Scholar] [CrossRef]
- Walters, R.N.; Hackett, S.M.; Lyon, R.E. Heats of combustion of high temperature polymers. Fire Mater. 2000, 24, 245–252. [Google Scholar] [CrossRef]
- Shemwell, B.E.; Levendi, Y.A. Particulates generated from combustion of polymers (Plastics). J. Air Waste Manag. Assoc. 2011, 1, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Express Elblog. Available online: https://www.expresselblag.pl/wiadomosci/s/15479,recykling-plastiku-jak-plastik-wraca-do-obiegu (accessed on 29 September 2021).
- Ptsp.pl. Available online: https://ptsp.pl/recykling/ (accessed on 22 February 2022).
- Przeglad-Spozywczy.pl. Available online: https://przeglad-spozywczy.pl/-inne-brane-/opakowania/102-wiadomoci-opakowania/1202537-technologie-recyklingu-tworzyw-sztucznych (accessed on 27 February 2018).
- Punkkinen, H.; Oasmaa, A.; Luntama, J.L.; Nieminen, M.; Laine-Ylijoki, J. Thermal Conversion of Plasticcontaining Waste: A Review; ARVI Material Value Chains: Helsinki, Finland, 2017; Available online: arvifinalreport.fi/files/Thermal%20conversion%20of%20plastic-containing%20waste%20A%20review.pdf (accessed on 9 January 2017).
- Wikipedia: Plastic Recycling. Available online: https://pl.abcdef.wiki/wiki/Plastic_recycling (accessed on 15 October 2021).
- Song, B.; Hall, P. Densification of biomass and waste plastic blends as a solid fuel: Hazards, advantages, and perspectives. Front. Energy Res. 2020, 8, 58. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).