Abstract
Metal hydroxides, owing to their catalytic active sites for the decomposition of O3 to Reactive Oxygen Species (ROS), have been adapted for catalytic ozonation of micropollutants in wastewater. In this study, commercial Mg (OH)2 was used for the degradation of cyclophosphamide (CYP) by ozone. The crystal phase was confirmed by X-ray powder Diffraction (XRD). Percent degradation of 10 ppm CYP after 30 min by O3 and Mg (OH)2/O3 was 56 and 93, respectively, suggesting enhanced decomposition of O3 to ROS by the catalyst. The presence of ROS was further confirmed using pCBA as a probe, which showed that the concentration of ROS was eight times higher in the presence of Mg (OH)2/O3 than O3 alone. Catalytic ozonation experiments in the presence of scavengers showed that OH· radicals play a significant role in the degradation of CYP. The catalyst was found to be reusable for at least three cycles without significant loss in degradation efficiency. To study the compatibility of Mg (OH)2 for wastewater treatment applications, synthetic effluent was spiked with CYP and subjected to ozonation by Mg(OH)2/O3. The TOC of CYP before and after the treatment showed that Mg (OH)2/O3 not only degrades CYP but also mineralizes to a certain extent unlike O3 alone.
1. Introduction
The persistence of pharmaceutical compounds in surface and ground water is a global concern [1]. The primary sources of these contaminants are effluents from hospitals and domestic sewage treatment plants [2]. Conventional wastewater treatment has limited removal efficiency for many recalcitrant pollutants [3]. Advanced oxidation processes (AOPs) have emerged as powerful techniques for the complete mineralization of organics or as preliminary treatment processes to destroy toxic and recalcitrant organics and pharmaceutical drugs in effluents [4,5,6,7,8,9]. In particular, ozone (O3) has long been used in water treatment for the disinfection and degradation of micropollutants in wastewater [10,11]. Ozone by itself is an environmentally friendly oxidant: during redox reactions, it decomposes to O2 without producing self-derived byproducts, and ozone-based AOPs (e.g., O3/H2O2) are reported to be more energy-efficient than UV-based processes (e.g., UV/H2O2, UV/O3) in terms of organic pollutant abatement [12]. Typically, there are two major pathways for the degradation of organics: (i) direct oxidation in aqueous solution by free O3 molecules possessing high oxidation potential (2.07 V) and (ii) reactive oxygen species (ROS) from O3 leading to decomposition and adsorption on the catalyst surface [13]. Nevertheless, direct oxidation of organic pollutants by O3 is a selective process resulting in slow and partial mineralization. Moreover, the low solubility and stability of ozone in aqueous solutions also hinders its efficient usage as an oxidant. Therefore, catalytic ozonation is generally the dominant degradation process for organics and pharmaceutical recalcitrants exposed to O3 [14,15].
Catalytic ozonation by metal oxides and hydroxides has been previously adapted for the degradation of various organic pollutants in wastewater [16]. Mg(OH)2 is an inexpensive and environmentally friendly powder with moderate solubility in water. In the present study, catalytic ozonation with Mg(OH)2 in synthetic effluents was investigated and the mechanism underlying the degradation was proposed. Cyclophosphamide (CYP) is one of the most prescribed alkylating agents for treating cancers and autoimmune diseases [17,18,19]. CYP is listed as a hazardous substance and is included in the “class 1 (carcinogenic impact for humans)” classification of the International Agency for Research on Cancer (IARC) [20]. Pharmacokinetics have shown that 10–20% of ingested CYP may be excreted undegraded in the urine [21]. Various studies proved the presence of CYP in hospital effluents, and surface waters in concentrations ranging from ng/L to ppm [22]. Wastewater treatment plants (WWTPs) do not remove CYP, which may contaminate aquatic ecosystems receiving treated effluents and impact human health via contamination of drinking water or bioaccumulation in the food chain [23]. Due to these environmental and public health implications, CYP was chosen as a model pollutant to study the catalytic ozonation properties of Mg(OH)2. The degradation of cyclophosphamide by Mg(OH)2 under ozone was reported for the first time to our knowledge.
2. Materials and Methods
2.1. Material Characterization
In the present study, commercial Mg (OH)2 from Sigma Aldrich, 95% was used for catalytic ozonation experiments. The phase and purity of the powder was confirmed by X-ray powder diffraction (XRD) in a D8 advanced diffractometer (Bruker AXS, Karlsruhe, Germany). The crystallite size was calculated using the Scherrer formula as shown in Equation (1):
where λ is 1.54060 Å, β is the full width at half maximum, and θ is the angle of diffraction.
D = 0.9λ/β Cosθ
2.2. Experimental Section
2.2.1. Effect of Catalyst Dosage
To understand the effect of Mg (OH)2 dosage on the degradation of CYP by catalytic ozonation, 10–100 mg catalyst was first added to 100 mL of 10 ppm CYP in the experimental reactor, after which O3 was diffused into the solution. Every 5 min for 30 min, a 1 mL suspension was extracted from the reactor and centrifuged, and the resulting supernatant was analyzed by HPLC-MS. Percent degradation of CYP was calculated as shown in Equation (2):
where C0 and Ct are the concentrations of CYP initially and at time (t), respectively.
% CYP = [(C0 − Ct)/C0] × 100
Ozone was generated using a benchtop ozone generator and diffused into the reactor via a PVC tube in an Erlenmeyer flask. The concentration of ozone passing into the reactor was estimated spectrophotometrically by indigo method at 600 nm.
2.2.2. Estimation of OH· by Mg (OH)2/O3
The concentration of OH· produced by ozonation and catalytic ozonation was estimated indirectly using pCBA as a probe. The catalyst was added to 3 ppm pCBA and O3 was passed into the reactor. Every 5 min for 30 min, 1 mL of the suspension was extracted, centrifuged and the concentration of OH· in the supernatant was analyzed by HPLC-UV. The concentration of OH· was calculated as shown in Equation (3):
where KOH pCBA is the rate constant of pCBA, 5 × 10−9 M−1 s−1 at ambient pressure and temperature, and [OH·] is the steady-state concentration of hydroxyl radicals.
−d[pCBA]/dt = KOH pCBA [OH·] ss
2.2.3. Reusability of Mg (OH)2 for Catalytic Ozonation
After CYP degradation, the Mg(OH)2 suspension was centrifuged, the pellet was dried, and the recovered powder was used again for further degradation of CYP. This cycle was repeated three times and percent CYP degradation was calculated as shown in Equation (2).
2.2.4. Analytical Methods
CYP after ozonation and catalytic ozonation was identified by HPLC-MS with a Synergy polar RP 2.1 × 250 mm, 4 mM column from Phenomenex. The column temperature was 50 °C; the gradient program, with 0.1% formic acid in water (solution A) and 0.1% formic acid in MeOH (solution B), was 95% A at 0 min, 10% A at 14 min, then 95% A at 21 min, with a flow rate of 0.45 mL/min and 100 μL injection volume. p-Chlorobenzoic acid (pCBA) was analyzed by high-performance liquid chromatography–ultraviolet (HPLC-UV; Agilent Technologies 1100 series instrument, Waldron, Germany) using a Kinetics® 2.6 mm EVO C18 column in an isocratic mode, with solvent composition of 40% water and 60% MeOH and a flow rate of 0.5 mL/min.
2.2.5. Total Organic Carbon (TOC)
TOC content of CYP before and after the treatment was analyzed by an Aurora TOC analyzer (OI Analytical, College Station, TX, USA). CYP samples were taken before and after 30 min of ozonation by O3, Mg(OH)2/O3, centrifuged, and then analyzed for TOC. DI water without CYP and DI water with catalyst alone served as controls.
2.2.6. Catalytic Degradation of CYP in Synthetic Effluent
To investigate the efficacy of Mg (OH)2 for wastewater treatment applications, catalytic ozonation experiments were carried out using synthetic effluent. Wastewater effluent would have been ideal, but due to the suspected presence of coronavirus in domestic sewage, synthetic effluent simulating the composition of wastewater effluent was used. The composition of the synthetic effluent is given in Table 1. Catalyst was added to synthetic effluent spiked with 10 ppm CYP and catalytic ozonation experiments were conducted as described in Section 2.2.1.

Table 1.
Composition of synthetic effluent.
3. Results and Discussion
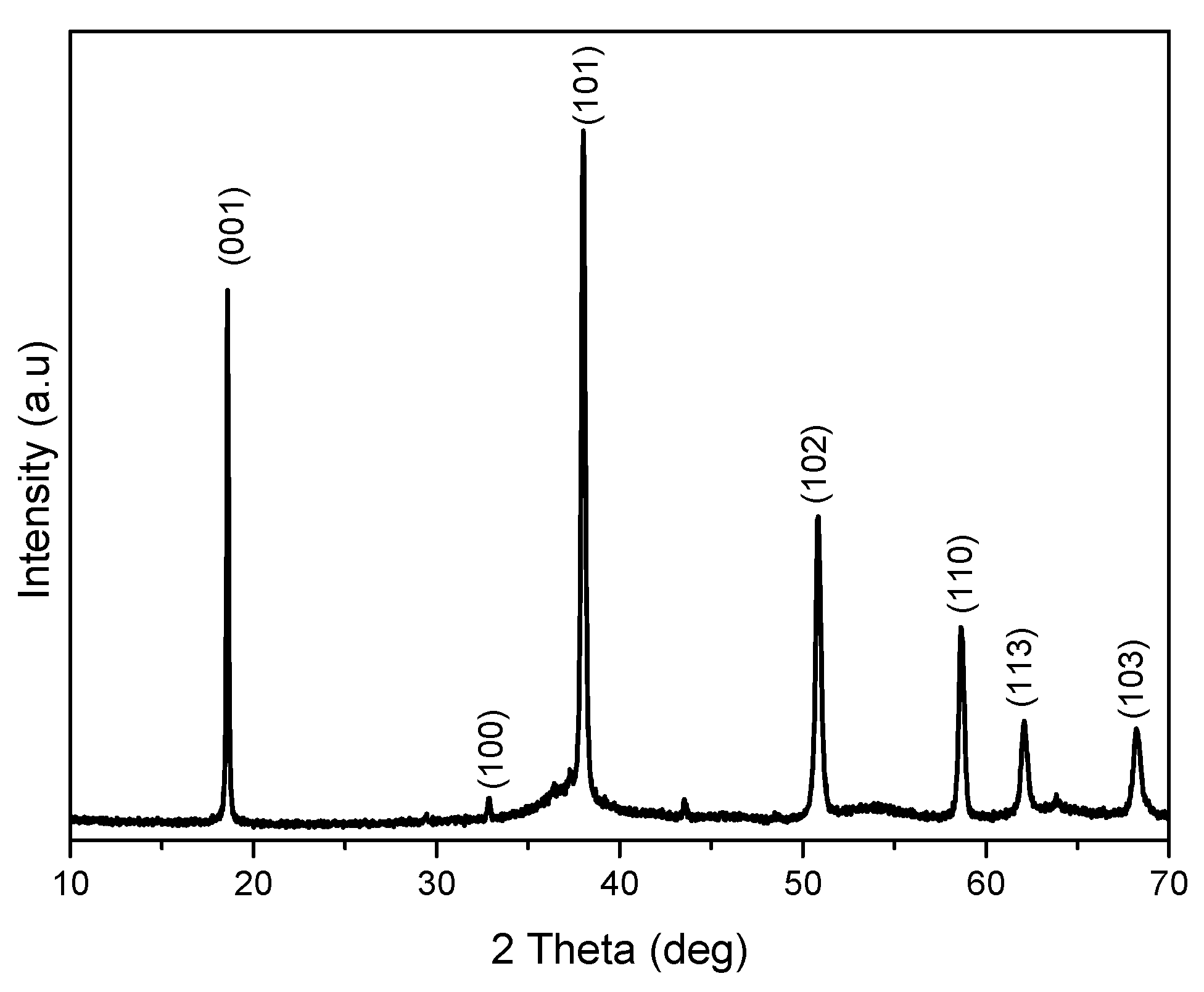
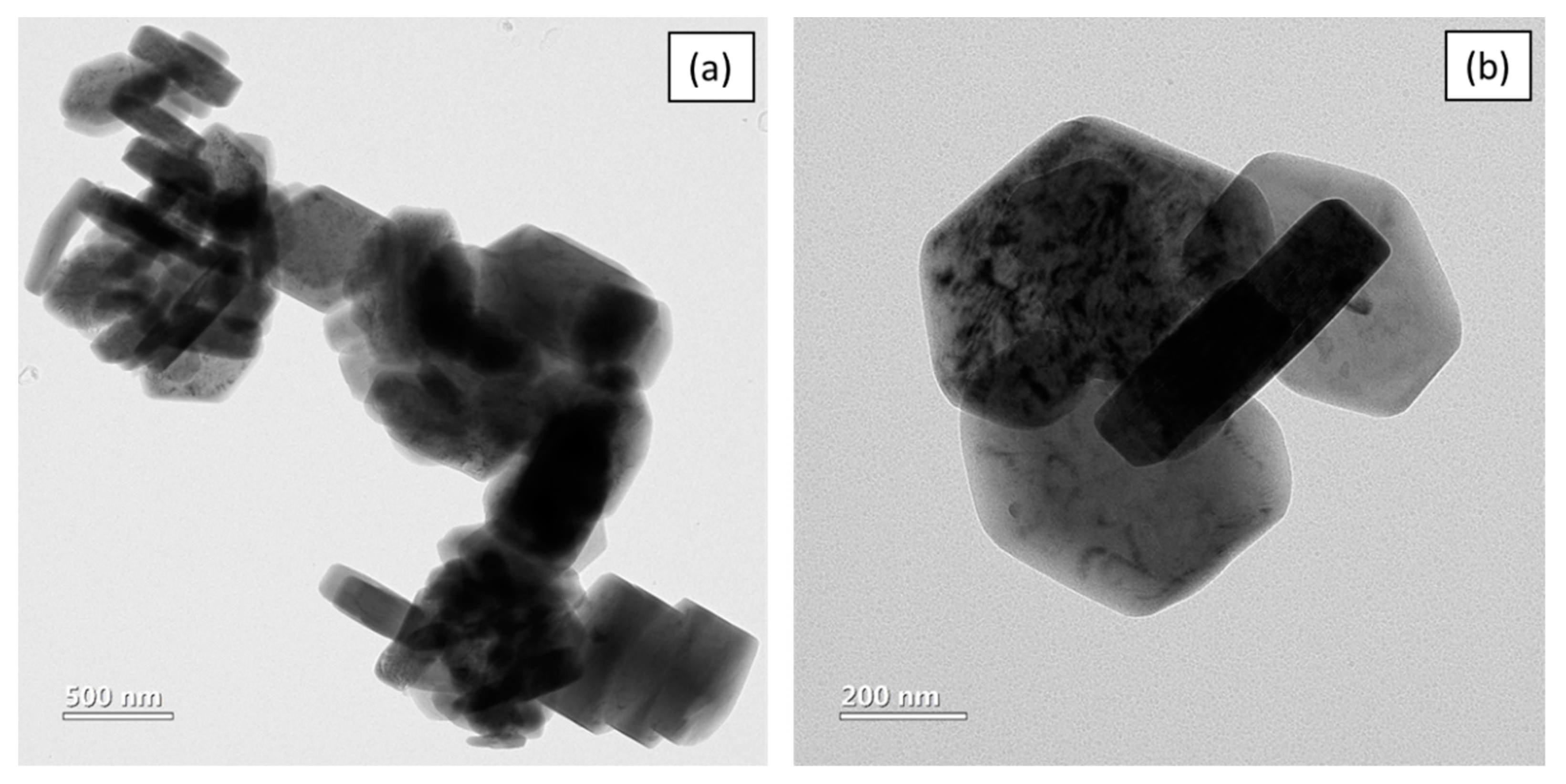
XRD (Figure 1) confirms pure Mg (OH)2 crystallizing in hexagonal phase with lattice parameters, a = 3.1475 Å and c = 4.7745 Å [20]. The crystallite calculated by the Scherrer equation as shown in Equation (1) is 41.6 nm. TEM (Figure 2) shows that Mg(OH)2 consists of rods with a length of about 357–470 nm and width of 96–109 nm. The powder also contains irregular shaped particles with diameters ranging from 250 to 310 nm.
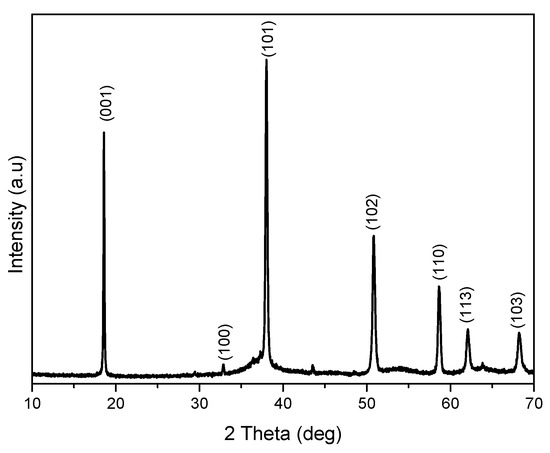
Figure 1.
XRD of Mg (OH)2.
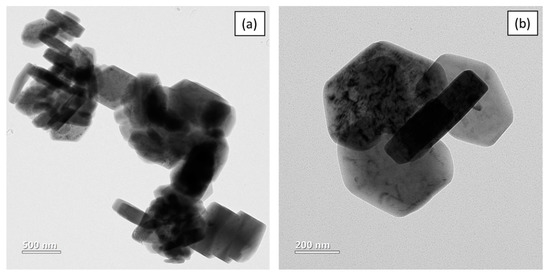
Figure 2.
(a,b) TEM of Mg(OH)2.
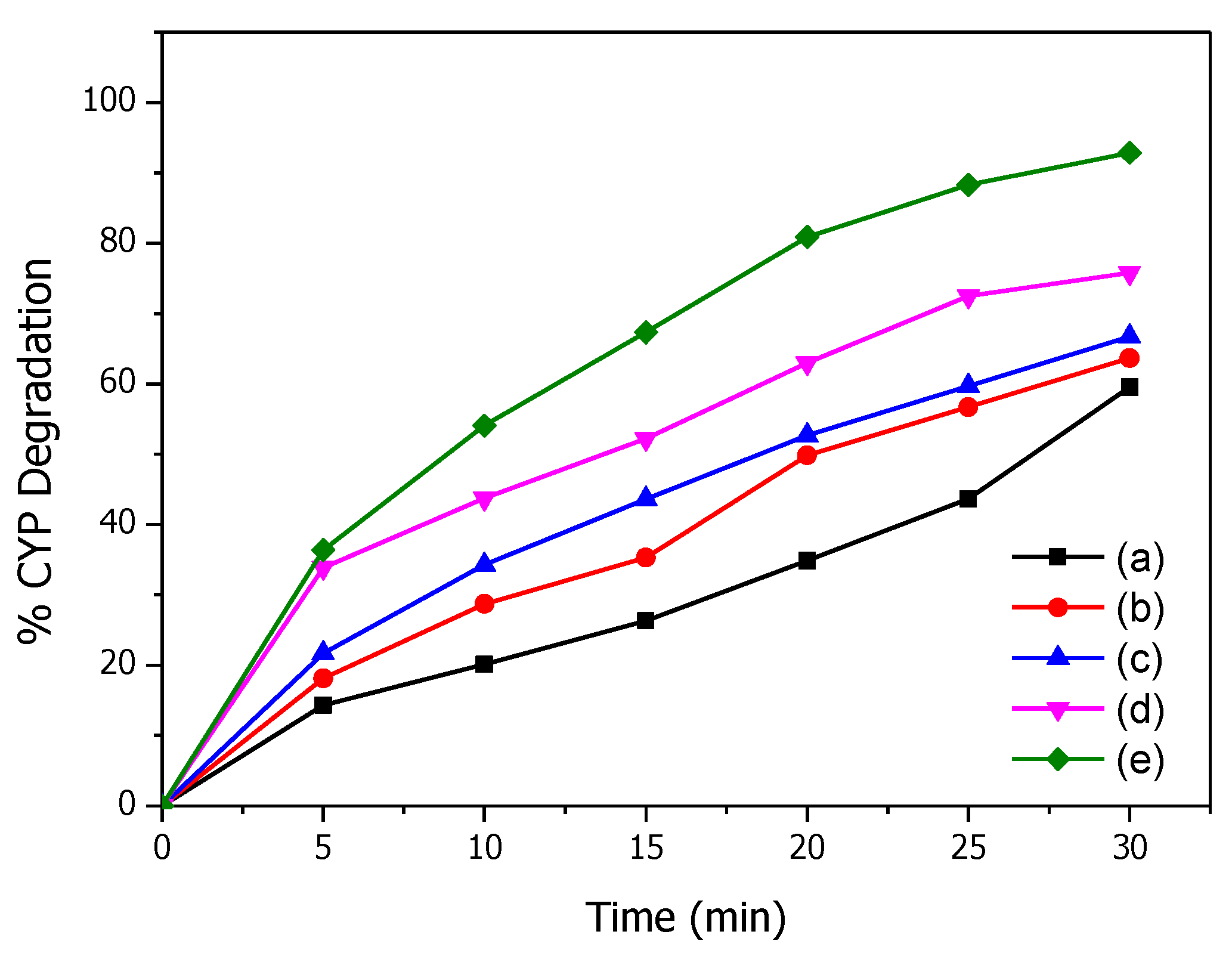
Figure 3 shows the kinetics of CYP degradation over time by O3 and Mg(OH)2/O3. A significant increase in percentage degradation was observed with an increase in the catalyst dosage from 10 mg to 100 mg Mg (OH)2. No change in degradation was observed when the catalyst dosage increased beyond 100 mg (data not given) and so this concentration was kept constant for further experiments. Percent CYP degradation after 30 min by O3 and Mg (OH)2 (100 mg)/O3 are 59.5 and 92.8, respectively. Significantly greater degradation of CYP was observed with Mg (OH)2/O3 than O3 suggesting that Mg(OH)2 enhances the decomposition of O3 to produce more ROS resulting in enhanced degradation of CYP [21].
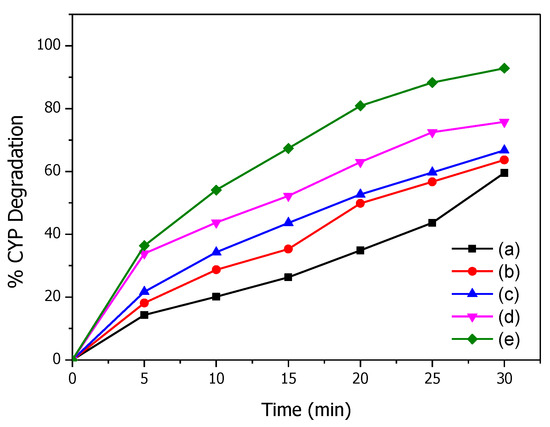
Figure 3.
Effect of catalyst dosage on degradation of CYP: (a) 0, (b) 10, (c) 25, (d) 50, and (e) 100 mg Mg(OH)2.
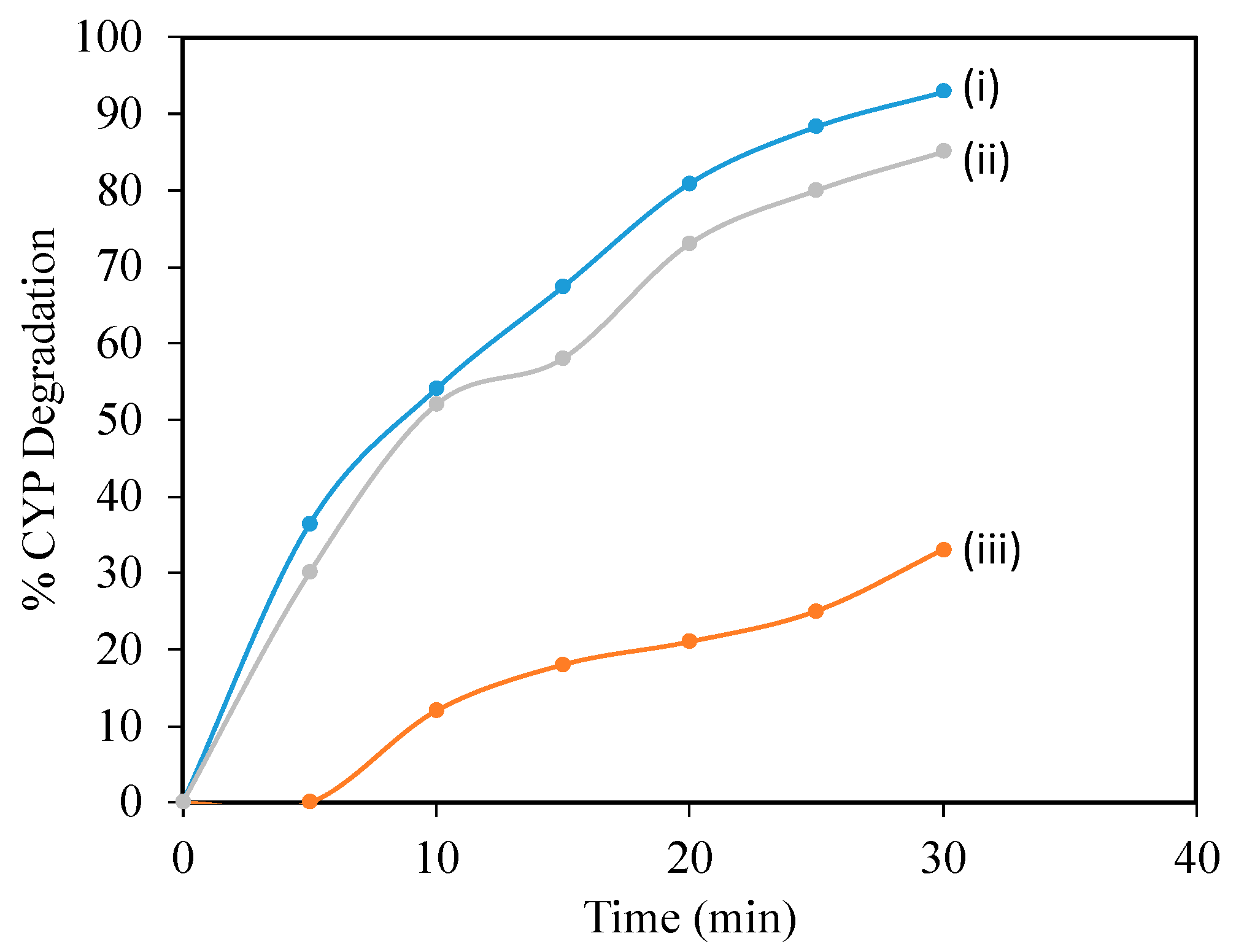
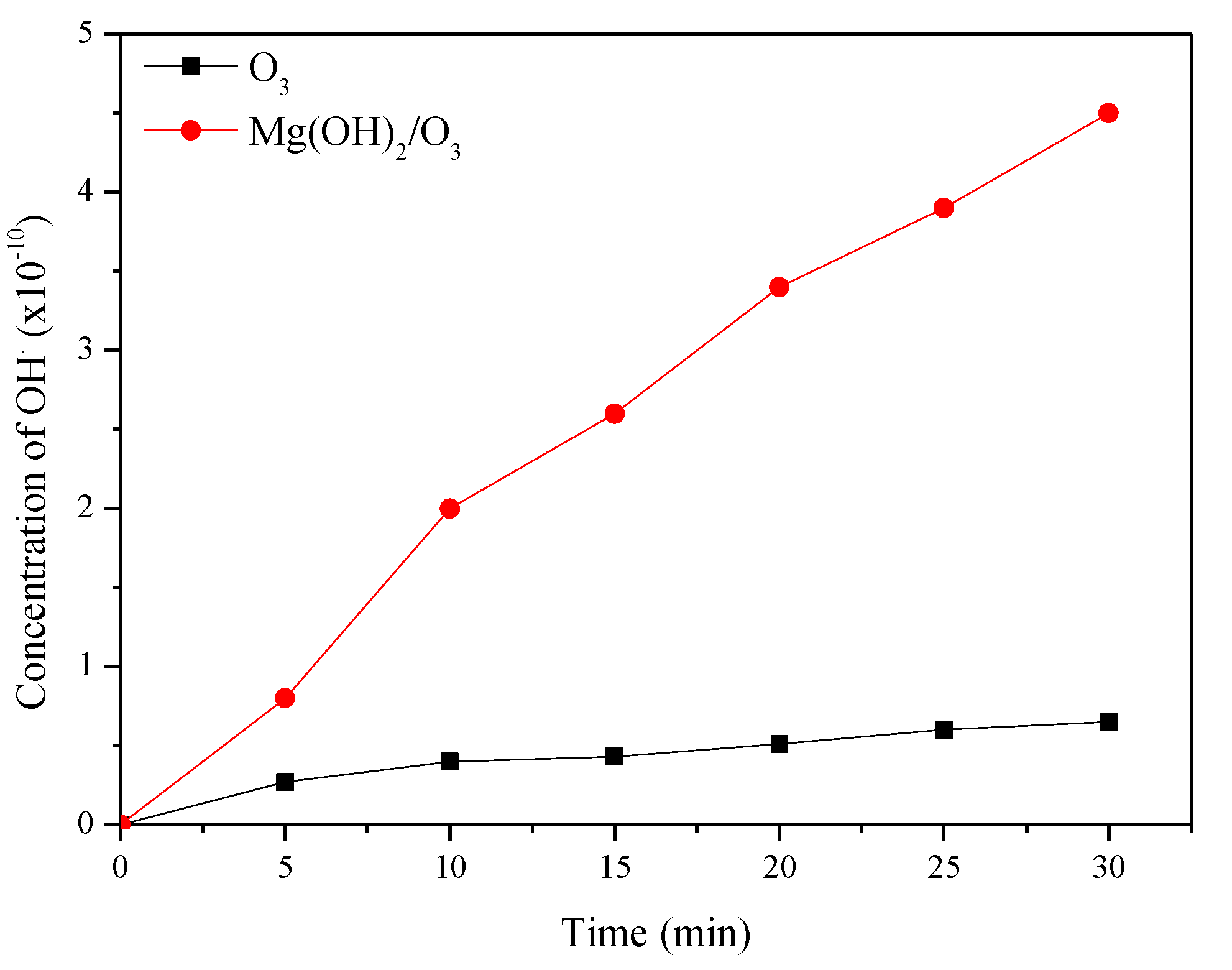
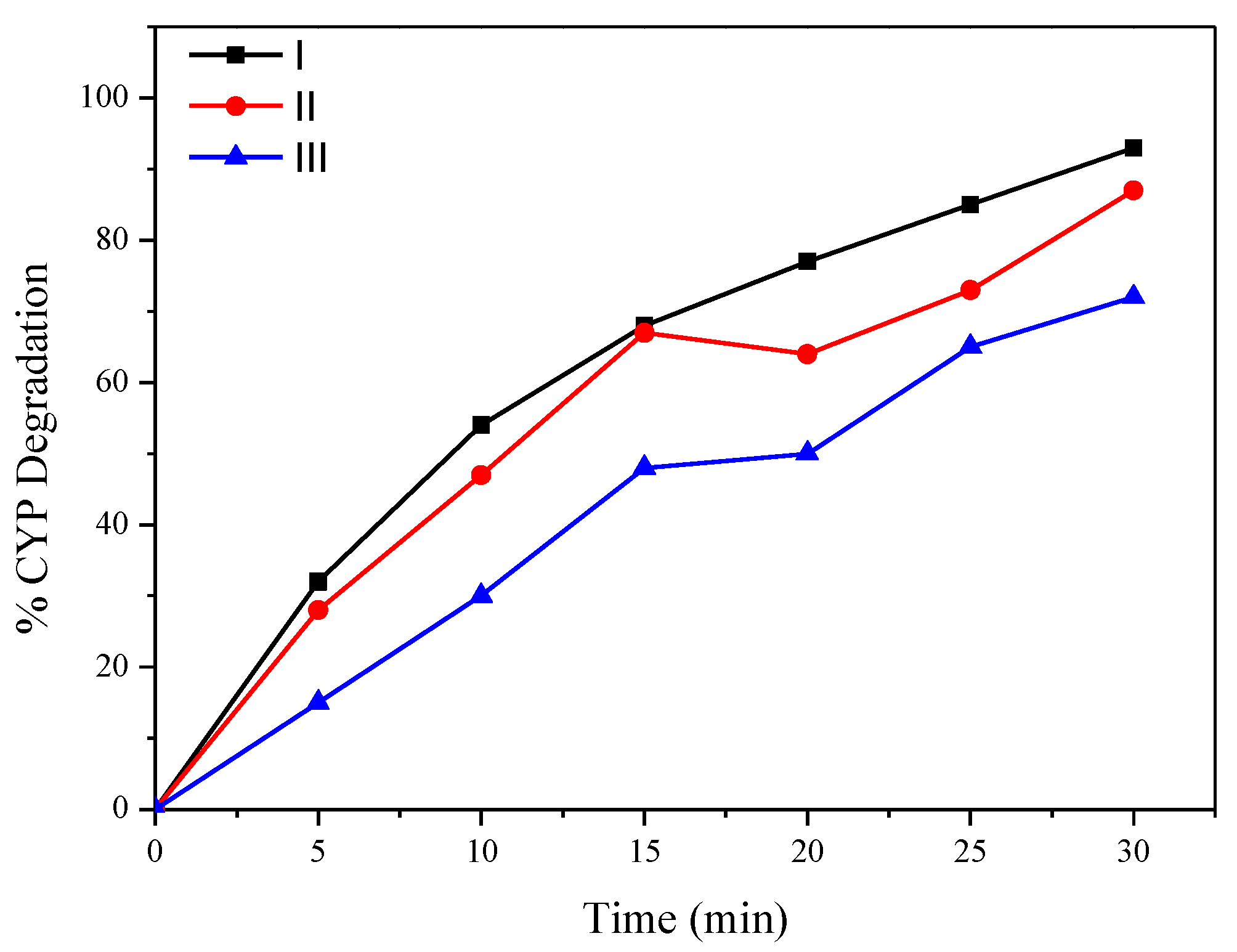
To confirm the degradation mechanism, catalytic ozonation experiments were conducted in the presence of ROS scavengers and the results are shown in Figure 4 [22]. In the present study, t-BuOH was applied as a hydroxyl scavenger to extinguish OH· radicals and also to limit the ozonation indirect radical pathway [24]. Besides OH·, superoxide radicals were also reported to play a significant role in degrading pollutants during ozonation and catalytic ozonation. So, to understand the role of −O2·, benzoquinone (BZQ) was added to the reaction mixture. The concentration of t-BuOH and BZQ in the reaction mixtures is 0.3 mmol/L. CYP degradation reduced from 92.8% to 85% in the presence of benzoquinone (BZQ), while in the presence of the OH· Scavenger t-BuOH, percentage degradation reduced from 92.8% to 33%, suggesting that OH· Radicals play a significant role in degrading CYP. To quantify ROS, pCBA was used as a probe. The degradation of pCBA is directly proportional to the concentration of OH·. After 30 min of ozonation with O3 and Mg (OH)2/O3, OH· Concentrations were 0.37 × 10−10 and 4.5 × 10−10 M, respectively (Figure 5).
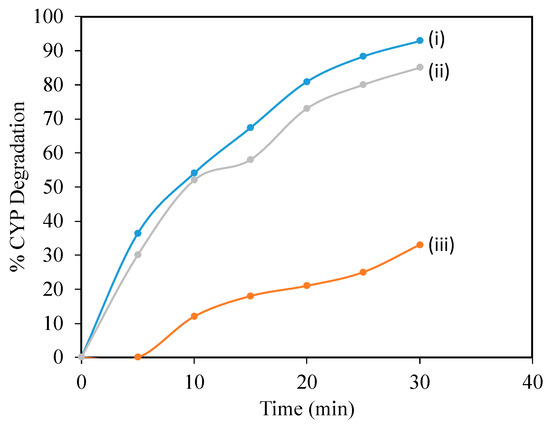
Figure 4.
Effect of scavengers on degradation of CYP by Mg (OH)2/O3: (i) no scavenger, (ii) BZQ, (iii) t-BuOH.
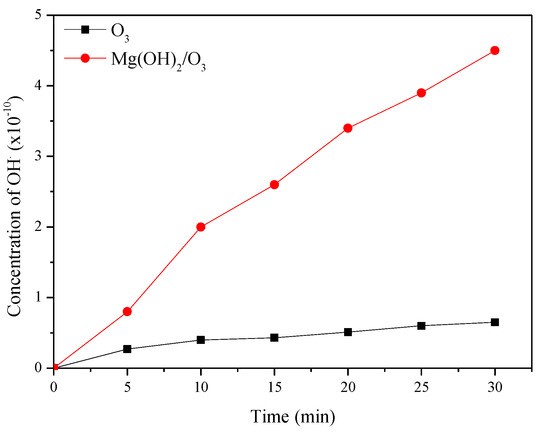
Figure 5.
The concentration of OH· produced during ozonation and catalytic ozonation.
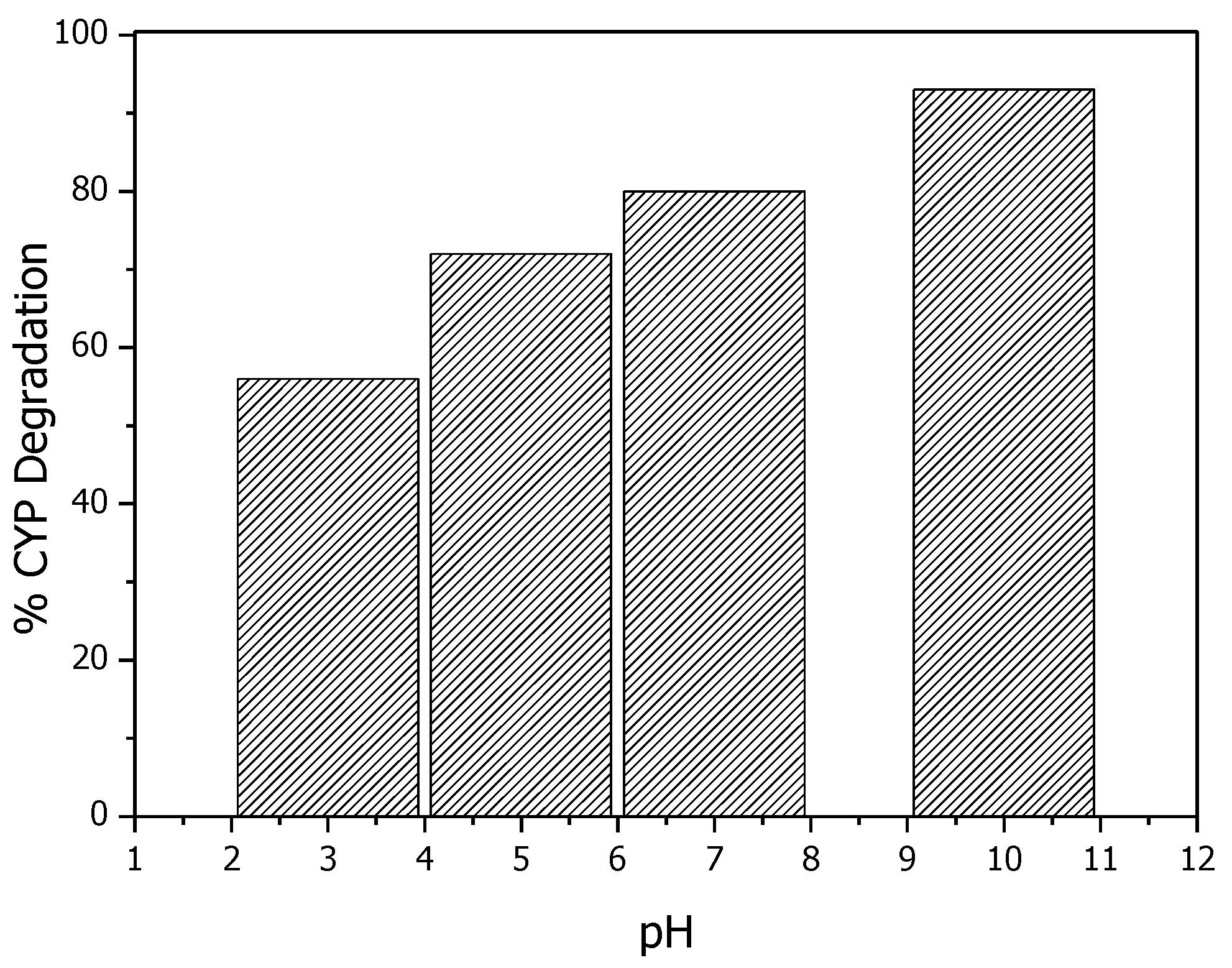
In catalytic ozonation experiments, solution pH effects the surface properties of the catalyst and further determines the catalytic activity. Extensive studies suggest that in the metal-based catalysts, the surface hydroxyl groups acts as catalytic active sites for the decomposition of O3. To better understand the mechanism of ROS production by Mg (OH)2/O3, catalytic ozonation experiments with pH values ranging from 3 to 7 were conducted. Due to the electrophilic properties of O3, surface hydroxyl groups on Mg(OH)2 act as catalytic active sites for the transformation of O3 to OH· [23]. Figure 6 shows that basic pH favors the degradation of CYP. As pH increases from 7 to 10, more hydroxyl groups will form on the surface of the catalyst, thereby enhancing the absorption and decomposition of O3 to OH as shown in Equations (4) and (5). As shown in Equations (4) and (5), Mg(OH)2 acts as an initiator in the process. O3 adsorbed on the surface of Mg(OH)2 and reacted with surface hydroxyl groups leading to the formation of Mg–OHO˙ as the first step; then Mg–OHO˙ was attacked by ozone producing OH· radicals [25,26]. These results suggest that surface hydroxyl groups on Mg (OH)2 act as catalytic sites for the decomposition of O3.
Mg-OH + O3 ⟶ Mg-OHo-o-o Mg–OHO˙· + O2
Mg–OHO˙ + 2H2O + O3 ⟶ Mg–OHOH˙· + 3·OH + O2
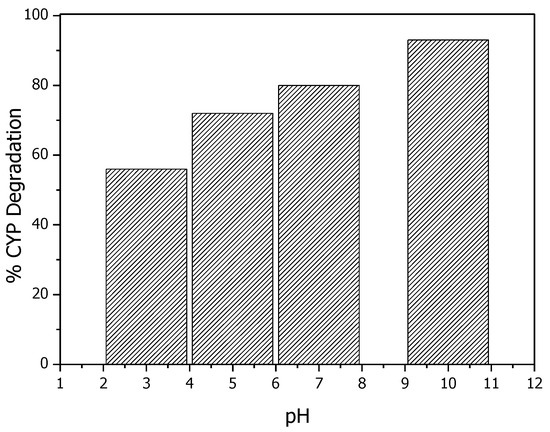
Figure 6.
Effect of pH on degradation of CYP by Mg (OH)2/O3.
Figure 7 shows the reusability of the catalyst for the degradation of CYP. To understand the effect of matrix components on CYP degradation by MgO2/O3, catalytic ozonation experiments were conducted using synthetic effluent. Percent degradation of 10 ppm CYP in synthetic effluent by O3 and Mg (OH)2/O3 was 35% and 82%, respectively, suggesting that the proposed process can be used for wastewater treatment applications. To understand the extent of mineralization, TOC of the CYP-spiked synthetic effluent was measured. The TOC of spiked effluent exposed to O3 was enhanced by 25%, suggesting that mineralization did not occur by ozonation alone. Conversely, Mg (OH)2/O3 treatment resulted in a TOC reduction of 12% in CYP-spiked effluent, indicating that some mineralization does occur by catalytic ozonation.
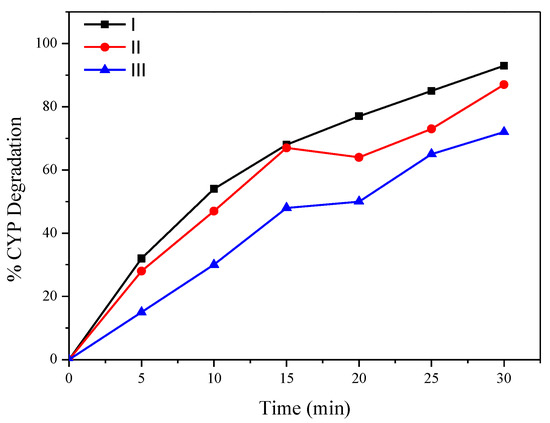
Figure 7.
Reusability of recovered Mg (OH)2 for catalytic ozonation.
4. Conclusions
Commercial Mg (OH)2 in the presence of O3 was used for the degradation of CYP in water. CYP was chosen as a model pollutant due to its ubiquitous presence in surface and ground water. A significant increase in CYP degradation by Mg (OH)2/O3 as compared to CYP degradation by O3 alone demonstrates that catalytic ozonation is more efficient than O3 alone. The production of ROS was confirmed by the degradation of pCBA, and results showed that Mg (OH)2/O3 produces more ROS than O3 alone. Surface hydroxyl groups on the surface of the metal hydroxide catalyst were proven to be the catalyst sites for the decomposition of O3 to ROS. Catalytic ozonation with Mg(OH)2 leading to the degradation of CYP in synthetic effluent demonstrated that this process can be used for wastewater treatment applications.
Author Contributions
Conceptualization: D.A. and L.P.; Methodology: D.A. and L.P.; Validation: L.P.; Formal analysis: D.A. and L.P.; Investigation: L.P.; Resources: D.A.; Data curation: D.A. and L.P.;Writing—original draft preparation: L.P.; Writing—review and editing: D.A.; Supervision: D.A.; Project administration: D.A.; Funding acquisition: D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, L.; Hu, C.; Nie, Y.; Qu, J. Catalytic ozonation of selected pharmaceuticals over mesoporous alumina-supported manganese oxide. Environ. Sci. Technol. 2009, 43, 2525–2529. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U.; Salhi, E.; Schmidt, C.K.; Arnold, W.A. Kinetics and mechanisms of N-nitrosodimethylamine formation upon ozonation of N, N-dimethylsulfamide-containing waters: Bromide catalysis. Environ. Sci. Technol. 2010, 44, 5762–5768. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bunian, M.; Chen, X.; Heald, S.; Yu, L.; Wen, J.; Lei, Y.; Wu, T. Plasmon-enhanced Catalytic Ozonation for Efficient Removal of Recalcitrant Water Pollutants. ACS EST Eng. 2021, 1, 874–883. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.Y.; Von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C.H. Advanced Oxidation Process with Peracetic Acid and Fe (II) for Contaminant Degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Tran, V.A.; Nguyen, T.P.; Le, V.T.; Kim, I.T.; Lee, S.-W.; Nguyen, C.T. Excellent photocatalytic activity of ternary Ag@WO3@rGO nanocomposites under solar simulation irradiation. J. Sci. Adv. Mater. Devices 2021, 6, 108–117. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Thi Vo, T.-T.; Huu Do, H.; Thuan Le, V.; Nguyen, T.D.; Ky Vo, T.; Nguyen, B.-S.; Nguyen, T.T.; Phung, T.K.; Tran, V.A. Ag@ZnO porous nanoparticle wrapped by rGO for the effective CO2 electrochemical reduction. Chem. Eng. Sci. 2021, 232, 116381. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Lam, T.C.; Khan, A.; Thi Vo, T.-T.; Nguyen, T.-Q.; Doan, V.D.; Tran, D.L.; Le, V.T.; Tran, V.A. TiO2/Ti3C2/g-C3N4 ternary heterojunction for photocatalytic hydrogen evolution. Chemosphere 2021, 285, 131429. [Google Scholar] [CrossRef]
- Anh Tran, V.; Khoa Phung, T.; Thuan Le, V.; Ky Vo, T.; Tai Nguyen, T.; Anh Nga Nguyen, T.; Quoc Viet, D.; Quang Hieu, V.; Thi Vo, T.-T. Solar-light-driven photocatalytic degradation of methyl orange dye over Co3O4-ZnO nanoparticles. Mater. Lett. 2021, 284, 128902. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- von Gunten, U. Oxidation Processes in Water Treatment: Are We on Track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Hermes, N.; Jewell, K.S.; Falås, P.; Lutze, H.V.; Wick, A.; Ternes, T.A. Ozonation of Sitagliptin: Removal Kinetics and Elucidation of Oxidative Transformation Products. Environ. Sci. Technol. 2020, 54, 10588–10598. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.M.; Kim, T.K.; Choi, K.; Zoh, K.D. Degradation of cyclophosphamide during UV/chlorine reaction: Kinetics, byproducts, and their toxicity. Chemosphere 2021, 268, 128817. [Google Scholar] [CrossRef]
- Emídio, E.S.; Hammer, P.; Nogueira, R.F.P. Simultaneous degradation of the anticancer drugs 5-fluorouracil and cyclophosphamide using a heterogeneous photo-Fenton process based on copper-containing magnetites (Fe3−xCuxO4). Chemosphere 2020, 241, 124990. [Google Scholar] [CrossRef]
- Steger-Hartmann, T.; Kümmerer, K.; Hartmann, A. Biological Degradation of Cyclophosphamide and Its Occurrence in Sewage Water. Ecotoxicol. Environ. Saf. 1997, 36, 174–179. [Google Scholar] [CrossRef]
- Lancharro, P.M.; De Castro-Acuña Iglesias, N.; González-Barcala, F.J.; González, J.D.M. Evidence of exposure to cytostatic drugs in healthcare staff: A review of recent literature. Farm. Hosp. 2016, 40, 604–621. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Photocatalytic degradation of cyclophosphamide and ifosfamide: Effects of wastewater matrix, transformation products and in silico toxicity prediction. Sci. Total Environ. 2019, 692, 503–510. [Google Scholar] [CrossRef]
- Česen, M.; Kosjek, T.; Busetti, F.; Kompare, B.; Heath, E. Human metabolites and transformation products of cyclophosphamide and ifosfamide: Analysis, occurrence and formation during abiotic treatments. Environ. Sci. Pollut. Res. 2016, 23, 11209–11223. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Advanced oxidation with ozone of 1,3,6-naphthalenetrisulfonic acid in aqueous solution. J. Chem. Technol. Biotechnol. 2002, 77, 148–154. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Lu, J.; Xiong, Y. Catalytic performance of MgO with different exposed crystal facets towards the ozonation of 4-chlorophenol. Appl. Catal. A Gen. 2015, 506, 118–125. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).