Accelerating Microbial Activity of Soil Aquifer Treatment by Hydrogen Peroxide
Abstract
1. Introduction
2. Materials and Methods
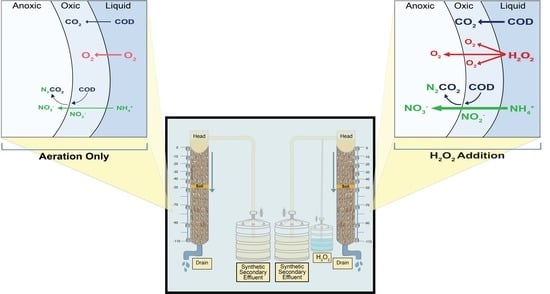
2.1. Accelerating Aerobic Microbial Activity by H2O2 Addition
2.2. Synthetic Secondary Effluent Column Feed
2.3. Verifying Microbial Only H2O2-Based Activity
2.4. Sampling and Analysis of Solute Quality and Soil Bacteria along Depth
2.5. Bacterial Population by qPCR and 16S rRNA Gene Analysis
2.6. Topsoil 16S rRNA Gene Sequencing, bacterial Population Analysis
3. Results
3.1. Evaluation of Soil Column Performance along with the Depth
3.2. The Difference in Community Structure of Topsoil Samples Due to Exposure to H2O2
3.3. Nitrogen Functional Group Dynamics
3.4. Influence of H2O2 on the Community Structure of NOB
4. Discussion
Potential for Application and Enviro-Economic Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aharoni, A.; Cikurel, H. Mekorot’s research activity in technological improvements for the production of unrestricted irrigation quality effluents. Desalination 2006, 187, 347–360. [Google Scholar] [CrossRef]
- Siegel Troubled Water, What is Wrong with What We Drink; Macmillan Publishers Limited: New York, NY, USA, 2019.
- Wojciechowska, E. Removal of persistent organic pollutants from landfill leachates treated in three constructed wetland systems. Water Sci. Technol. 2013, 68, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Chidichimo, F.; Bagetta, R.; Beneduci, A. BTX removal from open Aqueous systems by modified cellulose fibers and evaluation of competitive evaporation kinetics. Water 2020, 12, 3154. [Google Scholar] [CrossRef]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 2015, 77, 213–248. [Google Scholar] [CrossRef]
- Remy, C.; Miehe, U.; Lesjean, B.; Bartholomäus, C. Comparing environmental impacts of tertiary wastewater treatment technologies for advanced phosphorus removal and disinfection with life cycle assessment. Water Sci. Technol. 2014, 69, 1742–1750. [Google Scholar] [CrossRef]
- Fox, P.; Makam, R. Surface area and travel time relationships in aquifer treatment systems. Water Environ. Res. 2009, 81, 2337–2343. [Google Scholar] [CrossRef]
- Elkayam, R.; Sopliniak, A.; Gasser, G.; Lev, O. Oxidizer Demand in the Unsaturated Zone of a Surface-Spreading Soil Aquifer Treatment System. Vadose Zone J. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kennedy, M.D. Soil aquifer treatment for wastewater treatment and reuse. Int. Biodeterior. Biodegrad. 2017, 119, 671–677. [Google Scholar] [CrossRef]
- Besançon, A.; Pidou, M.; Jeffrey, P.; Jefferson, B.; Le Corre, K.S. Impact of pre-treatment technologies on soil aquifer treatment. J. Water Reuse Desalin. 2017, 7, 1–10. [Google Scholar] [CrossRef][Green Version]
- Ben Moshe, S.; Weisbrod, N.; Barquero, F.; Sallwey, J.; Orgad, O.; Furman, A. On the role of operational dynamics in biogeochemical efficiency of a soil aquifer treatment system. Hydrol. Earth Syst. Sci. 2020, 24, 417–426. [Google Scholar] [CrossRef]
- Ben-Noah, I.; Friedman, S.P. Review and Evaluation of Root Respiration and of Natural and Agricultural Processes of Soil Aeration. Vadose Zone J. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Schlegel, H.G. Aeration without air: Oxygen supply by hydrogen peroxide. Biotechnol. Bioeng. 1977, 19, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Mamane, H.; Avisar, D.; Chandran, K. The role of influent organic carbon-to-nitrogen (COD/N) ratio in removal rates and shaping microbial ecology in soil aquifer treatment (SAT). Water Res. 2018, 146, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Alidina, M.; Ouf, M.; Sharp, J.O.; Saikaly, P.; Drewes, J.E. Microbial community evolution during simulated managed aquifer recharge in response to different biodegradable dissolved organic carbon (BDOC) concentrations. Water Res. 2013, 56, 2421–2430. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Or, D. Hydration and diffusion processes shape microbial community organization and function in model soil aggregates. Water Resour. Res. 2015, 51, 9804–9827. [Google Scholar] [CrossRef]
- Bassin, J.P.; Abbas, B.; Vilela, C.L.S.; Kleerebezem, R.; Muyzer, G.; Rosado, A.S.; van Loosdrecht, M.C.M.; Dezotti, M. Tracking the dynamics of heterotrophs and nitrifiers in moving-bed biofilm reactors operated at different COD/N ratios. Bioresour. Technol. 2015, 192, 131–141. [Google Scholar] [CrossRef]
- Grady, C.P.L.; Daigger, G.T.; Love, N.G.; Filipe, C.D.M. Biological Wastewater Treatment; IWA Publishing-Co-Publication: London, UK, 2011; p. 3429. ISBN ISBN 978184339. [Google Scholar]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Palomo, A.J.; Fowler, S.; Gülay, A.; Rasmussen, S.; Sicheritz-Ponten, T.; Smets, B.F. Metagenomic analysis of rapid gravity sand filter microbial communities suggests novel physiology of Nitrospira spp. ISME J. 2016, 10, 2569–2581. [Google Scholar] [CrossRef]
- Stein, L.Y. Heterotrophic nitrification and nitrifier denitrification. Nitrification 2014, 95–114. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Or, D. Microbial community dynamics in soil aggregates shape biogeochemical gas fluxes from soil profiles–upscaling an aggregate biophysical model. Glob. Chang. Biol. 2016, 22, 3141–3156. [Google Scholar] [CrossRef] [PubMed]
- Goren, O.; Burg, A.; Gavrieli, I.; Negev, I.; Guttman, J.; Kraitzer, T.; Kloppmann, W.; Lazar, B. Biogeochemical processes in infiltration basins and their impact on the recharging effluent, the soil aquifer treatment (SAT) system of the Shafdan plant, Israel. Appl. Geochem. 2014, 48, 58–69. [Google Scholar] [CrossRef]
- Fiorenza, S.; Ward, C.H. Microbial adaptation to hydrogen peroxide and biodegradation of aromatic hydrocarbons. J. Ind. Microbiol. Biotechnol. 1997, 18, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ratnaweera, H.; Abdullah, J.; Olsbu, V. Statistical monitoring and dynamic simulation of a wastewater treatment plant: A combined approach to achieve model predictive control. J. Environ. Manag. 2017, 193, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Yoo, S.H.; Son, H.; Fish, K.E.; Douterelo, I.; Maeng, S.K. Effects of phosphate and hydrogen peroxide on the performance of a biological activated carbon filter for enhanced biofiltration. J. Hazard. Mater. 2020, 388, 121778. [Google Scholar] [CrossRef] [PubMed]
- Maduna, K.; Kumar, N.; Aho, A.; Wärnå, J.; Zrnčević, S.; Murzin, D.Y. Kinetics of Catalytic Wet Peroxide Oxidation of Phenolics in Olive Oil Mill Wastewaters over Copper Catalysts. ACS Omega 2018, 3, 7247–7260. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Fidaleo, M.; Lavecchia, R. Degradation and antibiotic activity reduction of chloramphenicol in aqueous solution by UV/H2O2 process. J. Environ. Manag. 2014, 133, 302–308. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Response surface methodology (RSM) analysis of photodegradation of sulfonated diazo dye Reactive Green 19 by UV/H2O2 process. J. Environ. Manag. 2013, 127, 28–35. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Evaluation of UV/H 2 O 2 advanced oxidation process (AOP) for the degradation of diazo dye Reactive Green 19 in aqueous solution. Desalin. Water Treat. 2014, 52, 1571–1577. [Google Scholar] [CrossRef]
- Wood, N.J.; Sørensen, J. Catalase and superoxide dismutase activity in ammonia-oxidising bacteria. FEMS Microbiol. Ecol. 2001, 38, 53–58. [Google Scholar] [CrossRef][Green Version]
- Chance, B.; Herbert, D. The enzyme-substrate compounds of bacterial catalase and peroxides. Biochem. J. 1950, 46, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Zappi, M.; White, K.; Hwang, H.-M.; Bajpai, R.; Qasim, M. The Fate of Hydrogen Peroxide as an Oxygen Source for Bioremediation Activities within Saturated Aquifer Systems. J. Air Waste Manag. Assoc. 2000, 50, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Mamane, H.; Chandran, K.; Jekel, M.; Cikurel, H.; Hübner, U.; Elgart, M.; Dagan, S.; Santo-Domingo, J.; Avisar, D. Stimulating Nitrogen Biokinetics with the Addition of Hydrogen Peroxide to Secondary Effluent Biofiltration. Clean Technol. 2020, 2, 53–73. [Google Scholar] [CrossRef]
- Zucker, I.; Lester, Y.; Avisar, D.; Hübner, U.; Jekel, M.; Weinberger, Y.; Mamane, H. Influence of wastewater particles on ozone degradation of trace organic contaminants. Environ. Sci. Technol. 2015, 49, 301–308. [Google Scholar] [CrossRef]
- Rotthauwe, J.; Witzel, K. The Ammonia Monooxygenase Structural Gene amoA as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Ma, Y.; Sundar, S.; Park, H.; Chandran, K. The effect of inorganic carbon on microbial interactions in a biofilm nitritation-anammox process. Water Res. 2015, 70, 246–254. [Google Scholar] [CrossRef]
- Graham, D.W.; Knapp, C.W.; Van Vleck, E.S.; Bloor, K.; Lane, T.B.; Graham, C.E. Experimental demonstration of chaotic instability in biological nitrification. ISME J. 2007, 1, 385–393. [Google Scholar] [CrossRef]
- Siegel Let There Be Water:Israel’s Solution for a Water-Starved World; Thomas Dunne Books/St. Martin’s Press: New York, NY, USA, 2015; ISBN ISBN 978-1-250-07395-2.
- Park, M.-R.; Park, H.; Chandran, K. Molecular and kinetic characterization of planktonic Nitrospira spp. selectively enriched from activated sludge. Environ. Sci. Technol. 2017, 51, 2720–2728. [Google Scholar] [CrossRef]
- Su, Y.; Sathyamoorthy, S.; Chandran, K. Bioaugmented methanol production using ammonia oxidizing bacteria in a continuous flow process Room 1045 Mudd Hall Current Address: Black & Veatch. Bioresour. Technol. 2019, 279, 101–107. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Wen, S.; Liu, H.; He, H.; Luo, L.; Li, X.; Zeng, G.; Zhou, Z.; Lou, W.; Yang, C. Treatment of anaerobically digested swine wastewater by Rhodobacter blasticus and Rhodobacter capsulatus. Bioresour. Technol. 2016, 222, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.D.; Chakraborty, R.; Lack, J.G.; Connor, S.M.O. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 2001, 411, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, C.; Li, F.; Luo, C.; Chen, M.; Hu, M. The key microorganisms for anaerobic degradation of pentachlorophenol in paddy soil as revealed by stable isotope probing. J. Hazard. Mater. 2015, 298, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Verbaendert, I.; De Vos, P.; Boon, N.; Heylen, K. Denitrification in Gram-positive bacteria: An underexplored trait. Biochem. Soc. Trans. 2011, 39, 254–258. [Google Scholar] [CrossRef][Green Version]
- Tatari, K.; Musovic, S.; Gülay, A.; Dechesne, A.; Smets, B.F. Density and distribution of nitrifying guilds in rapid sand filters for drinking water production: Dominance of Nitrospira spp. Water Res. 2017, 127, 239–248. [Google Scholar] [CrossRef]
- Lu, S.; Gao, X.; Wu, P.; Li, W.; Bai, X.; Sun, M.; Wang, A. Assessment of the treatment of domestic sewage by a vertical-flow artificial wetland at different operating water levels. J. Clean. Prod. 2019, 208, 649–655. [Google Scholar] [CrossRef]
- Aharoni, N.; Mamane, H.; Biran, D.; Lakretz, A.; Ron, E.Z. Gene expression in Pseudomonas aeruginosa exposed to hydroxyl-radicals. Chemosphere 2018, 199, 243–250. [Google Scholar] [CrossRef]
- Miller, J.H.; Ela, W.P.; Lansey, K.E.; Chipello, P.L.; Arnold, R.G. Nitrogen Transformations during Soil–Aquifer Treatment of Wastewater Effluent—Oxygen Effects in Field Studies. J. Environ. Eng. 2006, 132, 1298–1306. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Idelovitch, E. SAT (Soil Aquifer Treatment)–The Long-Term Performance of the Dan Region Reclamation Project Why Wastewater Reuse ? Water 2003. [Google Scholar]
- Zucker, I.; Mamane, H.; Cikurel, H.; Jekel, M.; Hubner, U.; Avisar, D. A hybrid process of biofiltration of secondary effluent followed by ozonation and short soil aquifer treatment for water reuse. Water Res. 2015, 84, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Priel, M.; Gelman, E.; Glueckstern, P.; Balkwill, A.; David, I.; Arviv, R. Optimization of wastewater desalination. Desalin. Water Treat. 2009, 7, 71–77. [Google Scholar] [CrossRef]
- Stanford, B.; Debroux, J.; Snyder, S.; Gerrity, D. Efficacy and Energy Requirements for Trace Contaminant Removal in Water Reuse Systems. In Proceedings of the IWA Water Reuse Conference, Namibia, 2013. [Google Scholar]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Niu, L.; Shen, F.; Wei, J.; Xiang, Y.; Yu, Z.; Zhang, G.; Ding, C.; Huang, Y.; Li, X. In Situ Hydrogen Peroxide Production for Selective Oxidation of Benzyl Alcohol over a Pd@Hierarchical Titanium Silicalite Catalyst. ACS Omega 2020, 5, 16865–16874. [Google Scholar] [CrossRef]
- Hutchinson, A.S.; Woodside, G.D.; Herndon, R.L. Increasing the Sustainable Yield of the Orange County Groundwater Basin with Managed Aquifer Recharge. Groundwater 2021. [Google Scholar] [CrossRef]
- Hafiz, M.A.; Hawari, A.H.; Alfahel, R.; Hassan, M.K.; Altaee, A. Comparison of nanofiltration with reverse osmosis in reclaiming tertiary treated municipal wastewater for irrigation purposes. Membranes 2021, 11, 32. [Google Scholar] [CrossRef]






| Compound | Concentration | Unit |
|---|---|---|
| NH4+ | 4.1 ± 0.2 | mg N/L |
| NO2− | 1.8 ± 0.05 | mg N/L |
| NO3− | 0.22 ± 0.01 | mg N/L |
| Organic nitrogen | 4.5 ± 0.1 | mg N/L |
| COD | 63.2 ± 1.1 | mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedman, L.; Chandran, K.; Avisar, D.; Taher, E.; Kirchmaier-Hurpia, A.; Mamane, H. Accelerating Microbial Activity of Soil Aquifer Treatment by Hydrogen Peroxide. Energies 2022, 15, 3852. https://doi.org/10.3390/en15113852
Friedman L, Chandran K, Avisar D, Taher E, Kirchmaier-Hurpia A, Mamane H. Accelerating Microbial Activity of Soil Aquifer Treatment by Hydrogen Peroxide. Energies. 2022; 15(11):3852. https://doi.org/10.3390/en15113852
Chicago/Turabian StyleFriedman, Liron, Kartik Chandran, Dror Avisar, Edris Taher, Amanda Kirchmaier-Hurpia, and Hadas Mamane. 2022. "Accelerating Microbial Activity of Soil Aquifer Treatment by Hydrogen Peroxide" Energies 15, no. 11: 3852. https://doi.org/10.3390/en15113852
APA StyleFriedman, L., Chandran, K., Avisar, D., Taher, E., Kirchmaier-Hurpia, A., & Mamane, H. (2022). Accelerating Microbial Activity of Soil Aquifer Treatment by Hydrogen Peroxide. Energies, 15(11), 3852. https://doi.org/10.3390/en15113852