Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage
Abstract
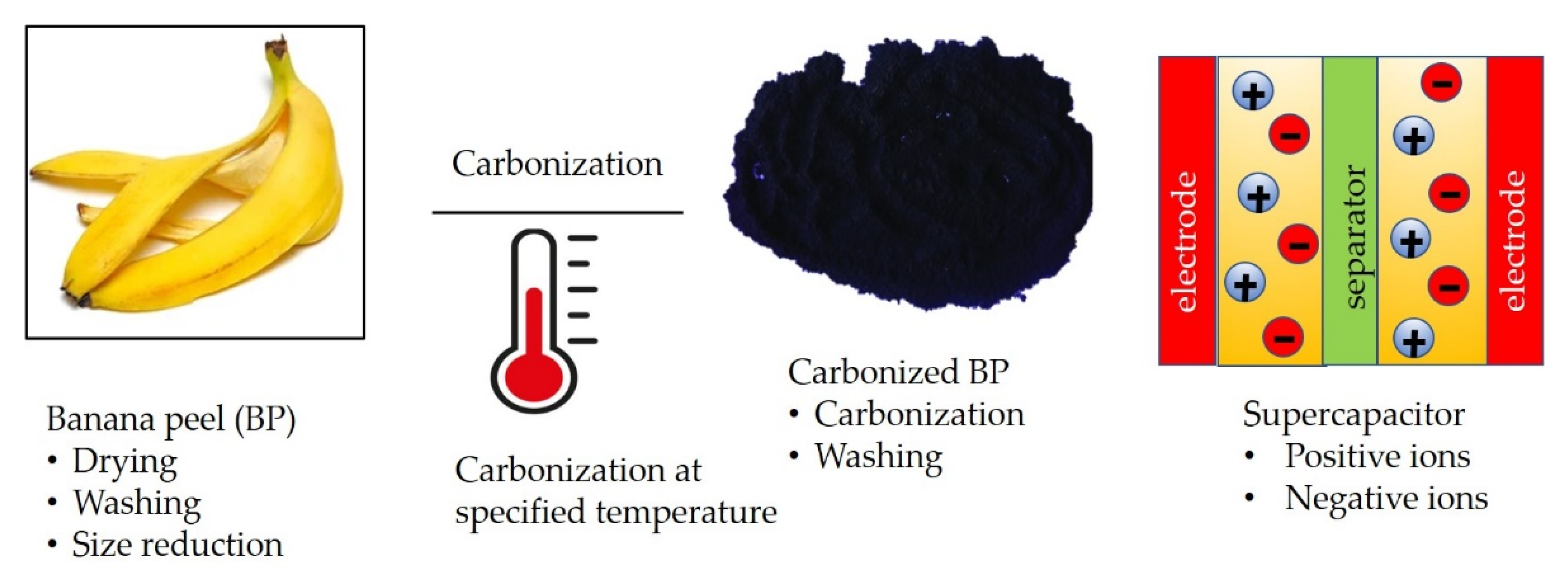
:1. Introduction
2. Supercapacitors Based on Carbonized Banana Peels
3. Supercapacitors Based on PEDOT:PSS
3.1. Introduction
3.2. Conductivity Enhancement Principle to Increase Capacitance Value
3.3. Textile Based Supercapacitors Using PEDOT:PSS
4. Supercapacitors Based on Polyaniline
5. Supercapacitors Based on PPy
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohsin, M.; Naseem, S.; Sarfraz, M.; Azam, T. Assessing the effects of fuel energy consumption, foreign direct investment and GDP on CO2 emission: New data science evidence from Europe & Central Asia. Fuel 2022, 314, 123098. [Google Scholar] [CrossRef]
- Brockway, P.E.; Owen, A.; Brand-Correa, L.I.; Hardt, L. Estimation of global final-stage energy-return-on-investment for fossil fuels with comparison to renewable energy sources. Nat. Energy 2019, 4, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Ravache, B.; Sartor, D. Building Innovation: A Guide For High-Performance Energy Efficient Buildings in India. Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2018. [Google Scholar]
- Thangavel, R.; Ahilan, V.; Moorthy, M.; Yoon, W.-S.; Shanmugam, S.; Lee, Y.-S. Flexible quasi-solid-state lithium-ion capacitors employing amorphous SiO2 nanospheres encapsulated in nitrogen-doped carbon shell as a high energy anode. J. Power Sour. 2021, 484, 229143. [Google Scholar] [CrossRef]
- Peddigari, M.; Park, J.H.; Han, J.H.; Jeong, C.K.; Jang, J.; Min, Y.; Kim, J.-W.; Ahn, C.-W.; Choi, J.-J.; Hahn, B.-D. Flexible self-charging, ultrafast, high-power-density ceramic capacitor system. ACS Energy Lett. 2021, 6, 1383–1391. [Google Scholar] [CrossRef]
- Tahir, M.B.; Abrar, M.; Tehseen, A.; Awan, T.I.; Bashir, A.; Nabi, G. Nanotechnology: The road ahead. In Chemistry of Nanomaterials; Almas, B., Ed.; Elsevier Ltd.: Oxford, UK, 2020; pp. 289–308. [Google Scholar] [CrossRef]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sour. 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Song, N.; Li, X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim. Acta 2016, 222, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas-Martínez, J.; España-Sánchez, B.L.; Esparza, R.; Ávila-Niño, J.A. Flexible and transparent supercapacitors using electrospun PEDOT:PSS electrodes. Synth. Met. 2020, 267, 116436. [Google Scholar] [CrossRef]
- Manjakkal, L.; Pullanchiyodan, A.; Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. A wearable supercapacitor based on conductive PEDOT:PSS-coated cloth and a sweat electrolyte. Adv. Mater. 2020, 32, 1907254. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, W.; Cheng, X.; Ren, J.; Weng, W.; Chen, P.; Fang, X.; Zhang, Z.; Peng, H. Flexible and stretchable lithium-ion batteries and supercapacitors based on electrically conducting carbon nanotube fiber springs. Angew. Chem. Int. Ed. 2014, 53, 14564–14568. [Google Scholar] [CrossRef]
- Pu, J.; Wang, X.; Xu, R.; Xu, S.; Komvopoulos, K. Highly flexible, foldable, and rollable microsupercapacitors on an ultrathin polyimide substrate with high power density. Microsyst. Nanoeng. 2018, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Heteroatom-doped and oxygen-functionalized nanocarbons for high-performance supercapacitors. Adv. Energy Mater. 2020, 10, 2001239. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Skinner, B.; Chen, T.; Loth, M.S.; Shklovskii, B.I. Theory of volumetric capacitance of an electric double-layer supercapacitor. Phys. Rev. E 2011, 83, 056102. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.-Z.; Zhang, J.-D.; Lai, W.-Y.; Huang, W. High-performance free-standing PEDOT:PSS electrodes for flexible and transparent all-solid-state supercapacitors. J. Mater. Chem. A 2016, 4, 10493–10499. [Google Scholar] [CrossRef]
- Yi, R.; Chen, S.; Song, J.; Gordin, M.L.; Manivannan, A.; Wang, D. High-performance hybrid supercapacitor enabled by a high-rate Si-based anode. Adv. Funct. Mater. 2014, 24, 7433–7439. [Google Scholar] [CrossRef]
- Wu, Q.; He, T.; Zhang, Y.; Zhang, J.; Wang, Z.; Liu, Y.; Zhao, L.; Wu, Y.; Ran, F. Cyclic stability of supercapacitors: Materials, energy storage mechanism, test methods, and device. J. Mater. Chem. A 2021, 9, 24094–24147. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Hasanin, M.S. Green and facile synthesis of nickel oxide-porous carbon composite as improved electrochemical electrodes for supercapacitor application from banana peel waste. Environ. Sci. Pollut. Res. 2021, 28, 66888–66900. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Qi, P.; Wang, G.; Shi, L.; Yu, F. Sulphur-doped banana peel-derived activated carbon as electrode materials for supercapacitors. Int. J. Nanomanuf. 2019, 15, 181–195. [Google Scholar] [CrossRef]
- Kaushal, I.; Maken, S.; Sharma, A.K. SnO2 mixed banana peel derived biochar composite for supercapacitor application. Korean Chem. Eng. Res. 2018, 56, 694–704. [Google Scholar] [CrossRef]
- Tripathy, A.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Renewable banana-peel-derived activated carbon as an inexpensive and efficient electrode material showing fascinating supercapacitive performance. J. Environ. Chem. Eng. 2021, 9, 106398. [Google Scholar] [CrossRef]
- Raji, A.; Thomas Nesakumar, J.I.E.; Mani, S.; Perumal, S.; Rajangam, V.; Thirunavukkarasu, S.; Lee, Y.R. Biowaste-originated heteroatom-doped porous carbonaceous material for electrochemical energy storage application. J. Ind. Eng. Chem. 2021, 98, 308–317. [Google Scholar] [CrossRef]
- Kandasamy, S.K.; Arumugam, C.; Sajitha, A.S.; Rao, S.P.; Selvaraj, S.; Vetrivel, R.; Selvarajan, R.; Alosaimi, A.M.; Khan, A.; Hussein, M.A.; et al. Paradisiaca/Solanum Tuberosum Biowaste Composited with Graphene Oxide for Flexible Supercapacitor. J. New Mater. Electrochem. Syst. 2021, 24, 21–28. [Google Scholar] [CrossRef]
- Singh, A.; Ghosh, K.; Kumar, S.; Agarwal, A.K.; Jassal, M.; Goswami, P.; Chaturvedi, H. Flexible planar asymmetric supercapacitor using synthesized few-layer graphene and activated carbon from biomass for wearable energy storage. Nanotechnol. Percept. 2019, 15, 183–188. [Google Scholar] [CrossRef]
- Ren, B.; Fan, M.; Yang, X.; Wang, L.; Yu, H. 3D Hierarchical structure Electrodes of MnO2 Nanosheets Decorated on Needle-like NiCo2O4 Nanocones on Ni Foam as a cathode material for Asymmetric Supercapacitors. ChemistrySelect 2019, 4, 5641–5650. [Google Scholar] [CrossRef]
- Kaushal, I.; Saharan, P.; Kumar, V.; Sharma, A.K.; Umar, A. Superb sono-adsorption and energy storage potential of multifunctional Ag-Biochar composite. J. Alloys Compd. 2019, 785, 240–249. [Google Scholar] [CrossRef]
- Taer, E.; Agustino, A.; Farma, R.; Taslim, R.; Awitdrus; Paiszal, M.; Ira, A.; Yardi, S.D.; Sari, Y.P.; Yusra, H.; et al. The relationship of surface area to cell capacitance for monolith carbon electrode from biomass materials for supercapacitor aplication. Proc. J. Phys. Conf. Ser. 2008, 1116, 032040. [Google Scholar] [CrossRef]
- Lian, Y.M.; Ni, M.; Zhou, L.; Chen, R.J.; Yang, W. Synthesis of Biomass-Derived Carbon Induced by Cellular Respiration in Yeast for Supercapacitor Applications. Chem.-A Eur. J. 2018, 24, 18068–18074. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, Y.; Ren, J.; Wu, H.; Lin, D.; Xie, F.; Jie, W.; Lam, K.H.; Xu, C.; Zheng, Q. An Eco-friendly Microorganism Method to Activate Biomass for Cathode Materials for High-Performance Lithium-Sulfur Batteries. Energy Fuels 2018, 32, 9997–10007. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, M.; Zhang, Y.; Liu, M.; Xiong, W.; Liu, S. Large surface area porous carbon materials synthesized by direct carbonization of banana peel and citrate salts for use as high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 4294–4300. [Google Scholar] [CrossRef]
- Fasakin, O.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Oyedotun, K.O.; Eleruja, M.A.; Manyala, N. Synthesis and characterization of porous carbon derived from activated banana peels with hierarchical porosity for improved electrochemical performance. Electrochim. Acta 2018, 262, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Taer, E.; Taslim, R.; Aini, Z.; Hartati, S.D.; Mustika, W.S. Activated carbon electrode from banana-peel waste for supercapacitor applications. Proc. AIP Conf. Proc. 2017, 1801, 040004. [Google Scholar]
- Sari, S.N.; Melati, A. Facile preparation of carbon nanofiber from banana peel waste. Mater. Today Proc. 2019, 13, 165–168. [Google Scholar] [CrossRef]
- Yusuf, J.Y.; Soleimani, H.; Chuan, L.K.; Sanusi, Y.K.; Adebayo, L.L. Physicochemical properties and microwave absorption performance of Co3O4 and banana peel-derived porous activated carbon composite at X-band frequency. J. Alloys Compd. 2021, 888. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sour. 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Mengistie, D.A.; Chen, Y.; Wang, L.; Loghin, C.; Nierstrasz, V. Electrically conductive highly elastic polyamide/lycra fabric treated with PEDOT:PSS and polyurethane. J. Mater. Sci. 2019, 54, 9591–9602. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, M.G.; Loghin, C.; Chen, Y.; Wang, L.; Catalin, D.; Nierstrasz, V. Effect of liquid immersion of PEDOT: PSS-coated polyester fabric on surface resistance and wettability. Smart Mater. Struct. 2017, 26, 065016. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable conductive polymers and composites based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadesse, M.G.; Dumitrescu, D.; Loghin, C.; Chen, Y.; Wang, L.; Nierstrasz, V. 3D Printing of NinjaFlex Filament onto PEDOT:PSS-Coated Textile Fabrics for Electroluminescence Applications. J. Electron. Mater. 2018, 47, 2082–2092. [Google Scholar] [CrossRef] [Green Version]
- Teng, W.; Zhou, Q.; Wang, X.; Gao, J.; Hu, P.; Du, Y.; Li, H.; Wang, J. Enhancing ions/electrons dual transport in rGO/PEDOT: PSS fiber for high-performance supercapaciton. Carbon 2022, 189, 284–292. [Google Scholar] [CrossRef]
- He, Y.; Liang, A.; Zhu, D.; Hu, M.; Xu, L.; Chao, S.; Zhou, W.; Wu, Y.; Xu, J.; Zhao, F. Organic-inorganic hybrid electrode engineering for high-performance asymmetric supercapacitor based on WO3-CeO2 nanowires with oxygen vacancies. Appl. Surf. Sci. 2022, 573, 151624. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Liu, K.; Parit, M.; Jiang, Z.; Zhang, X.; Li, B.; Si, C. Conductive PEDOT:PSS/cellulose nanofibril paper electrodes for flexible supercapacitors with superior areal capacitance and cycling stability. Chem. Eng. J 2022, 428, 131994. [Google Scholar] [CrossRef]
- Lai, H.; Bai, C.; Wang, Y.; Fan, Z.; Yuan, Y.; Jiao, H. Highly Crosslinked Conductive Polymer Nanofibrous Films for High-Rate Solid-State Supercapacitors and Electromagnetic Interference Shielding. Adv. Mater. Interfaces 2022, 9, 2102115. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Jafer, R.; Ramesh, S.; Ramesh, K. Self-healable poly (N, N-dimethylacrylamide)/poly (3, 4-ethylenedioxythiophene) polystyrene sulfonate composite hydrogel electrolytes for aqueous supercapacitors. J. Ener. Storage 2022, 45, 103760. [Google Scholar] [CrossRef]
- Li, Z.; Ruiz, V.; Mishukova, V.; Wan, Q.; Liu, H.; Xue, H.; Gao, Y.; Cao, G.; Li, Y.; Zhuang, X.; et al. Inkjet Printed Disposable High-Rate On-Paper Microsupercapacitors. Adv. Funct. Mater. 2022, 32, 2108773. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, J.; Yang, C.; Zang, L.; Zhang, G.; Sakai, E.; Wu, H.; Guo, S. Robust quasi-solid-state integrated asymmetric flexible supercapacitors with interchangeable positive and negative electrode based on all-conducting-polymer electrodes. J. Alloys Compd. 2021, 887, 161362. [Google Scholar] [CrossRef]
- Altin, Y.; Celik Bedeloglu, A. Poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate-coated carbon nanofiber electrodes via dip-coating method for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2021, 32, 28234–28244. [Google Scholar] [CrossRef]
- Li, D.; Yang, S.; Chen, X.; Lai, W.Y.; Huang, W. 3D Wearable Fabric-Based Micro-Supercapacitors with Ultra-High Areal Capacitance. Adv. Funct. Mater. 2021, 31, 2107484. [Google Scholar] [CrossRef]
- Song, J.; Li, W.; Xin, J.; Wang, W.; Song, K.; Chen, X.; Yin, G. The continuous porous PEDOT:PSS film improves wettability and flexibility of the rGO/CoFe2O4 paper electrodes for symmetric supercapacitors. Appl. Surf. Sci. 2021, 568, 150915. [Google Scholar] [CrossRef]
- Hina, M.; Bashir, S.; Kamran, K.; Iqbal, J.; Ramesh, S.; Ramesh, K. Fabrication of aqueous solid-state symmetric supercapacitors based on self-healable poly (acrylamide)/PEDOT:PSS composite hydrogel electrolytes. Mater. Chem. Phys. 2021, 273, 125125. [Google Scholar] [CrossRef]
- Song, J.; Li, W.; Song, K.; Qin, C.; Chen, X.; Sui, Y.; Zhao, Q.; Ye, Y. Synergistic effect of defects and porous structure in CoCCHH-CoSe heterogeneous-tube @PEDOT:PSS foam towards elastic supercapacitor with enhanced pseudocapacitances. J. Colloid Interface Sci. 2021, 602, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, T.; Li, S.; Shen, X. Electrodeposition Polyaniline Nanofiber on the PEDOT:PSS-Coated SiNWs for High Performance Supercapacitors. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4260–4271. [Google Scholar] [CrossRef]
- Pullanchiyodan, A.; Manjakkal, L.; Ntagios, M.; Dahiya, R. MnO x-Electrodeposited Fabric-Based Stretchable Supercapacitors with Intrinsic Strain Sensing. ACS Appl. Mater. Interfaces 2021, 13, 47581–47592. [Google Scholar] [CrossRef]
- Guan, X.; Pan, L.; Fan, Z. Flexible, transparent and highly conductive polymer film electrodes for all-solid-state transparent supercapacitor applications. Membranes 2021, 11, 788. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Q.; Tang, X.; Du, J.; Yu, T.; Xu, X.; Cai, D.; Guan, C.; Huang, W. 3D-Printed highly stretchable conducting polymer electrodes for flexible supercapacitors. J. Mater. Chem. A 2021, 9, 19649–19658. [Google Scholar] [CrossRef]
- Wang, P.; Du, X.; Wang, X.; Zhang, K.; Sun, J.; Chen, Z.; Xia, Y. Integrated fiber electrodes based on marine polysaccharide for ultrahigh-energy-density flexible supercapacitors. J. Power Sour. 2021, 506, 230130. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Liu, H.; Zhang, S.; Yang, J.; Zhou, J.; Yu, J.; Ji, M.; Zhu, C.; Xu, J. Tear resistant Tyvek/Ag/poly(3,4-ethylenedioxythiophene): Polystyrene sulfonate (PEDOT:PSS)/carbon nanotubes electrodes for flexible high-performance supercapacitors. Chem. Eng. J. 2021, 420, 127665. [Google Scholar] [CrossRef]
- Li, J.; Yan, W.; Zhang, G.; Sun, R.; Ho, D. Natively stretchable micro-supercapacitors based on a PEDOT:PSS hydrogel. J. Mater. Chem. C 2021, 9, 1685–1692. [Google Scholar] [CrossRef]
- Karade, S.S.; Sankapal, B.R. Room temperature PEDOT:PSS encapsulated MWCNTs thin film for electrochemical supercapacitor. J. Electroanal. Chem. 2016, 771, 80–86. [Google Scholar] [CrossRef]
- Lee, S.H.; Sohn, J.S.; Kulkarni, S.B.; Patil, U.M.; Jun, S.C.; Kim, J.H. Modified physico–chemical properties and supercapacitive performance via DMSO inducement to PEDOT:PSS active layer. Org. Electron. 2014, 15, 3423–3430. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X.; Liu, C.; Jiang, Q.; Jiang, F.; An, J.; Xu, J.; Liu, P. DMSO-treated flexible PEDOT:PSS/PANi fiber electrode for high performance supercapacitors. J. Mater. Sci. 2021, 56, 14632–14643. [Google Scholar] [CrossRef]
- Moraes, M.R.; Alves, A.C.; Toptan, F.; Martins, M.S.; Vieira, E.M.; Paleo, A.J.; Souto, A.P.; Santos, W.L.; Esteves, M.F.; Zille, A. Glycerol/PEDOT:PSS coated woven fabric as a flexible heating element on textiles. J. Mater. Chem. C 2017, 5, 3807–3822. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Wang, Y.-C.; Deng, J.; Dai, Y.; Zhang, S.L.; Zou, H.; Gu, B.; Sun, B.; Wang, Z.L. A highly stretchable and washable all-yarn-based self-charging knitting power textile composed of fiber triboelectric nanogenerators and supercapacitors. ACS Nano 2017, 11, 9490–9499. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Wang, P.; Zhou, W.; Chen, Z.; Gao, Q.; Shen, M.; Zhu, J. Urea-treated wet-spun PEDOT:PSS fibers for achieving high-performance wearable supercapacitors. Compos. Commun. 2021, 27, 100885. [Google Scholar] [CrossRef]
- Zhang, W.; Weng, J.; Xie, Y.; Li, X.; Ren, D.; Li, R. Flexible textile-based electronic materials assembled with hybrid PEDOT:PSS doped with anionic surfactant. J. Text. Inst. 2021, 1–9. [Google Scholar] [CrossRef]
- Kanth, S.; Narayanan, P.; Betty, C.A.; Rao, R.; Kumar, S. Investigations on performance of PEDOT:PSS/V2O5 hybrid symmetric supercapacitor with redox electrolyte. J. Appl. Polym. Sci. 2021, 138, 50838. [Google Scholar] [CrossRef]
- Song, J.; Ma, G.; Qin, F.; Hu, L.; Luo, B.; Liu, T.; Yin, X.; Su, Z.; Zeng, Z.; Li, Z.; et al. High-conductivity, flexible and transparent PEDOT: PSS electrodes for high performance semi-transparent supercapacitors. Polymers 2020, 12, 450. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Sui, Y.; Zhao, Q.; Ye, Y.; Qin, C.; Chen, X.; Song, K. A reinforced concrete structure rGO/CNTs/Fe2O3/PEDOT:PSS paper electrode with excellent wettability and flexibility for supercapacitors. New J. Chem. 2021, 45, 14483–14494. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Liu, Y.; Yang, J.; Liu, P.; Jiang, Q.; Jiang, F.; Liu, C.; Ding, W.; Xu, J. Heterostructural conductive polymer with multi-dimensional carbon materials for capacitive energy storage. Appl. Surf. Sci. 2021, 558. [Google Scholar] [CrossRef]
- Liang, J.; Sheng, H.; Wang, Q.; Yuan, J.; Zhang, X.; Su, Q.; Xie, E.; Lan, W.; Zhang, C. PEDOT:PSS-glued MoO3 nanowire network for all-solid-state flexible transparent supercapacitors. Nanoscale Adv. 2021, 3, 3502–3512. [Google Scholar] [CrossRef]
- Zhang, M.; Héraly, F.; Yi, M.; Yuan, J. Multitasking tartaric-acid-enabled, highly conductive, and stable MXene/conducting polymer composite for ultrafast supercapacitor. Cell Rep. Phys. Sci. 2021, 2, 100449. [Google Scholar] [CrossRef]
- Hareesh, K.; Rondiya, S.R.; Dzade, N.Y.; Dhole, S.D.; Williams, J.; Sergey, S. Polymer-wrapped reduced graphene oxide/nickel cobalt ferrite nanocomposites as tertiary hybrid supercapacitors: Insights from experiment and simulation. J. Sci. Adv. Mater. Devices 2021, 6, 291–301. [Google Scholar] [CrossRef]
- Babeli, I.; Ruano, G.; Puiggalí-Jou, A.; Ginebra, M.P.; Alemán, C.; Garcia-Torres, J. Self-Healable and Eco-Friendly Hydrogels for Flexible Supercapacitors. Adv. Sustain. Syst. 2021, 5, 2000273. [Google Scholar] [CrossRef]
- Badi, N.; Khasim, S.; Alatawi, A.S.; Pasha, A.; Al-Ghamdi, S.A.; Ignatiev, A. Fabrication and Testing of PEDOT:PSS Wrapped WO2/Au Ternary Nanocomposite Electrodes for High Performance Flexible Supercapacitor Applications. J. Electrochem. Soc. 2021, 168, 040526. [Google Scholar] [CrossRef]
- Zhu, W.C.; He, P.Q.; Tien, H.C.; Liu, H.L.; Chen, W.C.; Lv, W.; Lee, W.Y. Solvent-Enhanced Transparent Stretchable Polymer Nanocomposite Electrode for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 2266–2274. [Google Scholar] [CrossRef]
- Arthisree, D.; Madhuri, W.; Saravanan, N.; Dinesh, B.; Saikrithika, S.; Kumar, A.S. A ternary polymer nanocomposite film composed of green-synthesized graphene quantum dots, polyaniline, polyvinyl butyral and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate for supercapacitor application. J. Energy Storage 2021, 35, 102333. [Google Scholar] [CrossRef]
- Chao, Y.; Ge, Y.; Chen, Z.; Cui, X.; Zhao, C.; Wang, C.; Wallace, G.G. One-Pot Hydrothermal Synthesis of Solution-Processable MoS2/PEDOT:PSS Composites for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 7285–7296. [Google Scholar] [CrossRef]
- Bamgbopa, M.O.; Belaineh, D.; Mengistie, D.A.; Edberg, J.; Engquist, I.; Berggren, M.; Tybrandt, K. Modelling of heterogeneous ion transport in conducting polymer supercapacitors. J. Mater. Chem. A 2021, 9, 2184–2194. [Google Scholar] [CrossRef]
- Manjakkal, L.; Franco, F.F.; Pullanchiyodan, A.; González-Jiménez, M.; Dahiya, R. Natural Jute Fibre-Based Supercapacitors and Sensors for Eco-Friendly Energy Autonomous Systems. Adv. Sustain. Syst. 2021, 5, 2000286. [Google Scholar] [CrossRef]
- Mirabedini, A.; Lu, Z.; Mostafavian, S.; Foroughi, J. Triaxial carbon nanotube/conducting polymer wet-spun fibers supercapacitors for wearable electronics. Nanomaterials 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Schneegass, S.; Amft, O. Smart Textiles; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Ma, Y.; Wang, Q.; Liang, X.; Zhang, D.; Miao, M. Wearable supercapacitors based on conductive cotton yarns. J. Mater. Sci. 2018, 53, 14586–14597. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, C.; Sun, S.; Zhang, K. Electrochemical properties of PEDOT: PSS/V2O5 hybrid fiber based supercapacitors. J. Phys. Chem. Solids 2019, 129, 234–241. [Google Scholar] [CrossRef]
- Kumar, N.; Ginting, R.T.; Kang, J.W. Flexible, large-area, all-solid-state supercapacitors using spray deposited PEDOT:PSS/reduced-graphene oxide. Electrochim. Acta 2018, 270, 37–47. [Google Scholar] [CrossRef]
- Han, Y.; Dai, L. Conducting polymers for flexible supercapacitors. Macromol. Chem. Phys. 2019, 220, 1800355. [Google Scholar] [CrossRef]
- Moussa, M.; Shi, G.; Wu, H.; Zhao, Z.; Voelcker, N.H.; Losic, D.; Ma, J. Development of flexible supercapacitors using an inexpensive graphene/PEDOT/MnO2 sponge composite. Mater. Des. 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Rajesh, M.; Raj, C.J.; Manikandan, R.; Kim, B.C.; Park, S.Y.; Yu, K.H. A high performance PEDOT/PEDOT symmetric supercapacitor by facile in-situ hydrothermal polymerization of PEDOT nanostructures on flexible carbon fibre cloth electrodes. Mater. Today Energy 2017, 6, 96–104. [Google Scholar] [CrossRef]
- Su, F.; Miao, M. Flexible, high performance two-ply yarn supercapacitors based on irradiated carbon nanotube yarn and PEDOT/PSS. Electrochim. Acta 2014, 127, 433–438. [Google Scholar] [CrossRef]
- Yuan, D.; Li, B.; Cheng, J.; Guan, Q.; Wang, Z.; Ni, W.; Li, C.; Liu, H.; Wang, B. Twisted yarns for fiber-shaped supercapacitors based on wetspun PEDOT:PSS fibers from aqueous coagulation. J. Mater. Chem. A 2016, 4, 11616–11624. [Google Scholar] [CrossRef]
- Yuksel, R.; Unalan, H.E. Textile supercapacitors-based on MnO2/SWNT/conducting polymer ternary composites. Int. J. Energy Res. 2015, 39, 2042–2052. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, H.S.; Lee, C.G.; Park, S.J.; Lee, J.; Jung, S.; Shin, G.A. Application of PANI/TiO2 composite for photocatalytic degradation of contaminants from aqueous solution. Appl. Sci. 2020, 10, 6710. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Peng, C.; Gao, B.; Peng, X.; Zhang, X.; Tao, J.; Kong, J.; Fu, J. Fabrication of PANI/C-TiO2 Composite Nanotube Arrays Electrode for Supercapacitor. J. Nanomater. 2015, 2015, 140596. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, C.; Du, H.; Wang, W. Enhanced electrochemical performance of polyaniline/carbon/titanium nitride nanowire array for flexible supercapacitor. J. Power Sour. 2015, 286, 561–570. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Rajkumar, S.; Elanthamilan, E.; Merlin, J.P.; Sathiyan, A. Enhanced electrochemical behaviour of FeCo2O4/PANI electrode material for supercapacitors. J. Alloys Compd. 2021, 874, 159876. [Google Scholar] [CrossRef]
- Lu, X.F.; Chen, X.Y.; Zhou, W.; Tong, Y.X.; Li, G.R. α-Fe2O3@PANI core-shell nanowire arrays as negative electrodes for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 14843–14850. [Google Scholar]
- Srinivasan, R.; Elaiyappillai, E.; Nixon, E.J.; Lydia, I.S.; Johnson, P.M. Enhanced electrochemical behaviour of Co-MOF/PANI composite electrode for supercapacitors. Inorg. Chim. Acta 2020, 502, 119393. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Kim, H.; Im, H. Mesoporous Ni-PANI composite electrode for electrochromic energy storage applications. Sol. Energy Mater. Sol. Cells 2019, 201, 110121. [Google Scholar]
- Xinming, W.; Qiguan, W.; Wenzhi, Z.; Yan, W.; Weixing, C. Enhanced electrochemical performance of hydrogen-bonded graphene/polyaniline for electrochromo-supercapacitor. J. Mater. Sci. 2016, 51, 7731–7741. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, H.; Gao, X.; Yao, F.; Chen, H.; Lu, X.; Wang, Y.; Guo, X. High-performance supercapacitors based on polyaniline nanowire arrays grown on three-dimensional graphene with small pore sizes. Dalton Trans. 2020, 49, 3304–3311. [Google Scholar] [PubMed]
- Wang, K.; Wu, H.; Meng, Y.; Zhang, Y.; Wei, Z. Integrated energy storage and electrochromic function in one flexible device: An energy storage smart window. Energy Environ. Sci. 2012, 5, 8384–8389. [Google Scholar] [CrossRef]
- An, H.; Wang, Y.; Wang, X.; Li, N.; Zheng, L. The preparation of PANI/CA composite electrode material for supercapacitors and its electrochemical performance. J. Solid State Electrochem. 2010, 14, 651–657. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Yang, G. In situ growth of the Ni3V2O8@PANI composite electrode for flexible and transparent symmetric supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 20688–20695. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mele, G. PANI@Co-Porphyrins composite for the construction of supercapacitors. J. Energy Storage 2019, 26, 101013. [Google Scholar] [CrossRef]
- Ren, X.; Fan, H.; Ma, J.; Wang, C.; Zhang, M.; Zhao, N. Hierarchical Co3O4/PANI hollow nanocages: Synthesis and application for electrode materials of supercapacitors. Appl. Surf. Sci. 2018, 31, 194–203. [Google Scholar] [CrossRef]
- Jiang, F.; Li, W.; Zou, R.; Liu, Q.; Xu, K.; An, L.; Hu, J. MoO3/PANI coaxial heterostructure nanobelts by in situ polymerization for high performance supercapacitors. Nano Energy 2014, 7, 72–79. [Google Scholar] [CrossRef]
- Wang, W.-d.; Lin, X.-Q.; Zhao, H.B.; Lü, Q.F. Nitrogen-doped graphene prepared by pyrolysis of graphene oxide/polyaniline composites as supercapacitor electrodes. J. Anal. Appl. Pyrolysis 2016, 120, 27–36. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, R.; Wang, Z.; Liu, H. Carboxyl-functionalized graphene oxide–polyaniline composite as a promising supercapacitor material. J. Mater. Chem. 2012, 22, 13619–13624. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Farid, S.; Afzal, A.M. Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
- Wei, Y.; Luo, W.; Li, X.; Lin, Z.; Hou, C.; Ma, M.; Ding, J.; Li, T.; Ma, Y. PANI-MnO2 and Ti3C2Tx (MXene) as electrodes for high-performance flexible asymmetric supercapacitors. Electrochim. Acta 2022, 406, 139874. [Google Scholar] [CrossRef]
- Ben, J.; Song, Z.; Liu, X.; Lü, W.; Li, X. Fabrication and Electrochemical Performance of PVA/CNT/PANI Flexible Films as Electrodes for Supercapacitors. Nanoscale Res. Lett. 2020, 15, 151. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Fei, H.; Sapurina, I.; Ngwabebhoh, F.A.; Bubulinca, C.; Munster, L.; Bergerová, E.D.; Lengálová, A.; Jiang, H.; Dao, T.T.; et al. Electrochemical performance of composites made of rGO with Zn-MOF and PANI as electrodes for supercapacitors. Electrochim. Acta 2021, 367, 137563. [Google Scholar]
- He, H.; Ma, L.; Fu, S.; Gan, M.; Hu, L.; Zhang, H.; Xie, F.; Jiang, M. Fabrication of 3D ordered honeycomb-like nitrogen-doped carbon/PANI composite for high-performance supercapacitors. Appl. Surf. Sci. 2019, 484, 1288–1296. [Google Scholar] [CrossRef]
- Barik, R.; Barik, G.; Tanwar, V.; Ingole, P.P. Supercapacitor performance and charge storage mechanism of brannerite type CuV2O6/PANI nanocomposites synthesis with their theoretical aspects. Electrochim. Acta 2022, 410, 140015. [Google Scholar] [CrossRef]
- Atram, R.R.; Bhuse, V.M.; Atram, R.G.; Wu, C.-M.; Koinkar, P.; Kondawar, S.B. Novel carbon nanofibers/thionickel ferrite/polyaniline (CNF/NiFe2S4/PANI) ternary nanocomposite for high performance supercapacitor. Mater. Chem. Phys. 2021, 262, 124253. [Google Scholar] [CrossRef]
- Yasoda, K.Y.; Kumar, S.; Kumar, M.S.; Ghosh, K.; Batabyal, S.K. Fabrication of MnS/GO/PANI nanocomposites on a highly conducting graphite electrode for supercapacitor application. Mater. Today Chem. 2021, 19, 100394. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Prasanna, B.P.; Yogesh Kumar, K.; Asha, P.K.; Nautiyal, P.; Anusuya Devi, V.S.; Alharthi, F.A.; Parashuram, L.; Raghu, M.S. Fabrication of anode material for asymmetric supercapacitor device using polyaniline wrapped boroncarbonitride nanocomposite with enhanced capacitance. J. Alloys Compd. 2020, 848, 156602. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, L.; Ma, Z.; Xu, J.; Li, Y.; Wang, C. Hierarchical porous PANI/MIL-101 nanocomposites based solid-state flexible supercapacitor. Electrochim. Acta 2018, 281, 582–593. [Google Scholar] [CrossRef]
- Sadeghinia, M.; Shayeh, J.S.; Fatemi, F.; Rahmandoust, M.; Ehsani, A.; Rezaei, M. Electrochemical study of perlite-barium ferrite/conductive polymer nano composite for super capacitor applications. Int. J. Hydrogen Energy 2019, 44, 28088–28095. [Google Scholar] [CrossRef]
- Sun, P.-P.; Zhang, Y.-H.; Shi, H.; Shi, F.-N. Controllable one step electrochemical synthesis of PANI encapsulating 3d-4f bimetal MOFs heterostructures as electrode materials for high-performance supercapacitors. Chem. Eng. J. 2022, 427, 130836. [Google Scholar] [CrossRef]
- Pal, R.; Goyal, S.L.; Rawal, I.; Gupta, A.K.; Ruchi. Efficient energy storage performance of electrochemical supercapacitors based on polyaniline/graphene nanocomposite electrodes. J. Phys. Chem. Solids 2021, 154, 110057. [Google Scholar] [CrossRef]
- Poudel, M.B.; Shin, M.; Kim, H.J. Polyaniline-silver-manganese dioxide nanorod ternary composite for asymmetric supercapacitor with remarkable electrochemical performance. Int. J. Hydrogen Energy 2021, 46, 474–485. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Peng, T.; Liu, B.; Hou, Y.; Lei, B. Preparation and characterization of polyaniline/p-phenylenediamine grafted graphene oxide composites for supercapacitors. J. Mol. Struct. 2020, 1221, 128835. [Google Scholar] [CrossRef]
- Zeplin, G.; Neiva, E.G.C. One-pot green synthesis of graphene oxide/MnO2/polyaniline nanocomposites applied in aqueous and neutral supercapacitors and sensors. J. Electroanal. Chem. 2021, 902, 115776. [Google Scholar] [CrossRef]
- Ou, D.; Liu, J.; Yan, J.; Qin, Q.; Xu, J.; Wu, Y. Construction of three-dimensional graphene like carbon on carbon fibers and loading of polyaniline for high performance asymmetric supercapacitor. Electrochim. Acta 2020, 335, 135679. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Gao, N.; Yin, Z.; Zhou, H.; Yang, X.; Kuang, Y. Synthesis of 3D reduced graphene oxide/unzipped carbon nanotubes/polyaniline composite for high-performance supercapacitors. Electrochim. Acta 2018, 269, 649–656. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, H.; Xing, R.; Ge, X.; Sun, H.; Li, R.; Zhang, Q. Multi-growth site graphene/polyaniline composites with highly enhanced specific capacitance and rate capability for supercapacitor application. Electrochim. Acta 2018, 260, 504–513. [Google Scholar] [CrossRef]
- Babu, K.F.; Subramanian, S.S.; Kulandainathan, M.A. Functionalisation of fabrics with conducting polymer for tuning capacitance and fabrication of supercapacitor. Carbohydr. Polym. 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, C.; Yuan, Z. Facile and large-area preparation of polypyrrole film for low-haze transparent supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 41299–41311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, X.; Shang, Y.; Hua, C.; Song, P.; Li, X.; Zhang, Y.; Cao, A. Highly flexible all-solid-state supercapacitors based on carbon nanotube/polypyrrole composite films and fibers. RSC Adv. 2016, 6, 62062–62070. [Google Scholar] [CrossRef]
- Yan, J.; Ma, Y.; Zhang, C.; Li, X.; Liu, W.; Yao, X.; Yao, S.; Luo, S. Polypyrrole–MXene coated textile-based flexible energy storage device. RSC Adv. 2018, 8, 39742–39748. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Liu, Q.; Chen, J.; Wang, B.; Wang, Y.; Liu, K.; Li, M.; Jiang, H.; Lu, Z.; Wang, D. Hierarchically three-dimensional nanofiber based textile with high conductivity and biocompatibility as a microbial fuel cell anode. Environ. Sci. Technol. 2016, 50, 7889–7895. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hao, H.; Chen, Y.; Li, W.; Shen, W.; Shearing, P.R.; Brett, D.J.; He, G. Flexible all-solid-state supercapacitors based on PPy/rGO nanocomposite on cotton fabric. Nanotechnology 2021, 32, 305401. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, B.; Chen, J.; Li, F.; Liu, K.; Wang, Y.; Li, M.; Lu, Z.; Wang, W.; Wang, D. Facile synthesis of three-dimensional (3D) interconnecting polypyrrole (PPy) nanowires/nanofibrous textile composite electrode for high performance supercapacitors. Compos. Part A Appl. Sci. Manuf. 2017, 101, 30–40. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, Q.; Wu, Y.; Wang, Y.; Qing, X.; Li, M.; Liu, K.; Wang, W.; Wang, D. A nanofiber based artificial electronic skin with high pressure sensitivity and 3D conformability. Nanoscale 2016, 8, 12105–12112. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Q.; Yi, C.; Chen, J.; Wang, D.; Wang, Y.; Liu, X.; Li, M.; Liu, K.; Zhou, P. The construction of sea urchin spines-like polypyrrole arrays on cotton-based fabric electrode via a facile electropolymerization for high performance flexible solid-state supercapacitors. Electrochim. Acta 2020, 354, 136746. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Jiao, X.; Yuan, Z. Polypyrrole-based hybrid nanostructures grown on textile for wearable supercapacitors. Nano Res. 2019, 12, 1129–1137. [Google Scholar] [CrossRef]
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196. [Google Scholar] [CrossRef]
- Song, P.; Xi, C.; Premlatha, S.; Shen, X.; Ji, Z.; Yan, Z.; Yuan, A.; Kong, L.; Zhu, G. Sword/scabbard-shaped asymmetric all-solid-state supercapacitors based on PPy-MWCNTs-silk and hollow graphene tube for wearable applications. Chem. Eng. J. 2021, 411, 128522. [Google Scholar] [CrossRef]
- Wen, J.; Xu, B.; Zhou, J.; Chen, Y. Novel high-performance asymmetric supercapacitors based on nickel-cobalt composite and PPy for flexible and wearable energy storage. J. Power Sour. 2018, 402, 91–98. [Google Scholar] [CrossRef]
- Liu, J.-h.; Xu, X.-y.; Liu, C.; Chen, D.-Z. Thermal effect on the pseudocapacitive behavior of high-performance flexible supercapacitors based on polypyrrole-decorated carbon cloth electrodes. New J. Chem. 2021, 45, 12435–12447. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Chen, M.-Z.; Tien, Y.-C.; Kuo, C.-C.; Chen, E.-C.; Lin, Y.-C.; Chiang, T.-C.; Lee, R.-H. V2O5/Carbon Nanotube/Polypyrrole Based Freestanding Negative Electrodes for High-Performance Supercapacitors. Catalysts 2021, 11, 980. [Google Scholar] [CrossRef]
- Upadhyay, J.; Das, T.M.; Borah, R. Electrochemical performance study of polyaniline and polypyrrole based flexible electrodes. Int. J. Polym. Anal. Charact. 2021, 26, 354–363. [Google Scholar] [CrossRef]
- Arena, A.; Branca, C.; Ciofi, C.; D’Angelo, G.; Romano, V.; Scandurra, G. Polypyrrole and Graphene Nanoplatelets Inks as Electrodes for Flexible Solid-State Supercapacitor. Nanomaterials 2021, 11, 2589. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhu, J.; Lan, L.; Li, C.; Mao, J.; Wang, F.; Zhang, Z.; Wang, L. Ultra-low temperature flexible supercapacitor based on hierarchically structured pristine polypyrrole membranes. Chem. Eng. J. 2021, 420, 129712. [Google Scholar] [CrossRef]
- BoopathiRaja, R.; Vadivel, S.; Parthibavarman, M.; Prabhu, S.; Ramesh, R. Effect of polypyrrole incorporated sun flower like Mn2P2O7 with lab waste tissue paper derived activated carbon for asymmetric supercapacitor applications. Surf. Interfaces 2021, 26, 101409. [Google Scholar] [CrossRef]
- Yue, T.; Hou, R.; Liu, X.; Qi, K.; Chen, Z.; Qiu, Y.; Guo, X.; Xia, B.Y. Hybrid Architecture of a Porous Polypyrrole Scaffold Loaded with Metal–Organic Frameworks for Flexible Solid-State Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11920–11928. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Prakash, P. A new high-performance supercapacitor electrode of strategically integrated cerium vanadium oxide and polypyrrole nanocomposite. Int. J. Hydrogen Energy 2021, 46, 19323–19337. [Google Scholar] [CrossRef]
- Malik, R.; Lata, S.; Malik, R.S. Study of supercapacitive pursuance of polypyrrole/sulphonated poly (ether ether ketone)/multi walled carbon nanotubes composites for energy storage. J. Energy Storage 2020, 27, 101162. [Google Scholar] [CrossRef]



| Method of Carbonization | Purposes | Results Achieved | Ref. |
|---|---|---|---|
| One-step chemical activation | Improving electrochemical performance | Capacitance of 227 Fg−1 at 1 Ag−1 | [24] |
| Carbonization without activation | Green and cost-effective facile route | Notable specific capacitance (811 Fg−1) | [21] |
| one-step hydrothermal method | To get excellent electrochemical performance | Capacitance enduring 51 Fg−1 at 5.0 Ag−1 | [25] |
| Chemical activation | To improve conductivity and electrochemical performance | Specific capacitance of 90.23 Fg−1 at 10 mVs−1 | [26] |
| Heating banana peel soaked with KOH at high temperature | To check stability against multiple electronic cycling and bending | Devices displayed high areal capacitance of 88 mF/cm2 at 10 mV/s scan rate | [27] |
| two-step hydrothermal process | To reach easily to the active site and to shorten the ion transport path | Large capacitance of 816 Fg−1 at the current density of 5 mA cm−2 | [28] |
| Green pyrolysis | To check energy storage ability and environmental remediation | Capacitance of 655 Fg−1 in 1 M at a current density of 0.35 Ag−1 & excellent cyclic stability of 79.3% | [29] |
| Sulfur-doped (chemical carbonization) | Sustainable supercapacitor production | High Brunauer-Emmett-Teller surface area of 2224.9 m2/g, a large pore volume of 0.77 cm3/g | [22] |
| Carbonization with chemical activation | To see relationship of surface area to cell capacitance | SC increased from 59–~265 Fg−1 at 0.1 Ag−1 | [30] |
| Biological activation | Optimization of precursors and synthesis methods | Get specific capacitance of 476 Fg−1 in 1 M H2SO4 electrolyte | [31] |
| Chemical co-precipitation method | To get high electrochemical property | specific capacitance of 465 Fg−1 at a scan rate of 10 mV s−1 by CV | [23] |
| Biological fermentation | Stabilize the structure of electrodes | High-capacity hold of 58.35% after 100 cycles | [32] |
| Hydrothermal method | To increase the electrochemical performance | Had a specific capacitance of 139.6 Fg−1 at 300 mA g−1 | [33] |
| straightforward carbonization | To improve electrochemical performance | Specific capacitance improved from 59 to 258–273 Fg−1 at 0.1 Ag−1 | [34] |
| KOH pellets at different carbonization temperatures | To improve electrochemical performance | Specific capacitance of 165 Fg−1 at energy density of 18.6 Wh kg−1 at 0.5 Ag−1 | [35] |
| Carbonization followed by activation | To see the relationship between surface area and electrochemical property | Surface area had significant effect on electrochemical property (specific capacitance of 68 Fg−1) | [36] |
| H2SO4 activation | Carbon nanofiber synthesis | Carbon nanofiber formed at 700 °C) | [37] |
| KOH | Absorption study | reflection loss peak of −44.59 dB at 10.84 GHz | [38] |
| Precursor/Composite | Capacitance | Performance | Ref. |
|---|---|---|---|
| rGO | 226.5 F cm−3/279.3 mF cm−2 at 0.5 A cm−3 | 74.7% C retention at 50 A cm−3 | [45] |
| WO3 | 1139.6 mF cm−2 at 2 mA cm−2 | Working voltage of 1.6 V | [46] |
| Cellulose nanofibrils | 854.4 mF cm−2 at 5 mV/s | Areal ED of 30.86 μWh cm−2 | [47] |
| PANI, PPy | 156 mF cm−2 C at 1 mA cm−2 CD | 41% capacity persisted at 20 mA cm−2 | [48] |
| MgTf2 | 280 Fg−1 at 3 mV/s and 376.6 Fg−1 at 100 mA g−1 | PD ~100.08 Wkg−1 | [49] |
| Graphene | C of 2 mF cm−2 at a scan rate of 102 mV s−1 | >95% C retaining after 103 cycles | [50] |
| Polypyrrole | 12.4–10.5 F cm−3 at a CD of 40–320 mA cm−3 | C retention rate of 88.1% for 103 charges/discharge cycles | [51] |
| Carbon nanofibers | C of 1321 Fg−1, at a scanning speed of 1 mV/s | Retention of 80% of its performance after 2500 CV cycles | [52] |
| MnO2 microspheres | Capacitance of 135.4 mF cm−2 | 94% C maintenance after 3000 cycles | [53] |
| rGO/CoFe2O4 | Capacitance of 229.6 mF cm−2 | ED and PD of 25.9 Wh kg−1 and 135.3 W kg−1, respectively | [54] |
| Poly(acrylamide) | specific C of 327 Fg−1 at 3 mV/s | highest ionic conductivity of 13.7 × 10−3 S/cm at 22 ± 2 °C | [55] |
| CoCCHH-CoSe | C of 440.6 Fg−1 at 1 Ag−1 | ED of 137.7 Wh kg−1 | [56] |
| PANI Nanofiber | C of 301.71 mF cm−2 at CD of 1 mA cm−2 | ED of 0.023 mWh cm−2, with PD of 0.279 mW cm−2 at a lower current density of 1 mA cm−2 | [57] |
| nanoflower MnOx | C of 580 mF·cm−2 at 0.5 mA | >90% for 40% stretch | [58] |
| ---- | C of 3.92 mF/cm2 at 1 mA/cm2 | C retention > 90% after 3 × 103 cycles | [59] |
| ---- | Capacitance of 990 mF cm−2 | C retention of 74.7% after 14,000 cycles | [60] |
| Alginate/PPy | Capacitance of 246.4 mF cm−2 | 97% of initial values after 180° bending | [61] |
| Ag-coated Tyvek | Mass C (138.7 Fg−1) & volume C (544.2 F/cm3) at the scan rate of 50 mV/s. | 91.2% retention after 100 cycles | [62] |
| PVA/H2SO4 | Areal C of 44.5 mF cm−2 at PD of 0.04 mW cm−2 | 92% retention at 200% stretchability | [63] |
| MWCNT | specific capacitance of 235 Fg−1 at 5 mV s−1 | retention of about 92% in 1M H2SO4 electrolyte | [64] |
| No. | Super Capacitor Electrode or Devices Based on PANI | Method of Manufacturing | Capacitance Value | Ref. |
|---|---|---|---|---|
| 1 | PANI | chemical oxidative polymerization | 267 Fg−1 | [104] |
| 2 | H2 bonded graphene/PANI | chemical oxidation | 598 Fg−1 at 1.0 Ag−1 | [105] |
| 3 | PANI/carbon/titanium nitride nanowire composite | sequentially coating carbon and PANI on the surface of TiN Nano wire | 1093 Fg−1 at 1.0 Ag−1 | [99] |
| 4 | PANI/graphene | Chemical vapor deposition | 789.9 Fg−1 at 10 mVS−1 | [106] |
| 5 | PANI/nanowire | Spin-coated | Areal capacitance of 0.017 Fcm−2 at 5 mV S−1 | [107] |
| 6 | PANI/SWCNT composites | electro chemical polymerization of PANI onto SWCNTs | 485 Fg−1 | [100] |
| 8 | PANI/C-TiO2 NTAs | Ar atmosphere | 104.3 mF cm−2 | [98] |
| 9 | PANI)/carbon aerogel | chemical oxidation polymerization | 710.7 Fg−1 | [108] |
| 10 | Ni3V2O8@PANI composite | in situ chemical bath | 2565.7 Fg−1 at 5 mV/s | [109] |
| 11 | PANI/Co-Porphyrins composite | ------ | 823 Fg−1 at 0.5 Ag−1 | [110] |
| 12 | hollow Co3O4/PANI Nano cages | in situ surface polymerization | 1301 Fg−1 at CD of 1 Ag−1 | [111] |
| 13 | MoO3/PANI | in situ polymerization | 632 Fg−1 at a CD of 1 Ag−1 | [112] |
| 14 | Ni-PANI film electrode | Multi-step electrode position | 543 at 1 Ag−1 | [107] |
| 15 | PANI/graphene oxide composite | in situ polymerization of aniline monomers in the presence of GO | 206 Fg−1 at 1 Ag−1 | [113] |
| 16 | graphene oxide–polyaniline | in situ polymerization | 525 Fg−1 at 0.3 Ag−1 | [114] |
| 17 | Co-MOF/PANI composite | coupling | 162.5 Cg−1 at 0.4 Ag−1 | [115] |
| 18 | carbon cloth/PANI-MnO2 | electrochemical polymerization | 634.0 Fg−1 at 1 Ag−1 | [116] |
| 19 | PVA/carbon nanotubes/PANI film | in situ polymerization of PANI on the surface of PVA/CNT films | 196.5 mF cm−2 | [117] |
| 20 | reduced graphene oxide/Zn-Metal-organic frameworks@PANI | in situ polymerization | 372 Fg−1 at 0.1 A g−1 | [118] |
| 21 | Honeycomb-like nitrogen-doped carbon/PANI composite | in situ polymerization | 686 Fg−1 at 1 Ag−1 | [119] |
| 22 | brannerite type copper vanadate/PANI | in situ polymerization | 375 Fg−1 at 4 Ag−1 | [120] |
| 23 | CNF/thionickel ferrite/PANI ternary nanocomposite | in situ polymerization | 645 Fg−1 at CD of 1 Ag−1 | [121] |
| 24 | manganese sulfide/graphene oxide/PANI nanocomposite | in situ polymerization | 822 Fg−1 at 10 mV/s | [122] |
| 25 | PANI/Boron carbo nitride nanocomposite | in situ polymerization | 67.1 Fg−1 at a scan rate 5 mV S−1 | [123] |
| 26 | PANI/MIL-101 | as-synthesized | 1197 Fg−1 at 1 Ag−1 | [124] |
| 27 | PANI/perlite-barium ferrite nanoparticles composite | hydrothermal | 330 Fg−1 | [125] |
| 28 | CuCe-bimetal organic frameworks@PANI-1 | hydrothermal | 724.4 Fg−1 at 1 Ag−1 | [126] |
| 29 | PANI-graphene/PVA/PANI-graphene | chemical activation | 1412 Fg−1 | [127] |
| 30 | PANI/Ag@MnO2 | deposition | 1028.66 Fg−1 at 1 Ag−1 | [128] |
| 31 | PANI/p-phenylenediamine—GO composites | in situ polymerization | 635.2 Fg−1 at 1 Ag−1 | [129] |
| 32 | GO/MnO2/PANI nanocomposites | polymerization | 150 Fg−1 | [130] |
| 33 | 3D graphene oxide/PANI-carbon fiber paper | template method | 1013 Fg−1 at 1 Ag−1 | [131] |
| 34 | rGO/unzipped CNT/PANI | in situ polymerization | 359.3 Fg−1 at 1 Ag−1 | [132] |
| 35 | Multi-growth site graphene/PANI composites | oxidation | 912 Fg−1 at 1 Ag−1 | [133] |
| No. | PPy Based Electrode/ Super Capacitor | Method of Manufacturing | Investigated Properties | Values | Ref. |
|---|---|---|---|---|---|
| 1 | EPPy-PPy/CF | electropolymerization | SC | 617.5 mF cm−2 at 0.4 mA cm−2 CD | [143] |
| 2 | PPy/CNOs | template-degrading | SC | 64 Fg−1 | [144] |
| 3 | PET/Reduced graphene oxide/PPy composite electrode | oxidation polymerization | AC; VC; ED; PD and RC after 6000 cycles | 0.23 cm−2 at a scanning rate of 1 mV s−1; 5.5 F cm−3 at a discharge CD of 1.6 mA cm−3; 11 mWh cm−2; 0.03 mW cm−2 at 6.86 mg c2; 76% | [145] |
| 4 | PPy-Multi-Walled Carbon Nanotube-silk electrode | polymerization | SC; CR, after 3000 cycles | 676.9 mF cm−2 or 376.3 F cm−3; 81% | [146] |
| 5 | PPy/reduced graphene oxide Nano composite cotton fabric | chemical polymerization | SC; CR after 104 cycles | 9300 m−2 at 1 mA cm−2; 94.47% | [140] |
| 6 | Fabric based polyethylene terephthalate/reduced graphene oxide/PPy | dipping and drying | SC; CR after 6000 cycles; ED; PD | 230 mF cm−2 at 1 mV s−1; 76%; 11 μWh cm−2; 0.03 mW cm−2 | [145] |
| 7 | PPy-Cotton electrode | In situ polymerization | Specific capacitance | 268 Fg−1 at a scan rate of 5 mV s−1 | [134] |
| 8 | PPy-Viscose rayon electrode | In situ polymerization | Specific capacitance | 244 Fg−1 at a scan rate of 5 mV s−1 | [134] |
| 9 | Parallel CNT/PPy composite | electro chemical deposition | Specific capacitance | 139.2 Fg−1 (27.8 mF cm−2, 10 mV s−1) | [137] |
| 10 | Twisted carbon nanotube/PPy composite | electro chemical deposition | Specific capacitance | 331.4 Fg−1 at 5 mV s−1 | [137] |
| 11 | PPy@ acid-pre-treated stainless steel yarn electrode | electro chemical deposition | VC; ED; CR at 6000 cycles | 14.69 F cm−3 at CD of 25 mA cm−3; 3.83 mWh·cm−3 at a PD of 18.75 mW cm−3; 90% | [147] |
| 12 | PPy-carbonitrides coated textile electrode | dipping and drying | SC; ED; PD | 343.20 Fg−1; 1.30 mWh g−1; 41.1 mW g−1 | [138] |
| 13 | PPy/carbon cloth electrode | electro chemical | Areal specific capacitance | 174.5 mF cm−2 at scan rate of 5 mV s−1 | [148] |
| 14 | vanadium pentoxide/functionalized CNT/PPy composite electrode | VP polymerization | AC; CR after 103 charge-discharge cycles | 1266 cm−2 at a CD of 1 mA cm−2; 83% | [149] |
| 15 | PPy nanotubes/carbon cloth coated electrodes | interfacial polymerization | AC; CR after 500 cycles | 0.74 F cm−2 at constant discharge & CD of 10 mA cm−2; 79.5% | [150] |
| 16 | PPy/graphene nanoplatelets electrode | interfacial polymerization | AC | 250 mF cm−2 | [151] |
| 17 | pristine polypyrrole membrane electrode | MO-assisted polymerization | CR after 1000 cycles; SC | 88.9% cyclic stability; 509.8 Fg−1 at 0.5 Ag−1 | [152] |
| 18 | Paper derived activated carbon and bare/NF@PPy | hydrothermal & chemical polymerization | SC; ED | 658 Fg−1 at a CD of 1 Ag−1; 27.4 Wh kg−1 | [153] |
| 19 | porous PPy scaffold/conductive Cu3 (2,3,6,7,10,11-hexa hydroxyl triphenylene)2 catecholate electrode | polymerization | PD; ED; CR after 5000 cycles | 233 mF cm−2; 1.5 mW cm−2; 12 μWh cm−2; 85% | [154] |
| 20 | Cerium vanadate/PPy electrode | hydrothermal | SC; CR after 104 cycles | 1236 Fg−1 at CD of 0.75 Ag−1; 92.6% | [155] |
| 21 | PPy/sulfonated poly(ether ketone)/MWCNT electrode | In situ chemical oxidation | SC | 593 Fg−1 at scan rate of 2 mV/s | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadesse, M.G.; Kasaw, E.; Fentahun, B.; Loghin, E.; Lübben, J.F. Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage. Energies 2022, 15, 2471. https://doi.org/10.3390/en15072471
Tadesse MG, Kasaw E, Fentahun B, Loghin E, Lübben JF. Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage. Energies. 2022; 15(7):2471. https://doi.org/10.3390/en15072471
Chicago/Turabian StyleTadesse, Melkie Getnet, Esubalew Kasaw, Biruk Fentahun, Emil Loghin, and Jörn Felix Lübben. 2022. "Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage" Energies 15, no. 7: 2471. https://doi.org/10.3390/en15072471
APA StyleTadesse, M. G., Kasaw, E., Fentahun, B., Loghin, E., & Lübben, J. F. (2022). Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage. Energies, 15(7), 2471. https://doi.org/10.3390/en15072471









