Environmental and Economic Performance of CO2-Based Methanol Production Using Long-Distance Transport for H2 in Combination with CO2 Point Sources: A Case Study for Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location Selection
2.2. Environmental Assessment
2.3. Economic Assessment
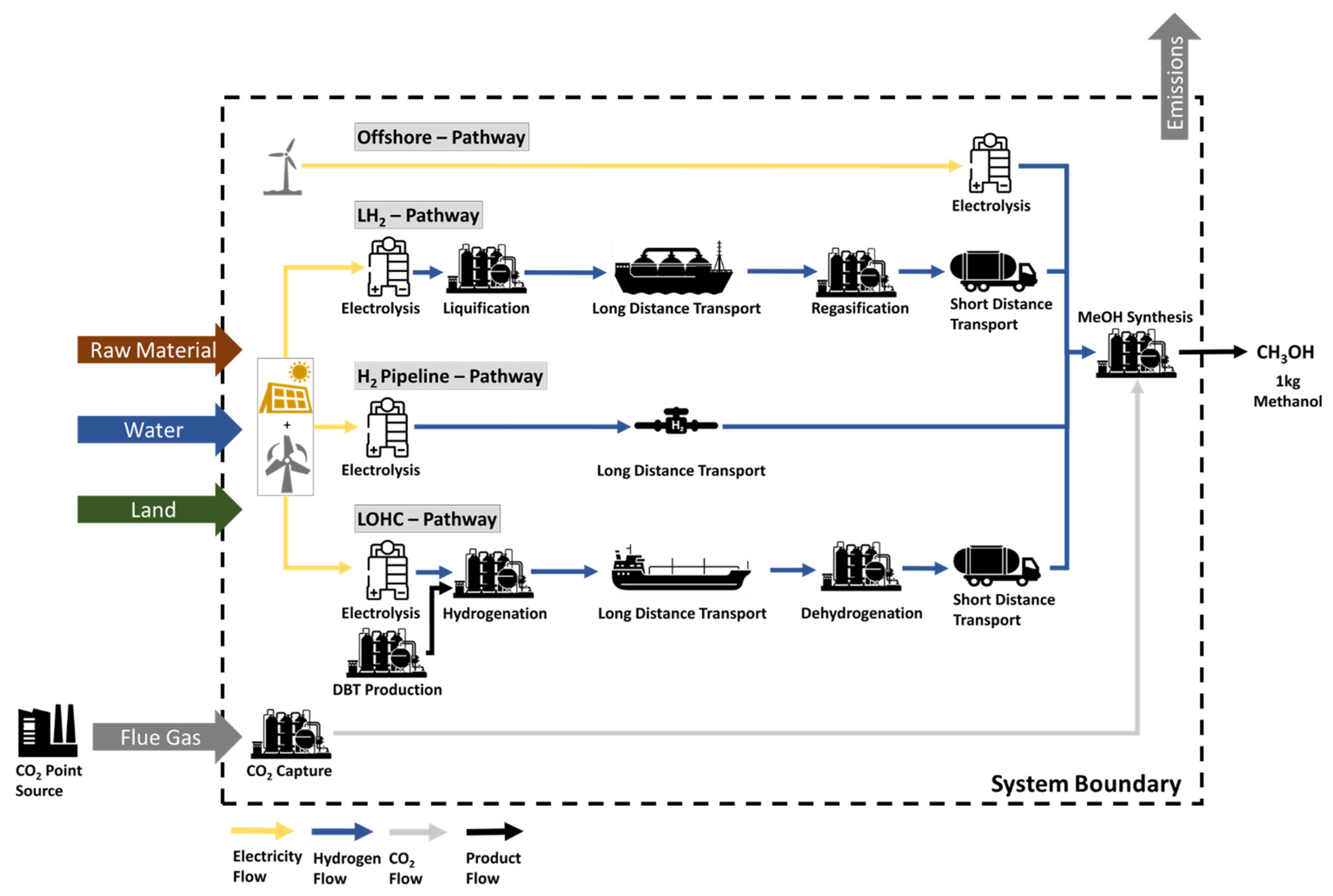
2.4. System Description
- Offshore: H2 production in Germany using electricity from an offshore wind park
- Pipeline: H2 production and import via Pipeline from Morocco
- LH2: H2 production and import via ship from Australia
- LOHC: H2 production and import via ship from Australia
- LOHCwh: H2 production and import via ship from Australia using a waste heat (wh) source in Germany
2.4.1. Renewable Electricity Production
2.4.2. Water Production and Purification
2.4.3. Water Electrolysis
2.4.4. Pipeline Transport
2.4.5. Liquefied H2 (LH2)
2.4.6. LOHC
2.4.7. CO2 Capture and MeOH Synthesis
3. Results
3.1. Energy Balances
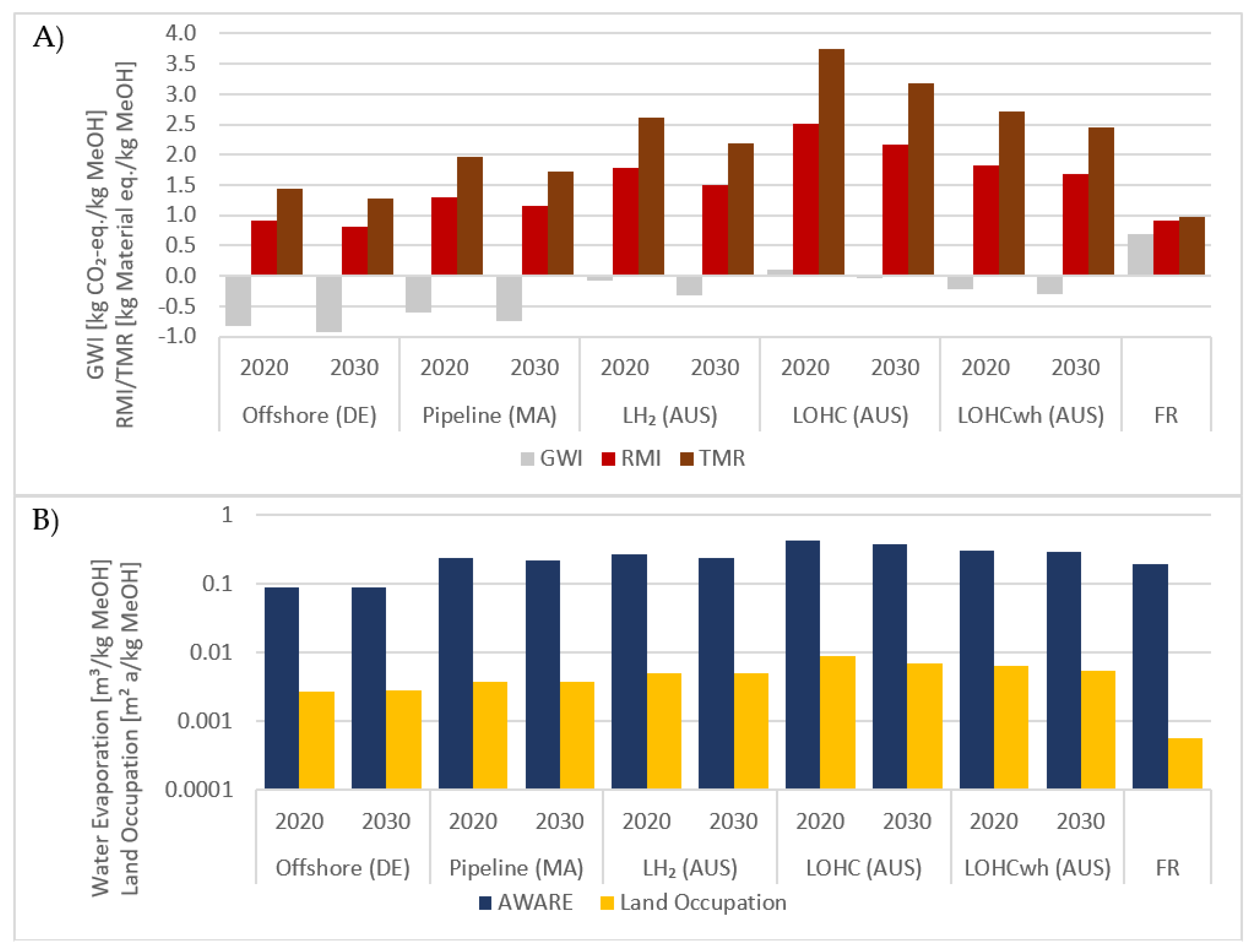
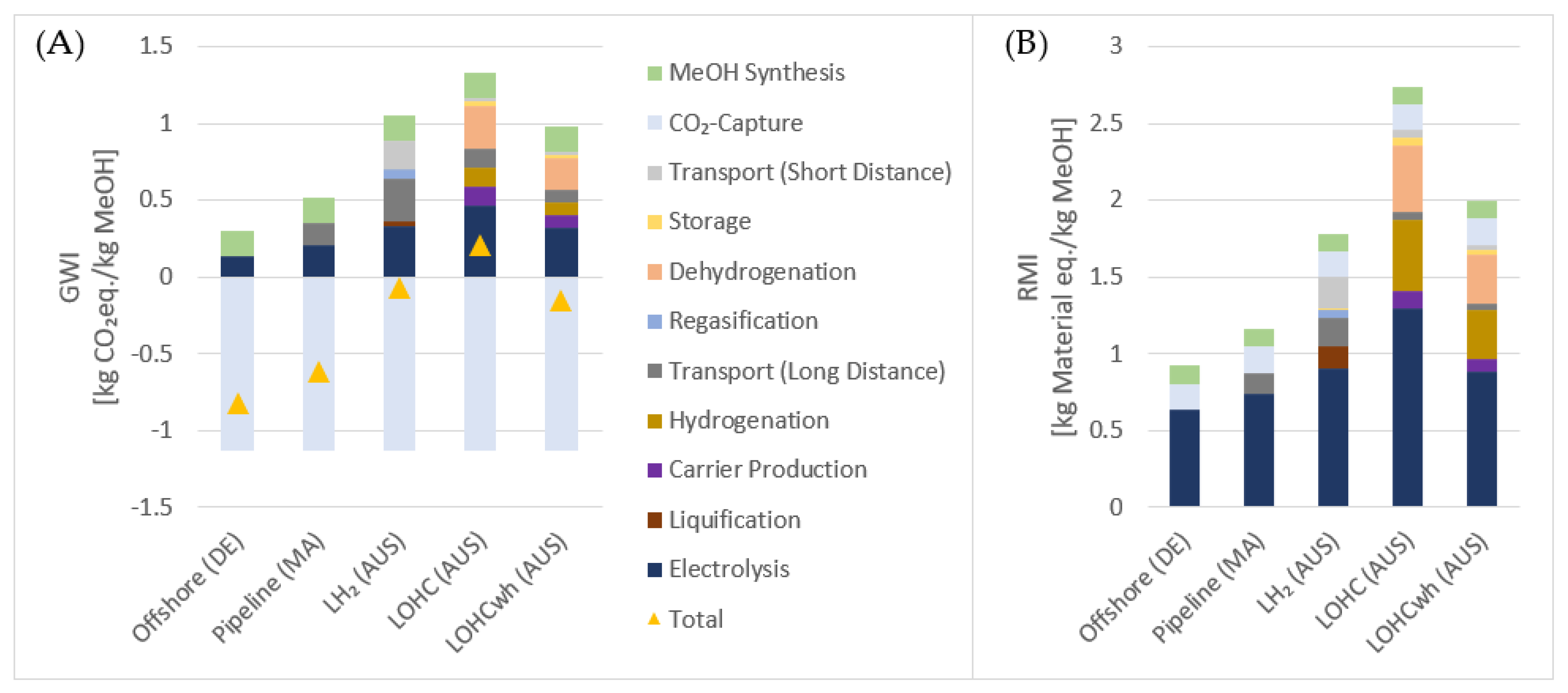
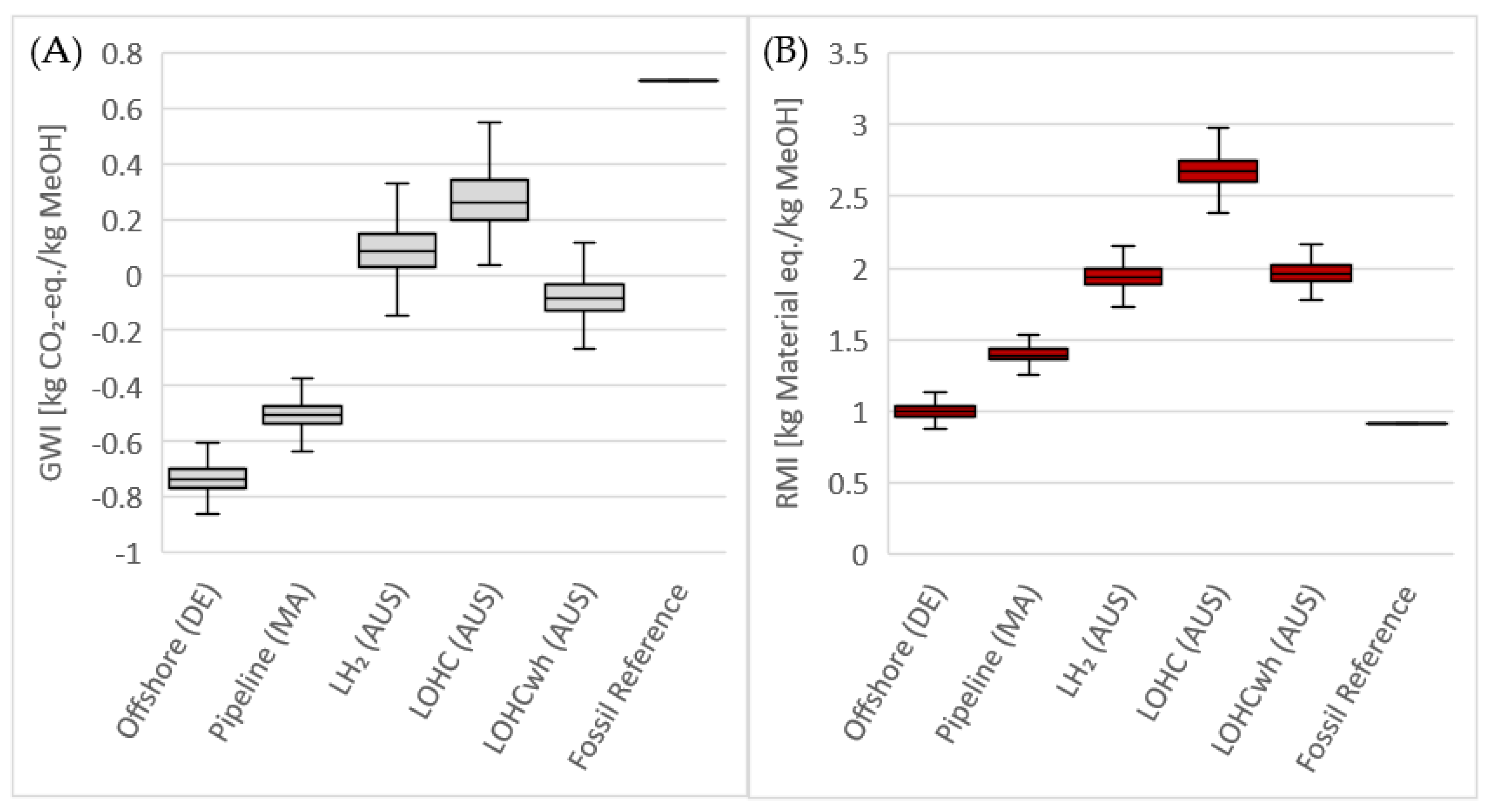
3.2. LCA Results
3.2.1. Environmental Footprints
3.2.2. Process Contribution to Footprints
3.2.3. Parameter Distribution
3.3. Economic Results
3.3.1. Production Costs
3.3.2. CO2-Avoidance Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AC | CO2 avoidance Costs |
| AUS | Australia |
| AWARE | Available Water Remaining |
| CCU | Carbon Dioxide Capture and Utilization |
| DE | Germany |
| FLH | Full Load Hours |
| FR | Fossil Reference |
| GHG | Green House Gases |
| GWI | Global Warming Impact |
| LCA | Life Cycle Assessment |
| LH2 | Liquefied H2 |
| LOHC | Liquid Organic Hydrogen Carriers |
| MCA | Monte Carlo Analysis |
| MeOH | Methanol |
| PV | Photovoltaic |
| PC | Production Costs |
| RMI | Raw Material Input |
| TMR | Total Material Requirement |
| wh | waste heat |
| WACC | Weighted Average Costs of Capital |
References
- IPCC. Climate Change 2014 Mitigation of Climate Change Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- IRP. Assessing Global Resource Use: A Systems Approach to Resource Efficiency and Pollution Reduction; International Resource Panel: Paris, France, 2019. [Google Scholar]
- Bataille, C.; Waisman, H.; Colombier, M.; Segafredo, L.; Williams, J.; Jotzo, F. The need for national deep decarbonization pathways for effective climate policy. Clim. Policy 2016, 16, S7–S26. [Google Scholar] [CrossRef]
- Wimbadi, R.W.; Djalante, R. From decarbonization to low carbon development and transition: A systematic literature review of the conceptualization of moving toward net-zero carbon dioxide emission (1995–2019). J. Clean. Prod. 2020, 256, 120307. [Google Scholar] [CrossRef]
- Kaiser, S.; Bringezu, S. Use of carbon dioxide as raw material to close the carbon cycle for the German chemical and polymer industries. J. Clean. Prod. 2020, 271, 122775. [Google Scholar] [CrossRef] [PubMed]
- IEA. The Future of Hydrogen; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Bampaou, M.; Panopoulos, K.; Seferlis, P.; Voutetakis, S.; Matino, I.; Petrucciani, A.; Zaccara, A.; Colla, V.; Dettori, S.; Branca, T.A.; et al. Integration of Renewable Hydrogen Production in Steelworks Off-Gases for the Synthesis of Methanol and Methane. Energies 2021, 14, 2904. [Google Scholar] [CrossRef]
- Matino, I.; Dettori, S.; Zaccara, A.; Petrucciani, A.; Iannino, V.; Colla, V.; Bampaou, M.; Panopoulos, K.; Rechberger, K.; Kolb, S.; et al. Hydrogen role in the valorization of integrated steelworks process off-gases through methane and methanol syntheses. Matériaux Tech. 2021, 109, 308. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2021; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Bareiß, K.; de la Rúa, C.; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- IEA. The Future of Petrochemicals towards More Sustainable Plastics and Fertilisers; Intenrational Energy Agency: Paris, France, 2018. [Google Scholar]
- Bazzanella, A.; Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemical Industry; DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie e.V.: Frankfurt am Main, Germany, 2017. [Google Scholar]
- Meys, R.; Kätelhön, A.; Bachmann, M.; Winter, B.; Zibunas, C.; Suh, S.; Bardow, A. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 2021, 374, 71–76. [Google Scholar] [CrossRef]
- Kätelhön, A.; Meys, R.; Deutz, S.; Suh, S.; Bardow, A. Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proc. Natl. Acad. Sci. USA 2019, 116, 11187–11194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Bringezu, S.; Distelkamp, M.; Lutz, C.; Wimmer, F.; Schaldach, R.; Hennenberg, K.J.; Böttcher, H.; Egenolf, V. Environmental and socioeconomic footprints of the German bioeconomy. Nat. Sustain. 2021, 4, 775–783. [Google Scholar] [CrossRef]
- Engström, G.; Gars, J.; Krishnamurthy, C.; Spiro, D.; Calel, R.; Lindahl, T.; Narayanan, B. Carbon pricing and planetary boundaries. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Galán-Martín, Á.; Tulus, V.; Díaz, I.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Sustainability footprints of a renewable carbon transition for the petrochemical sector within planetary boundaries. One Earth 2021, 4, 565–583. [Google Scholar] [CrossRef]
- Olfe-Kräutlein, B. Advancing CCU Technologies Pursuant to the SDGs: A Challenge for Policy Making. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Wyndorps, J.; Ostovari, H.; von der Assen, N. Is electrochemical CO2 reduction the future technology for power-to-chemicals? An environmental comparison with H2-based pathways. Sustain. Energy Fuels 2021, 5, 5748–5761. [Google Scholar] [CrossRef]
- Hoppe, W.; Thonemann, N.; Bringezu, S. Life Cycle Assessment of Carbon Dioxide-Based Production of Methane and Methanol and Derived Polymers. J. Ind. Ecol. 2017, 22, 327–340. [Google Scholar] [CrossRef]
- Sternberg, A.; Jens, C.M.; Bardow, A. Life cycle assessment of CO2-based C1-chemicals. Green Chem. 2017, 19, 2244–2259. [Google Scholar] [CrossRef]
- von der Assen, N.; Jung, J.; Bardow, A. Life-cycle assessment of carbon dioxide capture and utilization: Avoiding the pitfalls. Energy Environ. Sci. 2013, 6, 2721–2734. [Google Scholar] [CrossRef]
- Hoppe, W.; Bringezu, S.; Wachter, N. Economic assessment of CO2-based methane, methanol and polyoxymethylene production. J. CO2 Util. 2018, 27, 170–178. [Google Scholar] [CrossRef]
- Kaiser, S.; Prontnicki, K.; Bringezu, S. Environmental and economic assessment of global and German production locations for CO2-based methanol and naphtha. Green Chem. 2021, 23, 7659–7673. [Google Scholar] [CrossRef]
- Kaiser, S.; Gold, S.; Bringezu, S. Environmental and economic assessment of CO2-based value chains for a circular carbon use in consumer products. 2022; in Review. [Google Scholar]
- VCI. At a Glance—Chemical Industry 2020 (Orig. Title: Auf einen Blick—Chemische Industrie 2020); Verband der Chemischen Industrie: Frankfurt am Main, Germany, 2020; Available online: https://www.vci.de/vci/downloads-vci/publikation/chemische-industrie-auf-einen-blick.pdf (accessed on 5 February 2022).
- Müller, L.J.; Kätelhön, A.; Bringezu, S.; McCoy, S.; Suh, S.; Edwards, R.; Sick, V.; Kaiser, S.; Cuéllar-Franca, R.; El Khamlichi, A.; et al. The carbon footprint of the carbon feedstock CO. Energy Environ. Sci. 2020, 13, 2979–2992. [Google Scholar] [CrossRef]
- Geres, R.; Kohn, A.; Lenz, S.; Ausfelder, F.; Bazzanella, A.; Möller, A. Roadmap Chemie 2050 on the Way torwards a Carbon Neutral Chemical Industry in Germany (Orig. Title: Auf dem Weg zu Einer Treibhausgasneutralen Chemischen Industrie in Deutschland); VCI: Frankfurt am Main, Germany, 2019; Available online: https://www.vci.de/vci/downloads-vci/publikation/2019-10-09-studie-roadmap-chemie-2050-treibhausgasneutralitaet.pdf (accessed on 5 February 2022).
- DECHEMA. Roadmap of the Kopernikus Project P2X Phase II – Options for a Sustainable Energy System with Power-to-X Technologies. (Orig. Title: Roadmap des Kopernikus-Projektes P2X Phase II Optionen für eine Nachhaltiges Energiesystem mit Power-to-X Technologien); DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie e.V.: Frankfurt am Main, Germany, 2021. [Google Scholar]
- Chemie Technik. Covestro Buys Green Hydrogen from the Australien Company FFI (orig. Title: Covestro Kauft Grünen Wasserstoff vom Australischen Unternehmen FFI). 2022. Available online: https://www.chemietechnik.de/energie-utilities/covestro-kauft-gruenen-wasserstoff-vom-australischen-unternehmen-ffi-725.html (accessed on 5 February 2022).
- BASF. BASF Finishes Acquisition of 49.5 Percent of Offshore-Windpark Hollandse Kust Zuid Owned by Vattenfall (Orig. Title: BASF Schließt Erwerb von 49,5 Prozent des Offshore-Windparks Hollandse Kust Zuid von Vattenfall ab) 2021. Available online: https://www.basf.com/global/de/media/news-releases/2021/09/p-21-297.html (accessed on 5 February 2022).
- Wang, A.; van der Leun, K.; Peters, D.; Buseman, M. European Hydrogen Backbone; Guidehouse: Utrecht, The Netherlands, 2020. [Google Scholar]
- Perner, J.; Bothe, D. International Aspects of a Power-to-X Roadmap. A Report Prepared for the World Energy Council Germany; Frontier Economics: Cologne, Germany, 2018. [Google Scholar]
- Jensterle, M.; Narita, J.; Piria, R.; Schröder, J.; Steinbacher, K.; Wahabzada, F.; Zeller, T.; Crone, K.; Löchle, S. Green Hydrogen: International Cooperation Potential for Germany (Orig. Title: Grüner Wasserstoff: Internationale Kooperationspotenziale für Deutschland); Adelphi: Berlin, Germany, 2020. [Google Scholar]
- Merten, F.; Scholz, A.; Krüger, C.; Heck, S.; Girard, Y.; Mecke, M.; Goerge, M. Assessment of Advantages and Disadvantages of H2-Imports Compared to Domestic Production (Orig. Title: Bewertung der Vor- und Nachteile von Wasserstoffimporten im Vergleich zur heimischen Erzeugung); Wuppertal Institut; DIW Econ GmbH: Wuppertal, Germany, 2020. [Google Scholar]
- Heuser, P.-M.; Ryberg, D.S.; Grube, T.; Robinius, M.; Stolten, D. Techno-economic analysis of a potential energy trading link between Patagonia and Japan based on CO2 free hydrogen. Int. J. Hydrog. Energy 2019, 44, 12733–12747. [Google Scholar] [CrossRef]
- Wulf, C.; Zapp, P. Assessment of system variations for hydrogen transport by liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2018, 43, 11884–11895. [Google Scholar] [CrossRef]
- Krieg, D. Concept and Costs for a Pipeline System for a Supply of the German Traffic Sector with Hydrogen (Orig. Title: Konzept und Kosten eines Pipelinesystems zur Versorgung des Deutschen Straßenverkehrs mit Wasserstoff); Forschungszentrum Jülich: Jülich, Germany, 2012; ISBN 9783893368006. [Google Scholar]
- Gerhardt, N.; Bard, J.; Schmitz, R.; Beil, M.; Pfennig, M.; Kneiske, D.T. Hydrogen in the Future Energy System: Focus Heating of Buildings (Orig. Title: Wasserstoff im Zukünftigen Energiesystem: Fokus Gebäudewärme); Fraunhofer IEE: Kassel, Germany, 2020. [Google Scholar]
- Australian Government. Hydrogen. 2021. Available online: https://www.ga.gov.au/scientific-topics/energy/resources/hydrogen (accessed on 4 December 2021).
- Fasihi, M.; Breyer, C. Baseload electricity and hydrogen supply based on hybrid PV-wind power plants. J. Clean. Prod. 2019, 243, 118466. [Google Scholar] [CrossRef]
- Global Solar Atlas 3.0.2019. Available online: www.globalsolaratlas.info (accessed on 1 December 2021).
- Global Wind Atlas 3.0.2019. Available online: www.globalwindatlas.info (accessed on 1 December 2021).
- DIN EN ISO 14044; Environmental Management—Life Cycle Assessment Requirements and Guidelines. German Institut for Standardization: Berlin, Germany, 2021.
- DIN EN ISO 14045:2012; Environmental Management—Eco-Efficiency Assessment of Product Systems—Principles, Requirements and Guidelines. German Institut for Standardization: Berlin, Germany, 2021.
- Müller, L.J.; Kätelhön, A.; Bachmann, M.; Zimmermann, A.; Sternberg, A.; Bardow, A. A Guideline for Life Cycle Assessment of Carbon Capture and Utilization. Front. Energy Res. 2020, 8, 15. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Olah, G.; Goeppert, A. Beyond Oil and Gas: The Methanol Economy. ECS Trans. 2011, 35, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, Z.J.N.; Schipper, A.M.; Hauck, M.; Huijbregts, M.A.J. How Many Environmental Impact Indicators Are Needed in the Evaluation of Product Life Cycles? Environ. Sci. Technol. 2016, 50, 3913–3919. [Google Scholar] [CrossRef]
- Stocker, T.; Tignor, M. Climate Change 2013 the Physical Science Basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Mostert, C.; Bringezu, S. Measuring Product Material Footprint as New Life Cycle Impact Assessment Method: Indicators and Abiotic Characterization Factors. Resources 2019, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Schomberg, A.C.; Bringezu, S.; Flörke, M. Extended life cycle assessment reveals the spatially-explicit water scarcity footprint of a lithium-ion battery storage. Commun. Earth Environ. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Zimmermann, A.; Müller, L.J.; Marxen, A.; Armstrong, K.; Buchner, G.; Wunderlich, J.; Kätelhön, A.; Bachmann, M.; Sternberg, A.; Michailos, S.; et al. Techno-Economic Assessment & Life-Cycle Assessment Guidelines for CO2 Utilization Version 1.1; Global CO2 Initiative: Ann Arbour, MI, USA, 2020; Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/162573/TEA%26LCA%20Guidelines%20for%20CO2%20Utilization%20v1.1.pdf (accessed on 5 February 2022).
- Steffen, B. Estimating the cost of capital for renewable energy projects. Energy Econ. 2020, 88, 104783. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2020; International Energy Agency: Paris, France, 2020. [Google Scholar]
- UBA. Actualization and Assessment of LCAs for Wind Energy and Photovoltaic Plants Considering Current Technology Developments (Orig. Title: Aktualisierung und Bewertung der Ökobilanzen von Windenergie- und Photovoltaikanlagen unter Berücksichtigung Aktueller Technologieentwicklungen); German Environment Agency: Dessau-Roßlau, Germany, 2010.
- Frontier Economics. The Future Costs of Synthetic Fuels (Orig. Title Die Zukünftigen Kosten Strombasierter Synthetischer Brennstoffe); Agora Verkehrswende; Agora Energiewende: Berlin, Germany, 2018. [Google Scholar]
- Boulay, A.-M.; Bare, J.; Benini, L.; Berger, M.; Lathuillière, M.J.; Manzardo, A.; Margni, M.; Motoshita, M.; Nunez, M.; Pastor, A.V.; et al. The WULCA consensus characterization model for water scarcity footprints: Assessing impacts of water consumption based on available water remaining (AWARE). Int. J. Life Cycle Assess. 2017, 23, 368–378. [Google Scholar] [CrossRef] [Green Version]
- NOW GmbH. IndWEDe Industrialization of Water Electrolysis in Germany (Orig. Title Industrialisierung der Wasser Elektrolyse in Deutschland); Nationale Organisation Wasserstoff- und Brennstoffzellentechnologie: Berlin, Germany, 2018. [Google Scholar]
- Iskov, H.; Kneck, S. Using the natural gas network for transporting hydrogen–ten years of experience. In Proceedings of the International Gas Union Research Conference Proceedings, Rio de Janeiro, Brazil, 24–26 May 2017. [Google Scholar]
- Cerniauskas, S.; Junco, A.J.C.; Grube, T.; Robinius, M.; Stolten, D. Options of natural gas pipeline reassignment for hydrogen: Cost assessment for a Germany case study. Int. J. Hydrogen Energy 2020, 45, 12095–12107. [Google Scholar] [CrossRef] [Green Version]
- Fekete, J.; Sowards, J.W.; Amaro, R.L. Economic impact of applying high strength steels in hydrogen gas pipelines. Int. J. Hydrogen Energy 2015, 40, 10547–10558. [Google Scholar] [CrossRef] [Green Version]
- Wulf, C.; Reuß, M.; Grube, T.; Zapp, P.; Robinius, M.; Hake, J.-F.; Stolten, D. Life Cycle Assessment of hydrogen transport and distribution options. J. Clean. Prod. 2018, 199, 431–443. [Google Scholar] [CrossRef]
- Sea Distance. Ports Distances. Available online: https://sea-distances.org/ (accessed on 25 January 2022).
- Stolzenburg, K.; Mubbala, R. Hydrogen Liquefcation Report Integrated Design for Demonstration of Efficient Liquefaction of Hydrogen; Fuel Cells and Hydrogen Joint Undertaking: Brussels, Belgium, 2013. [Google Scholar]
- Aasadnia, M.; Mehrpooya, M. Large-scale liquid hydrogen production methods and approaches: A review. Appl. Energy 2018, 212, 57–83. [Google Scholar] [CrossRef]
- Ahn, J.; You, H.; Ryu, J.; Chang, D. Strategy for selecting an optimal propulsion system of a liquefied hydrogen tanker. Int. J. Hydrogen Energy 2017, 42, 5366–5380. [Google Scholar] [CrossRef]
- Kamiya, S.; Nishimura, M.; Harada, E. Study on Introduction of CO2 Free Energy to Japan with Liquid Hydrogen. Phys. Procedia 2015, 67, 11–19. [Google Scholar] [CrossRef] [Green Version]
- DOE. Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan Section 3.2 Hydrogen Delivery; U.S. Department of Energy: Washington, DC, USA, 2015. Available online: https://www.energy.gov/sites/default/files/2015/08/f25/fcto_myrdd_delivery.pdf (accessed on 5 February 2022).
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renew. Sustain. Energy Rev. 2020, 135, 110171. [Google Scholar] [CrossRef]
- Adland, R.; Cariou, P.; Wolff, F.-C. Optimal ship speed and the cubic law revisited: Empirical evidence from an oil tanker fleet. Transp. Res. Part E Logist. Transp. Rev. 2020, 140, 101972. [Google Scholar] [CrossRef]
- Harrison, P. Global Crude Oil Voyage Losses Fall. Petroleum Review. 2016. Available online: http://www.oil-transport.info/admin/resources/petrev20.pdf (accessed on 5 February 2022).
- Schneider, M.J. Hydrogen storage and distribution via liquid organic carriers. In Bridging Renewable Electricity with Transportation Fuels Workshop; Brown Palace Hotel: Denver, CO, USA, 2015. [Google Scholar]
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative production of methanol from industrial CO. Renew. Energy 2019, 146, 1192–1203. [Google Scholar] [CrossRef]
- Otto, A. Chemical, Technical and Economic Assessment of CO2 as Raw Material in the Chemical Industry (Orig. Title: Chemische, Verfahrenstechnische und ökonomische Bewertung von Kohlendioxid als Rohstoff in der Chemischen Industrie); Forschungszentrum Jülich: Aachen, Germany, 2015; ISBN 9783958060647. [Google Scholar]
- Carbon Recycling International. Shunli Project. 2020. Available online: https://www.carbonrecycling.is/projects#projects-shunli (accessed on 2 April 2021).
- Hank, C.; Sternberg, A.; Köppel, N.; Holst, M.; Smolinka, T.; Schaadt, A.; Hebling, C.; Henning, H.-M. Energy efficiency and economic assessment of imported energy carriers based on renewable electricity. Sustain. Energy Fuels 2020, 4, 2256–2273. [Google Scholar] [CrossRef]
- Bachmann, M.; Kätelhön, A.; Winter, B.; Meys, R.; Müller, L.J.; Bardow, A. Renewable carbon feedstock for polymers: Environmental benefits from synergistic use of biomass and CO. Faraday Discuss. 2021, 230, 227–246. [Google Scholar] [CrossRef]
- EU ETS Certificate Prices. 2021. Available online: https://ember-climate.org/data/carbon-price-viewer/ (accessed on 28 October 2021).
- Bloomberg Green. Europe CO2 Prices May Rise More Than 50% by 2030, EU Draft Shows. 2021. Available online: https://www.bloomberg.com/news/articles/2021-06-29/europe-co2-prices-may-rise-more-than-50-by-2030-eu-draft-shows (accessed on 5 February 2022).
- IEA. CCUS in Clean Energy Transitions; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, T.; Blömer, S.; Münter, D.; Brischke, L.-A. CO2 Quellen für die PtX Herstellung in Deutschland Technologien, Umweltwir-kung, Verfügbarkeit. 2019. Available online: https://www.ifeu.de/fileadmin/uploads/ifeu_paper_03_2019_CO2-Quellen-f%c3%bcr-PtX.pdf (accessed on 5 February 2022).
- RTE. Energy Data. 2022. Available online: https://www.rte-france.com/en/eco2mix/energy-data (accessed on 2 February 2022).
- European Commission. Integrated National Energy and Climate Plan for France. 2020. Available online: https://ec.europa.eu/info/energy-climate-change-environment/implementation-eu-countries/energy-and-climate-governance-and-reporting/national-energy-and-climate-plans_en (accessed on 5 March 2022).
- Fraunhofer ISE. Energy Charts: Öffentliche Nettostromerzeugung in Deutschland in 2021. 2022. Available online: https://energy-charts.info/charts/energy_pie/chart.htm?l=de&c=DE&interval=year&year=2021 (accessed on 2 February 2022).
- BMU. Klimaschutzplan 2050—Klimaschutzpolitische Grundsätze und Ziele der Bundesregierung; German Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer ProtectionBerlin: Berlin, Germany, 2016.
- RED, REData – Generation. 2022. Available online: https://www.ree.es/en/datos/generation (accessed on 2 February 2022).
- IRENA. Future of Solar Photovoltaic: A Global Energy Transformation Paper; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- IRENA. Future of Wind: A Global Energy Transformation Paper; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- IRENA. Renewable Power Generation Costs in 2019; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Fraunhofer ISE. Stromgestehungskosten Erneuerbare Energien (Levelized Costs for Electricity of Renewable Energies): June 2021; Fraunhofer ISE: Freiburg, Germany, 2021. [Google Scholar]
- Eurostat. Electricity Prices. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Electricity_price_statistics#Electricity_prices_for_non-household_consumers (accessed on 11 February 2022).
- Energy Brainpool. EU Energy Outlook 2050. 2019. Available online: https://blog.energybrainpool.com/en/update-december-2019-eu-energy-outlook-2050/ (accessed on 16 December 2021).
- Vos, M.; Douma, J.; van den Noort, A. Study on the Import of Liquid Renewable Energy: Technology Cost Assessment. 2020. Available online: https://www.gie.eu/wp-content/uploads/filr/2598/DNV-GL_Study-GLE-Technologies-and-costs-analysis-on-imports-of-liquid-renewable-energy.pdf (accessed on 5 March 2022).
- Chemical Engineering. Chemical Engineering Plant Cost Index. 2021. Available online: https://www.chemengonline.com/2019-chemical-engineering-plant-cost-index-annual-average/ (accessed on 27 October 2021).
- UBA. Roadmap Gas for the Energiewende—Sustainable Climate Contribution of the Gas Sector: (Orig. Title: Roadmap Gas für Die Energiewende—Nachhaltiger Klimabeitrag des Gassektors); German Environmental Agency: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Hurskainen, M. Seminar on the Finnish Needs and Research Highlights on Hydrogen—Focus on Liquid LOHC “Batteries” and Hydrogen Legislation: Liquid Organic Hydrogen Carriers (LOHC)—Concept Evaluation and Technoeconomics: Seminar on the Finnish Needs and Research Highlights on Hydrogen—Focus on Liquid LOHC “Batteries” and Hydrogen Legislation (Espoo, 2018). Available online: https://projectsites.vtt.fi/sites/lohcness/files/LOHCNESS_WP1_Hurskainen_H2seminar_7.11.2018_public.pdf (accessed on 5 February 2022).
- Saadi, F.H.; Lewis, N.S.; McFarland, E.W. Relative costs of transporting electrical and chemical energy. Energy Environ. Sci. 2018, 11, 469–475. [Google Scholar] [CrossRef] [Green Version]


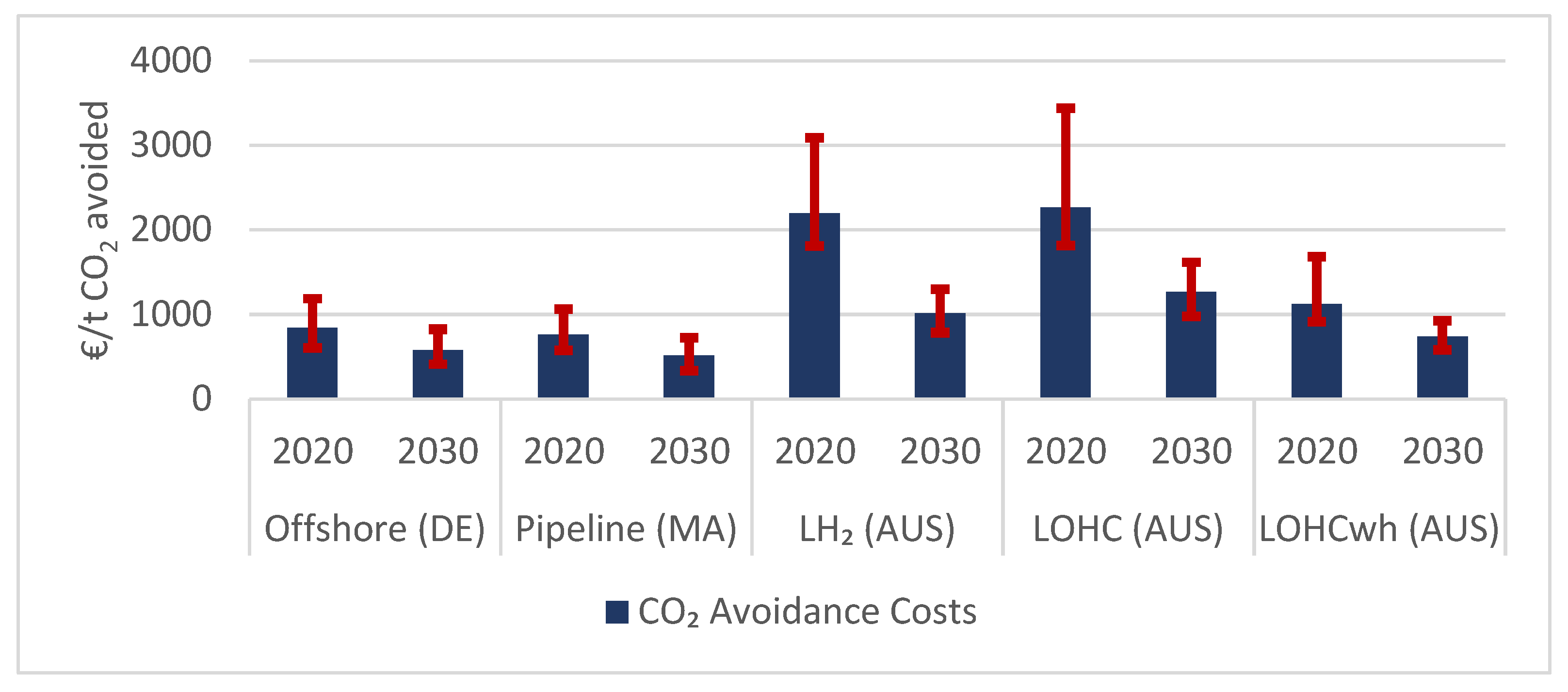
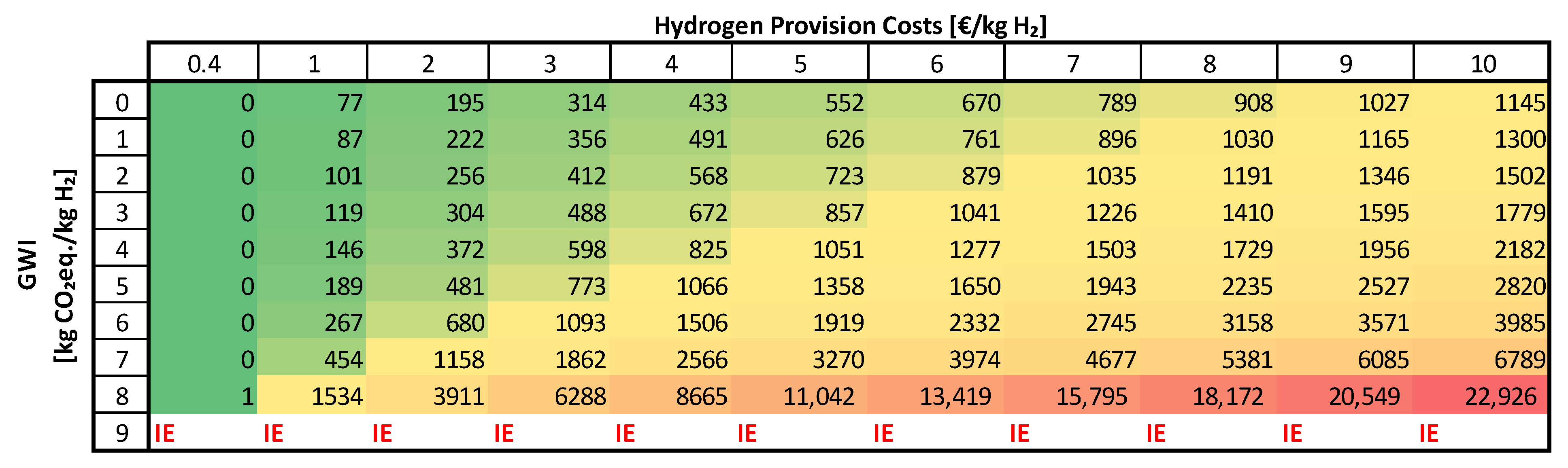
| System Element | 2020 | 2030 |
|---|---|---|
| Grid Mixes 1 | Based on actual data for grid composition in every country | Based on the national energy and climate action plans |
| Short Distance Transport | Via tube trailers or trucks | Via H2 pipeline distribution net |
| CO2-Capture | Heat provision via fossil fuels | Heat provision via heat pumps |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, S.; Siems, F.; Mostert, C.; Bringezu, S. Environmental and Economic Performance of CO2-Based Methanol Production Using Long-Distance Transport for H2 in Combination with CO2 Point Sources: A Case Study for Germany. Energies 2022, 15, 2507. https://doi.org/10.3390/en15072507
Kaiser S, Siems F, Mostert C, Bringezu S. Environmental and Economic Performance of CO2-Based Methanol Production Using Long-Distance Transport for H2 in Combination with CO2 Point Sources: A Case Study for Germany. Energies. 2022; 15(7):2507. https://doi.org/10.3390/en15072507
Chicago/Turabian StyleKaiser, Simon, Felix Siems, Clemens Mostert, and Stefan Bringezu. 2022. "Environmental and Economic Performance of CO2-Based Methanol Production Using Long-Distance Transport for H2 in Combination with CO2 Point Sources: A Case Study for Germany" Energies 15, no. 7: 2507. https://doi.org/10.3390/en15072507






