Processing Studies on Banded Hematite Quartzite’s of Sandur Sciht, Karnataka, India
Abstract
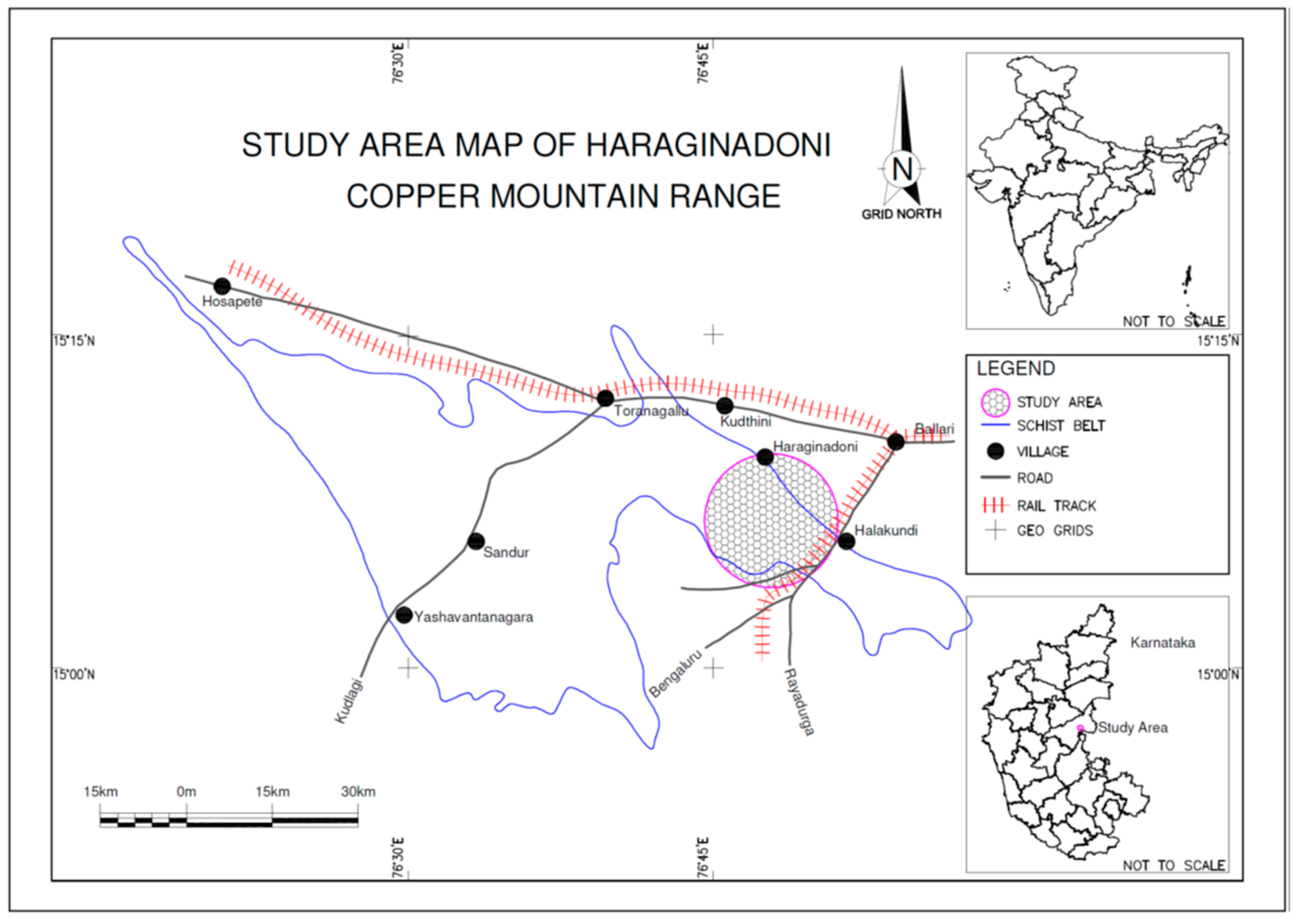
:1. Introduction
2. Materials and Methods
3. Results
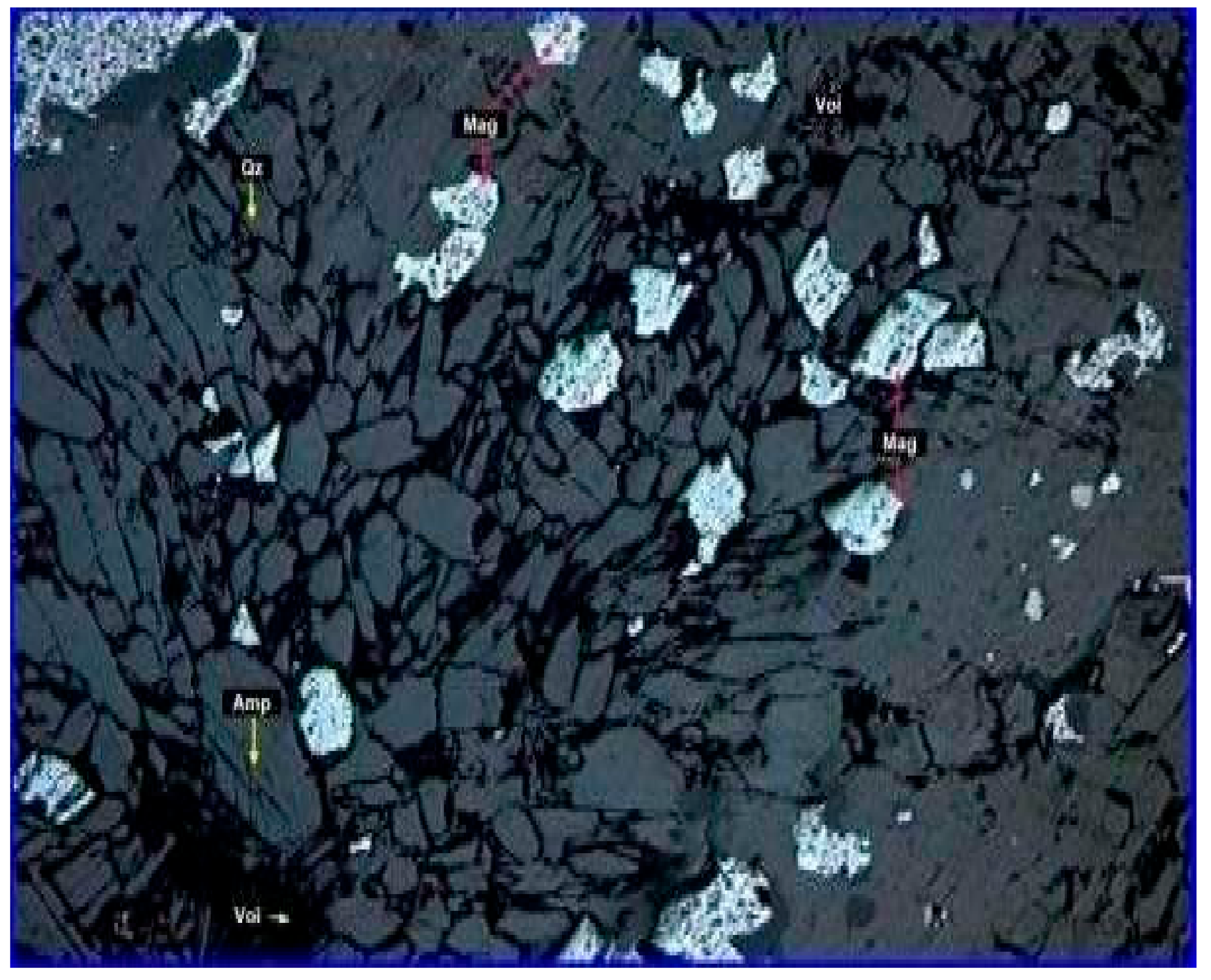
3.1. Characterization Studies
3.2. Effect of Mesh of Grind (MOG) on Tabling
3.3. Effect of MOG on Reverse Flotation Tests
3.4. Effect of Feed Particle Size on WHIMS Test
3.5. Amenability of BHQ to Gravity Concentration, WHIMS, and Reverse Flotation
3.6. Wet High-Intensity Magnetic Separation (WHIMS) Tests to Optimize Machine Parameters
3.6.1. Effect of WHIMS Matrix on % Fe Grade and % Fe Recovery
3.6.2. Effect of Intensity on % Fe Grade and % Fe Recovery
3.6.3. WHIMS Test under Optimal Conditions
3.7. WHIMS Followed by Reverse Flotation
3.7.1. Effect of MOG on Flotation Performance
3.7.2. Effect of Depressant Dosage on % Fe Grade and Recovery
3.7.3. Effect of (Dodecylamine) Collector Dosage on % Fe Grade and % Fe Recovery
3.8. Amenability to the Conventional Process of Tabling, WHIMS of Table Tailings, and Flotation of WHIMS Concentrates
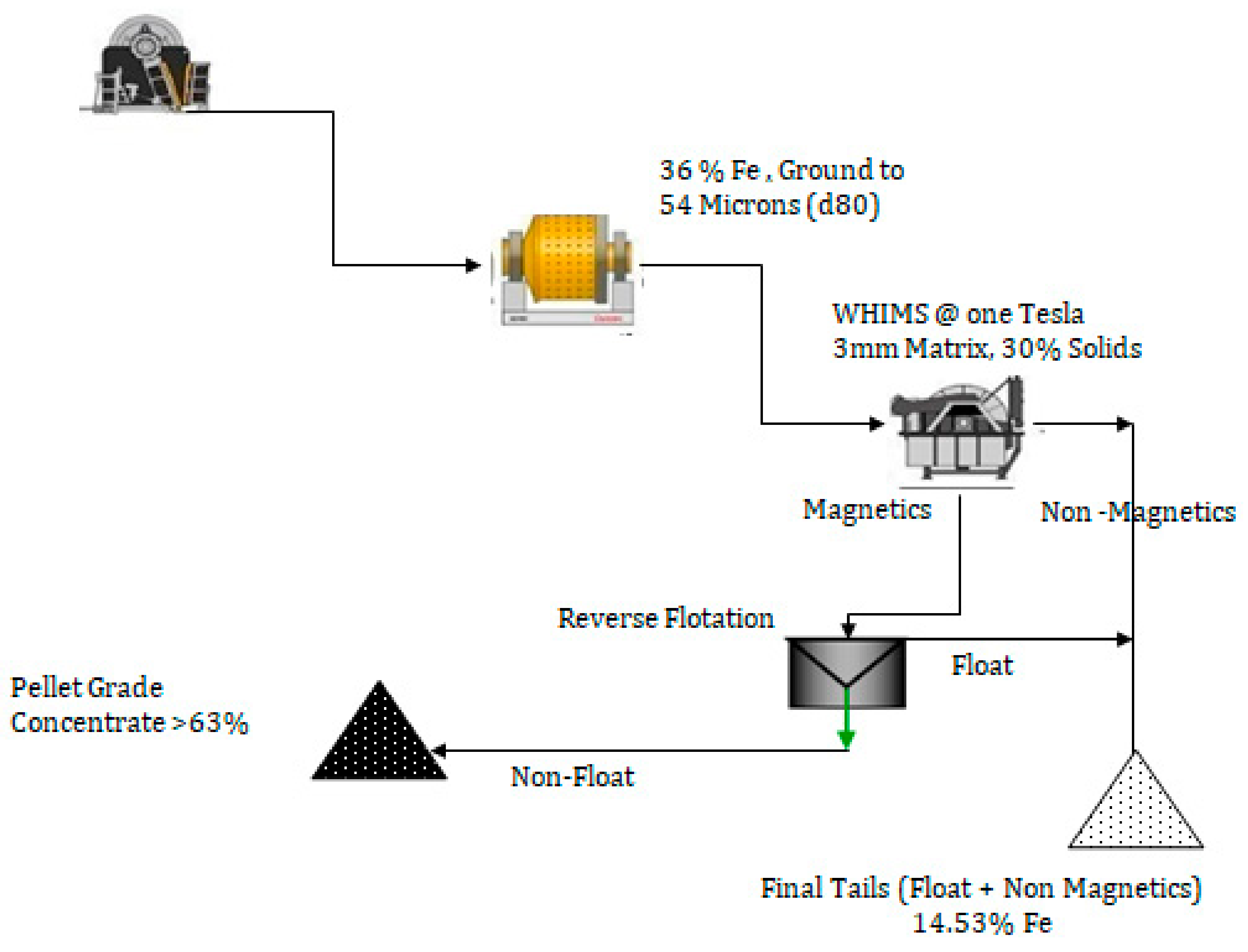
3.9. The Final Process of WHIMS and Flotation of WHIMS Concentrate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BHQ | Banded hematite quartzite |
| WHIMS | Wet high-intensity magnetic separator |
| LOI | Loss on ignition |
| ROM | Run of mine |
| DDA | Dodecylamine |
| MOG | Mesh of grind |
| D80 | 80% passing size |
| Kwh | Kilowatt hour |
| XRD | X-ray diffraction |
| EPMA | Electron probe microscopic analysis |
| CE | Concentration efficiency |
| MPE | Mineral processing equipment |
| IBM | Indian Bureau of Mines |
References
- Das, B.; Mishra, P.K.; Prakash, S.; Das, S.K.; Reddy, P.S.R.; Angadi, S.L. Magnetic and flotation studies of BHQ ore for pelletgrade concentrate. Int. J. Miner. Metall. Mater. 2010, 17, 675–682. [Google Scholar] [CrossRef]
- Sadasivaiah, M.S.; Karisiddaiah, S.M. Occurrence of Aegirine and Riebeckite in the Banded FerruginiousQuartziteofSanjeevarayanakota, Bellary, Karnataka. J. Kar. Uni. 1976, 21, 65–70. [Google Scholar]
- Roy, A.; Biswas, A.K. Stratigraphy and structure of Sandurschist belt, Karnataka. J. GSI 1983, 24, 19–28. [Google Scholar]
- IBM. Monographon Iron Ore; IBM: Nagpur, India, 1997. [Google Scholar]
- IBM. Ironand Steel Vision 2020; IBM: Nagpur, India, 2011. [Google Scholar]
- Khosravi, R.; Dehghani, F.; Siavoshi, H.; Pazoki, A.; Jahanian, R.; Ghosh, T. The Application of Numerical Taxonomy Technique in the Iron Ore Flotation to Determine Appropriate pH and Particle Size Distribution. Am. J. Eng. Appl. Sci. 2020, 13, 827–836. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ravi, B.P.; Sivrikaya, O.; Nanda, R.K. The Study of Pelletizing of Mixed Hematite and Magnetite Ores. Sci. Sinter. 2019, 51, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.K.; Reddy, P.S.; Das, B.; Biswal, S.K.; Prakash, S.; Das, S.K. Issues Relating to Characterization and Beneficiation of Low Grade Iron Ore Fines. Available online: www.worldsteel.org (accessed on 15 December 2007).
- Sherell, I.; Nevens, M. Iron ore-mineral processing overview. In Proceedings of the XXV International Mineral Processing Congress, Queensland, Australia, 6–10 September 2010; pp. 1227–1234. [Google Scholar]
- Anupam, A.; Singh, G.; Raghav, P.K.; Suresh, N. Studies on beneficiation of BHQ samples by different methods. In Proceedings of the XI International Seminar on Mineral Processing Technology (MPT—2010), Jamshedpur, India, 15–17 December 2010; p. 572. [Google Scholar]
- Gurulaxmi, S.N.; Ghosh, T.K.; Mukherjee, A.K. State of Art Characterization Study of Banded Hematite Jasper for Beneficiation. In Proceedings of the International Seminar on Mineral Processing Technology (MPT—2010), Jamshedpur, India, 15–17 December 2010; p. 440. [Google Scholar]
- Das, B.; Rath, S.S.; Reddy, P.S.; Das, S.K.; Mishra, B.K. The flotation response of Banded Iron Ore of Karnataka Region, India. In Proceedings of the 26th International Mineral Processing Congress (IMPC2012), New Delhi, India, 24–28 September 2012; pp. 1030–1040. [Google Scholar]
- Mohanrao, A.; Rudramuniyappa, M.V.; Hiremath, M.S.; Naganoor, P.C. Flotation studies on Banded Hematite Quartzite from Sandur Schist Belt, Bellary district, Karnataka. In Proceedings of the XXVI International Mineral Processing Congress (IMPC 2012), New Delhi, India, 24–28 September 2012; pp. 3452–3459. [Google Scholar]
- Nanda, N.K.; Gopalkrishna, S.J. Development of Process for Beneficiation of low grade iron ore fines from Karnataka. J. Mines Met. Fuels 2015, 62, 5–9. [Google Scholar]
- Sharath Kumar, P.S.; Ravi, B.P.; Krishna, S.J.G.; Patil, M.R.; Reddy, U.M. Processing of BMQ for pellet making. IJIR 2016, 2, 1–3. [Google Scholar]
- IBM. Manual of Classical and Instrumental Analysis of Ores, Mineral, and Environmental Samples; IBM: Nagpur, India, 2012. [Google Scholar]
- Weiss, N. Handbook of Mineral Processing; SME: New York, NY, USA, 1980. [Google Scholar]
- Krishna, S.J.G.; Patil, M.R.; Rudrappa, C.; Kumar, S.P.; Ravi, B.P. Characterization and processing of some Iron ores of India. J. Inst. Eng. India Ser. D 2013, 94, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.K.; Pani, S.; Beura, D. Recovery of iron values through conventional beneficiation techniques from BHJ of eastern India with reference to mineralogical and chemical characterization. Ind. J. Sci. Technol. 2020, 13, 3960–3969. [Google Scholar] [CrossRef]
- Nadezhda, V.N.; Tatiana, N.A.; Elena, L.C.; Anastasia, A. Mineral and Technological Features of Magnetite–Hematite Ores and Their Influence on the Choice of Processing Technology. Am. Chem. Soc. Omega 2021, 6, 9077–9085. [Google Scholar]
- Nayak, N.; Das, A.; Paul, B.K. Feasibility of beneficiation of BHJ of eastern India. Int. J. Eng. Res. Technol. 2012, 1, 1–11. [Google Scholar]
- Vijaya Kumar, T.V.; Prabhakar, S.; Gopalkrishna, S.J. Selection of cationic collector for reduction of alumina and silica in Iron ore slimes of an operating plant by flotation. Int. J. Eng. Sci. Res. Technol. 2013, 2, 3295–3302. [Google Scholar]
- Wills, B.A.; Napier-Munn, T.J. Mineral Processing Technology—An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2006; p. 18. [Google Scholar]
- Raj, B.; Rao, B.S.S.; Mathur, N. Development of a Process flow sheet for Beneficiation of Indian Banded Hematite Quartz (BHQ) Iron Ore. In Proceedings of the Iron Ore 2007 Conference Perth: AUS IMM, Perth, Australia, 20–22 August 2007; pp. 375–380. [Google Scholar]
- Tang, Y.; Zhang, J.; Wernham, J. The research on the application of new technologies in High Gradient Magnetic Separator with horizontal magnetic line. In Proceedings of the International Mineral Processing Congress, Brisbane, Australia, 6–10 September 2010; pp. 1281–1286. [Google Scholar]
- Svoboda, J. Magnetic Methods for the Treatment of Minerals; Elsevier: Amsterdam, The Netherlands, 1987; pp. 516–527. [Google Scholar]
- Lima, N.P.; Valadão, G.E.S.; Peres, A.E.C. Effect of amine and starch dosages on the reverse cationic flotation of iron ore. Miner. Eng. 2013, 45, 180–184. [Google Scholar] [CrossRef]




| Feed Particle Size | Product | wt% | %Fe | CE | |
|---|---|---|---|---|---|
| Grade | Recovery | ||||
| <210 µm D80 106 µm | Concentrate | 19.8 | 57.5 | 31.7 | 24.3 |
| Tailings | 80.2 | 30.7 | |||
| Feed | 100.0 | 36.0 | |||
| <106 µm D80 80 µm | Concentrate | 28.2 | 59.2 | 46.4 | 37.4 |
| Tailings | 71.8 | 26.9 | |||
| Feed | 100.0 | 36.0 | |||
| <74 µm D80 54 µm | Concentrate | 25.7 | 63.0 | 45.0 | 39.7 |
| Tailings | 74.3 | 26.7 | |||
| Feed | 100.0 | 36.0 | |||
| Feed Particle Size | Product | %Fe | CE | ||
|---|---|---|---|---|---|
| wt% | Grade | Recovery | |||
| <210 µm D80 106 µm | Concentrate | 29.4 | 50.2 | 41.0 | 23.9 |
| Tailings | 70.6 | 30.1 | |||
| Feed | 100.0 | 36.0 | |||
| <−106 µm D80 80 µm | Concentrate | 34.7 | 59.7 | 56.8 | 46.1 |
| Tailings | 65.3 | 24.2 | |||
| Feed | 100.0 | 36.5 | |||
| <−74 µm D80 54 µm | Concentrate | 32.9 | 63.0 | 56.0 | 49.0 |
| Tailings | 67.1 | 24.3 | |||
| Feed | 100.0 | 37.0 | |||
| Feed Particle Size | Product | %Fe | CE | ||
|---|---|---|---|---|---|
| wt% | Grade | Recovery | |||
| −210 µm D80 106 µm | Concentrate | 69.4 | 46.0 | 90.0 | 41.7 |
| Tailings | 30.6 | 11.6 | |||
| Feed | 100.0 | 35.5 | |||
| −106 µm D80 80 µm | Concentrate | 57.9 | 50.1 | 80.0 | 45.9 |
| Tailings | 22.1 | 22.7 | |||
| Feed | 100.0 | 36.3 | |||
| −74 µm D80 54 µm | Concentrate | 45.6 | 60.0 | 75.0 | 61.3 |
| Tailings | 54.4 | 16.8 | |||
| Feed | 100.0 | 36.5 | |||
| Ball Size in Matrix | Product | wt% | % Fe | ||
|---|---|---|---|---|---|
| Grade | Recovery | CE | |||
| 9 mm | Concentrate | 35.0 | 63.5 | 60.0 | 53.2 |
| Tailings | 65.0 | 22.8 | |||
| Feed | 100.0 | 37.0 | |||
| 6 mm | Concentrate | 45.6 | 60.0 | 78.0 | 61.3 |
| Tailings | 54.4 | 16.8 | |||
| Feed | 100.0 | 36.5 | |||
| 3 mm | Concentrate | 61.7 | 50.8 | 87.0 | 52.1 |
| Tailings | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Magnetic Intensity (Gauss) | Product | % Fe | |||
|---|---|---|---|---|---|
| wt% | Grade | Recovery | CE | ||
| 4965 | Concentrate | 24.5 | 66.5 | 45.3 | 42.7 |
| Tailings | 75.5 | 26.1 | |||
| Feed | 100.0 | 36.0 | |||
| 8800 | Concentrate | 45.6 | 59.4 | 75.3 | 61.1 |
| Tailings | 73.5 | 16.4 | |||
| Feed | 100.0 | 36.0 | |||
| 10,000 | Concentrate | 61.7 | 50.8 | 87.0 | 52.1 |
| Tailings | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Feed Particle Size | Product | % Fe | CE | ||
|---|---|---|---|---|---|
| wt% | Grade | Recovery | |||
| −210 µm D80 106 µm | Concentrate | 68.4 | 48.7 | 92.5 | 49.6 |
| Flotationtailings | 15.2 | 8.8 | |||
| WHIMS non-mag | 16.4 | 8.3 | |||
| Feed | 100 | 36 | |||
| Flotation feed/WHIMS mag (Cal) | 83.6 | 41.4 | 96.2 | ||
| −106 µm D80 80 µm | Concentrate | 48.7 | 61.5 | 92.2 | 71 |
| Flotationtailings | 11.1 | 36 | |||
| WHIMSnon-mag | 20 | 7.2 | |||
| Feed | 100 | 36 | |||
| Flotation feed/WHIMS mag (Cal) | 80 | 43.2 | 96 | ||
| −74 µm D80 54 µm | Concentrate | 37.7 | 66 | 69.1 | 64.7 |
| Flotation tailings | 24 | 26.9 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100 | 36 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87 | ||
| Depressant Caustic Starch Dosage kg/t | Product | %Fe | CE | ||
|---|---|---|---|---|---|
| wt% | Grade | Recovery | |||
| 0.5 | Concentrate | 34.1 | 59.3 | 56.1 | 45.4 |
| Flotation tailings | 27.6 | 40.3 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87.0 | ||
| 1 | Concentrate | 44.1 | 63.5 | 77.78 | 69.4 |
| Flotation tailings | 18.5 | 26.6 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87.0 | ||
| 1.5 | Concentrate | 37.7 | 66.0 | 69.1 | 64.7 |
| Flotation tailings | 24.0 | 26.9 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87.0 | ||
| Collector Dosage (kg/t) | Product | % Fe | CE | ||
|---|---|---|---|---|---|
| wt% | Grade | Recovery | |||
| 0.05 | Concentrate | 44.0 | 63.3 | 77.4 | 68.8 |
| Flotation tailings | 17.7 | 17.5 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87.0 | ||
| 0.15 | Concentrate | 37.7 | 66.0 | 69.1 | 64.7 |
| Flotation tailings | 24.0 | 26.9 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87.0 | ||
| 0.25 | Concentrate | 36.0 | 67.0 | 67.0 | 63.9 |
| Flotation tailings | 25.7 | 28.0 | |||
| WHIMS non-mag | 38.3 | 12.1 | |||
| Feed | 100.0 | 36.0 | |||
| Flotation feed/WHIMS mag (Cal) | 61.7 | 50.8 | 87.0 | ||
| Product | wt% | % FeGrade | % FeDist. |
|---|---|---|---|
| Table concentrate | 25.70 | 63.03 | 45.00 |
| Flotation concentrate | 13.90 | 65.00 | 25.10 |
| WHIMS tails | 38.30 | 12.12 | |
| Flotation floattailings | 24.00 | 26.85 | |
| HeadCal | 100.00 | 36.00 | |
| Final concentrate | 39.60 | 63.73 | 70.10 |
| Finaltails (WHIMS-non-mag + flotation tailings) | 60.40 | 17.82 | |
| Table tailings (WHIMS feed) | 74.30 | 26.05 | |
| Flotation feed/WHIMS conc | 26.80 | 48.40 |
| Product | wt% | Grade (% Fe) | Recovery (% Fe) |
|---|---|---|---|
| Reverse flotation concentrate (final) | 44.00 | 63.34 | 77.40 |
| Reverse flotation tails | 17.70 | 17.49 | |
| WHIMS non-mag (tails) | 38.30 | 12.12 | |
| Head Cal | 100.00 | 36.00 | |
| WHIMS mag conc (flotation feed) | 61.70 | 50.76 | |
| Final tails (flotation tailings + WHIMS non-mag) | 56.00 | 14.53 |
| Product | Physico-Chemical | Mineralogy | Photo |
|---|---|---|---|
| Final Concentrate | Black fine powder,4.5 SG, 2.9 t/m3 BD, −0.074 mm, D80 0.045 mm, 63.34% Fe, 6.30% SiO2, 0.20% Al2O3, 0.02% P, 0.02% S (0.03 Al2O3/SiO2), and 0.20% LOI with 77.4% Fe recovery, 68.8% concentration efficiency at 44.0 wt% yield. | It contained fine-grained (<100 µm) martitized magnetite (30–35%), fine-grained (<74 µm) hematite (60–65%), and extremely fine-grained (<40 µm) cherty quartz (5–10%). Most of the grains are free. |  |
| Product | Physico-Chemical | Mineralogy | Photo |
|---|---|---|---|
| Final tails | Gray fine powder, 3 S.G., 1.9 t/m3 BD0.074 mm, D80 0.06 mm, 14.53% Fe, 79.75% SiO2, 1.18% Al2O3, and LOI 1.33% with 23.6% Fe recovery at a wt% of 56.0. | It contained fine-grained (<74 µm) hematite (20–25%) and extremely fine-grained (<40 µm) cherty quartz (75–80%). Most of the grains are free. Some hematite grains are seen as inclusions in quartz. | 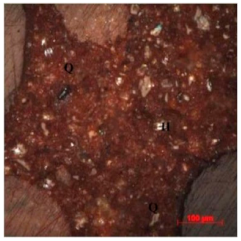 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swamy, A.K.; Nikkam, S.; Kumar, P.S. Processing Studies on Banded Hematite Quartzite’s of Sandur Sciht, Karnataka, India. Energies 2022, 15, 2542. https://doi.org/10.3390/en15072542
Swamy AK, Nikkam S, Kumar PS. Processing Studies on Banded Hematite Quartzite’s of Sandur Sciht, Karnataka, India. Energies. 2022; 15(7):2542. https://doi.org/10.3390/en15072542
Chicago/Turabian StyleSwamy, Aspari Kumara, Suresh Nikkam, and Palthur Sharath Kumar. 2022. "Processing Studies on Banded Hematite Quartzite’s of Sandur Sciht, Karnataka, India" Energies 15, no. 7: 2542. https://doi.org/10.3390/en15072542
APA StyleSwamy, A. K., Nikkam, S., & Kumar, P. S. (2022). Processing Studies on Banded Hematite Quartzite’s of Sandur Sciht, Karnataka, India. Energies, 15(7), 2542. https://doi.org/10.3390/en15072542





