Predicting Adsorption of Methane and Carbon Dioxide Mixture in Shale Using Simplified Local-Density Model: Implications for Enhanced Gas Recovery and Carbon Dioxide Sequestration
Abstract
:1. Introduction
2. SLD Model
2.1. SLD Model for Single Component Gas
2.2. SLD Model for Multicomponent Gas
3. Calculation Workflow for Adsorption of Multicomponent Gas
4. Results and Discussion
4.1. Validation of Developed Calculation Workflow
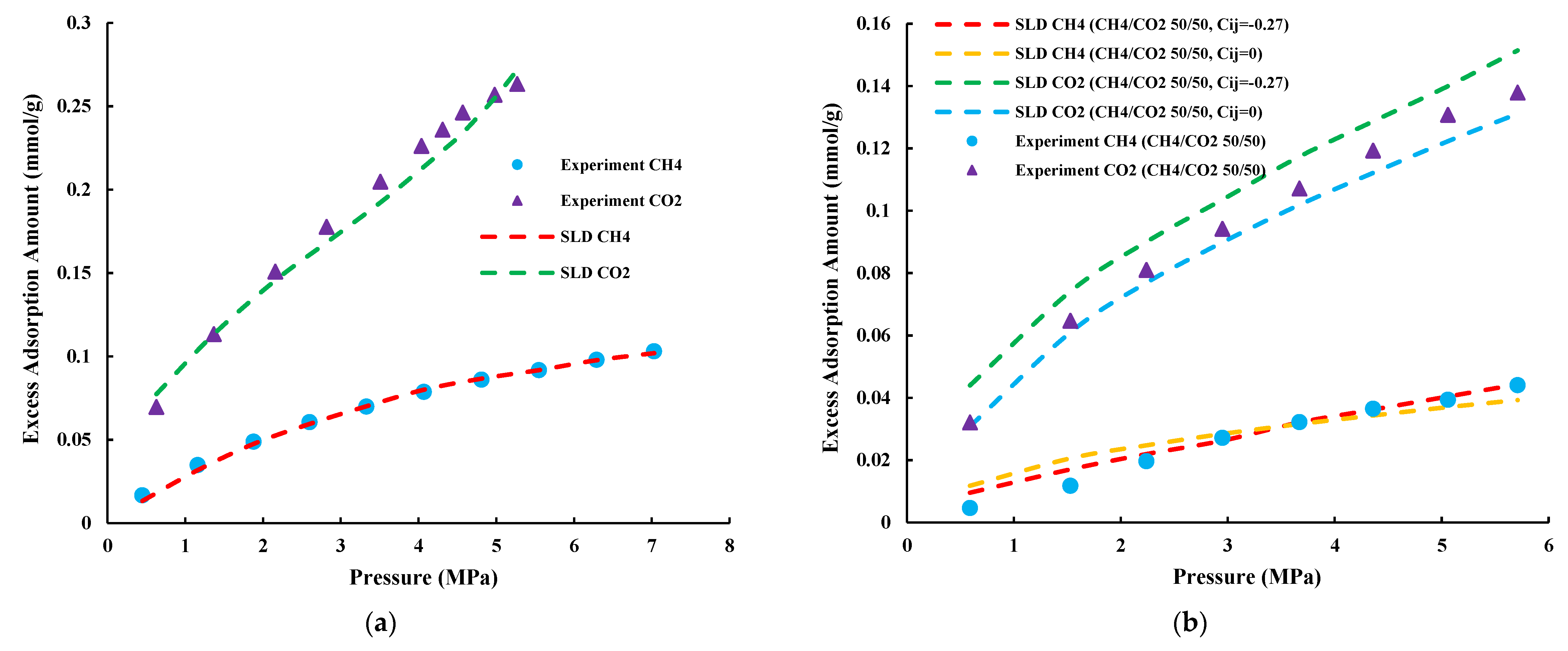
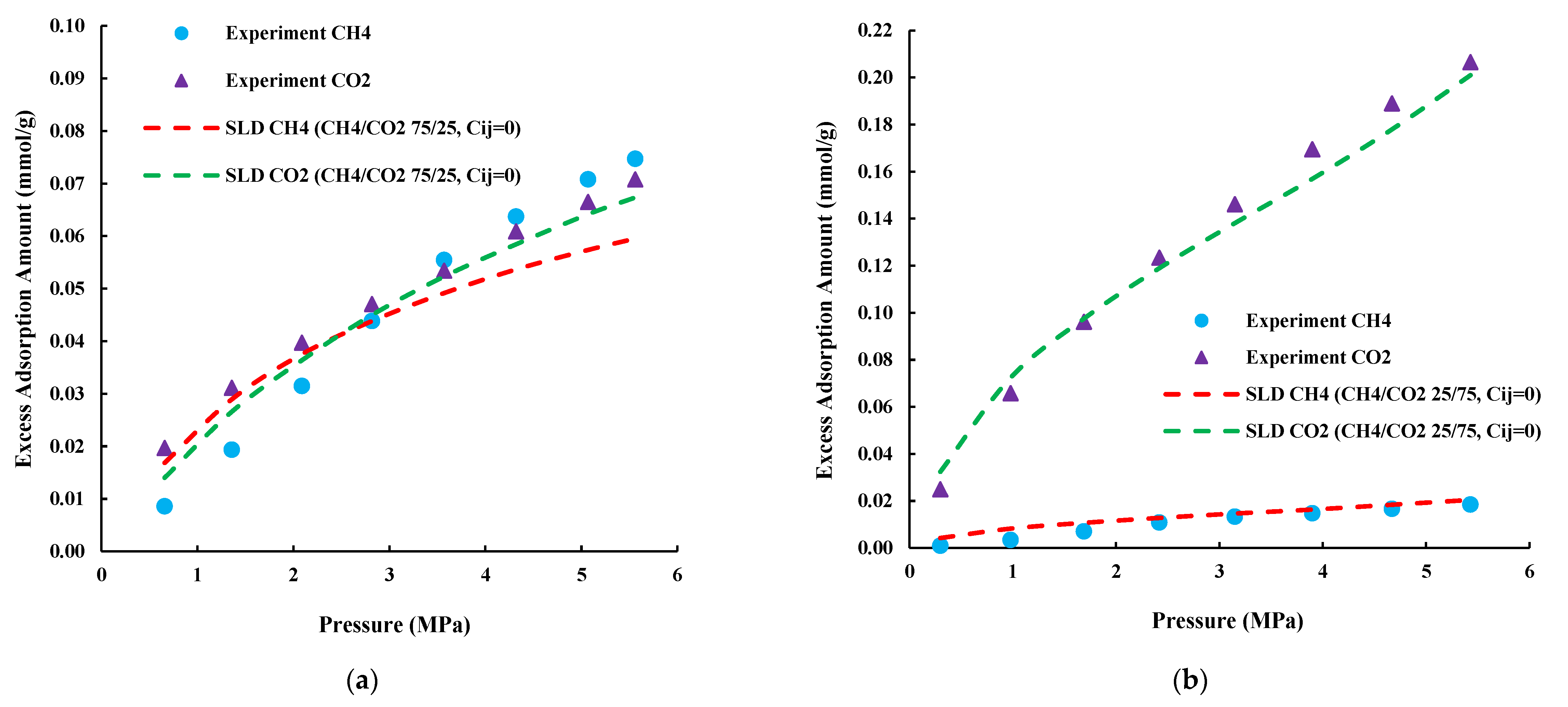
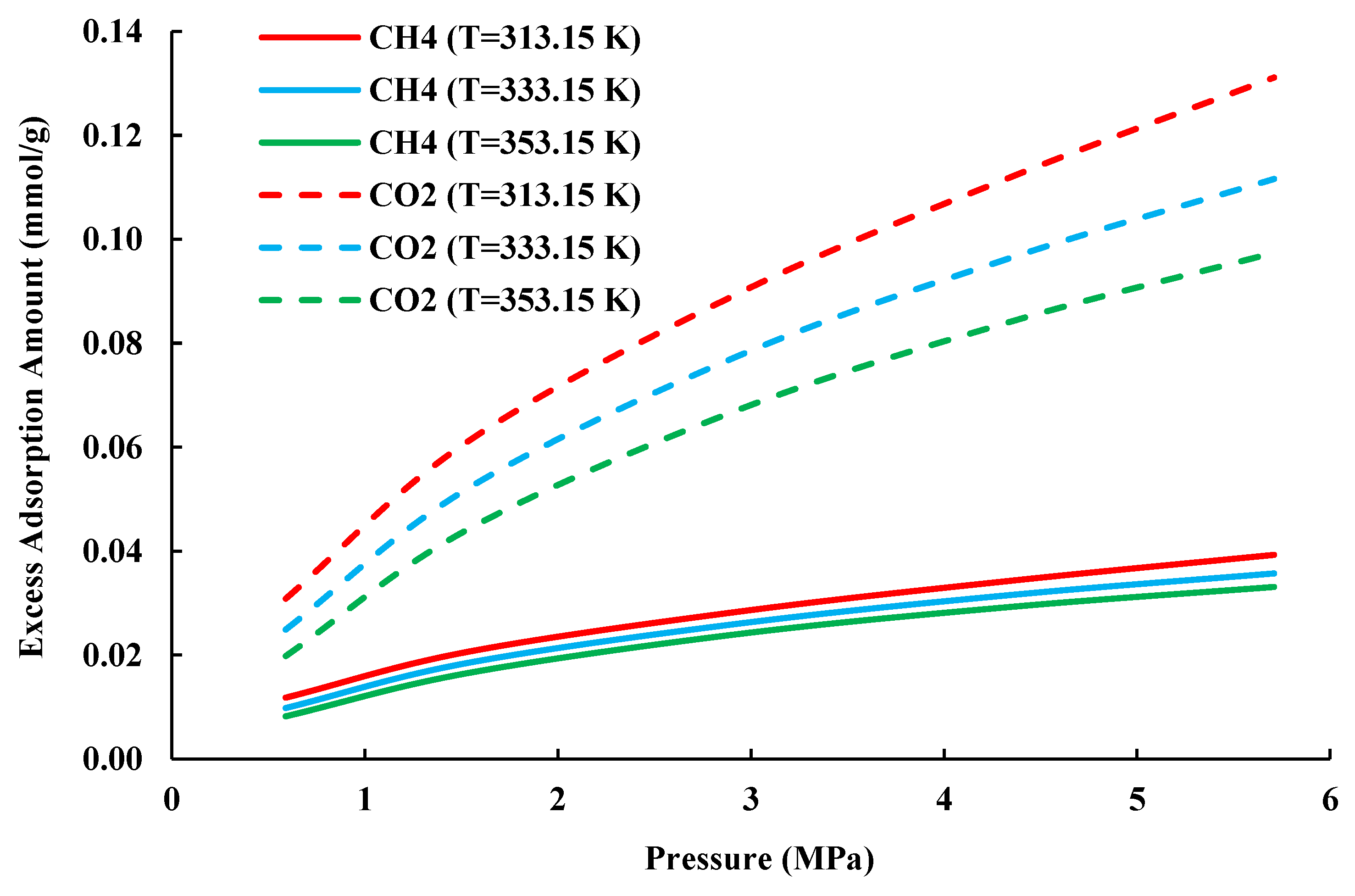
4.2. Prediction of CH4/CO2 Competitive Adsorption
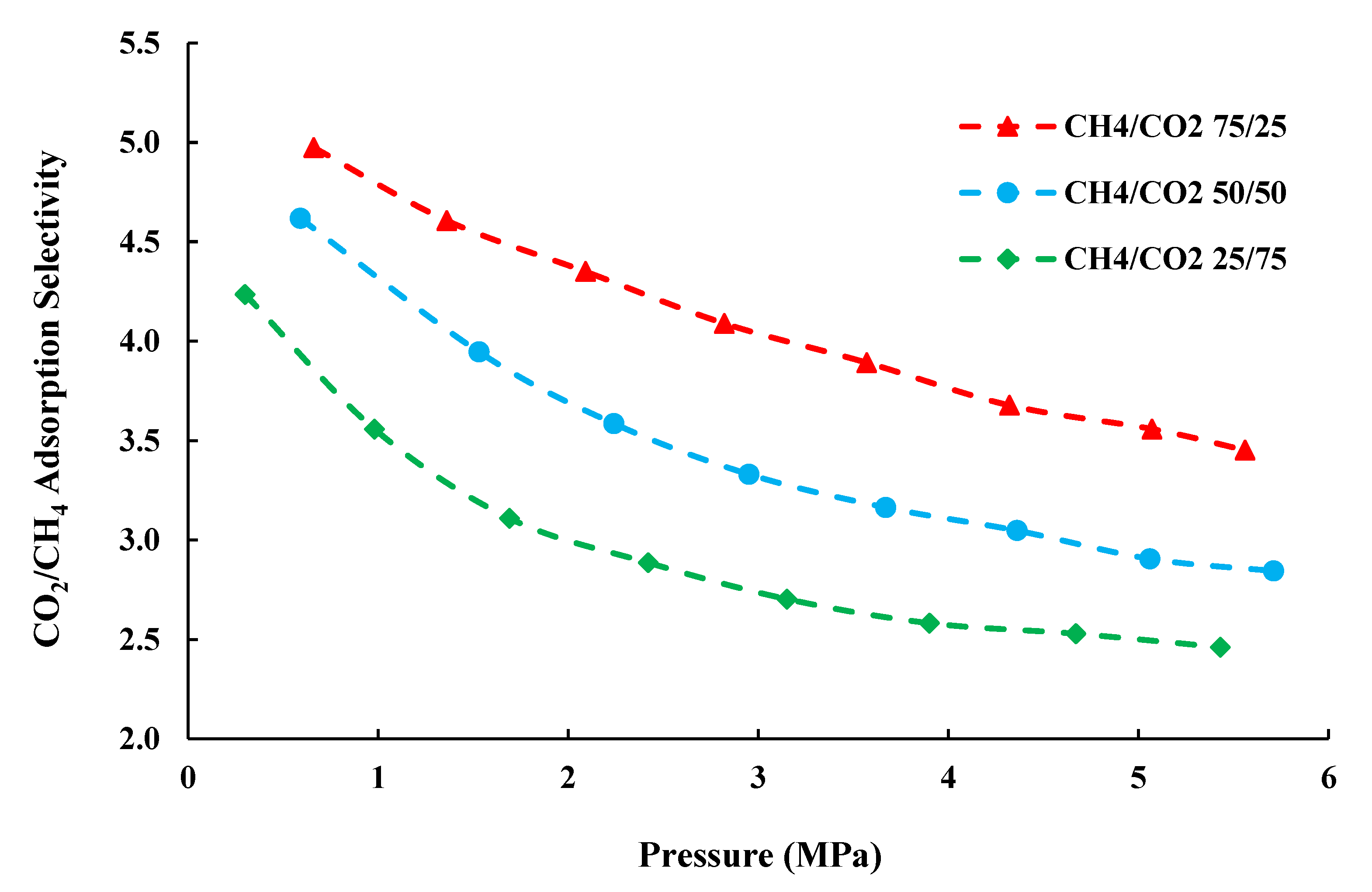
4.3. CO2/CH4 Adsorption Selectivity
5. Conclusions
- The developed iterative calculation workflow is able to characterize and predict the excess and absolute adsorption of a gas mixture with various feed-gas mole fractions through manipulating the multicomponent SLD model to implement regression analysis on the measured single-component adsorption results simultaneously, thus bypassing the demands of the mole fraction of each component in the gas phase at the equilibrium in experiments and the binary interaction parameters obtained from the curve-fitting process.
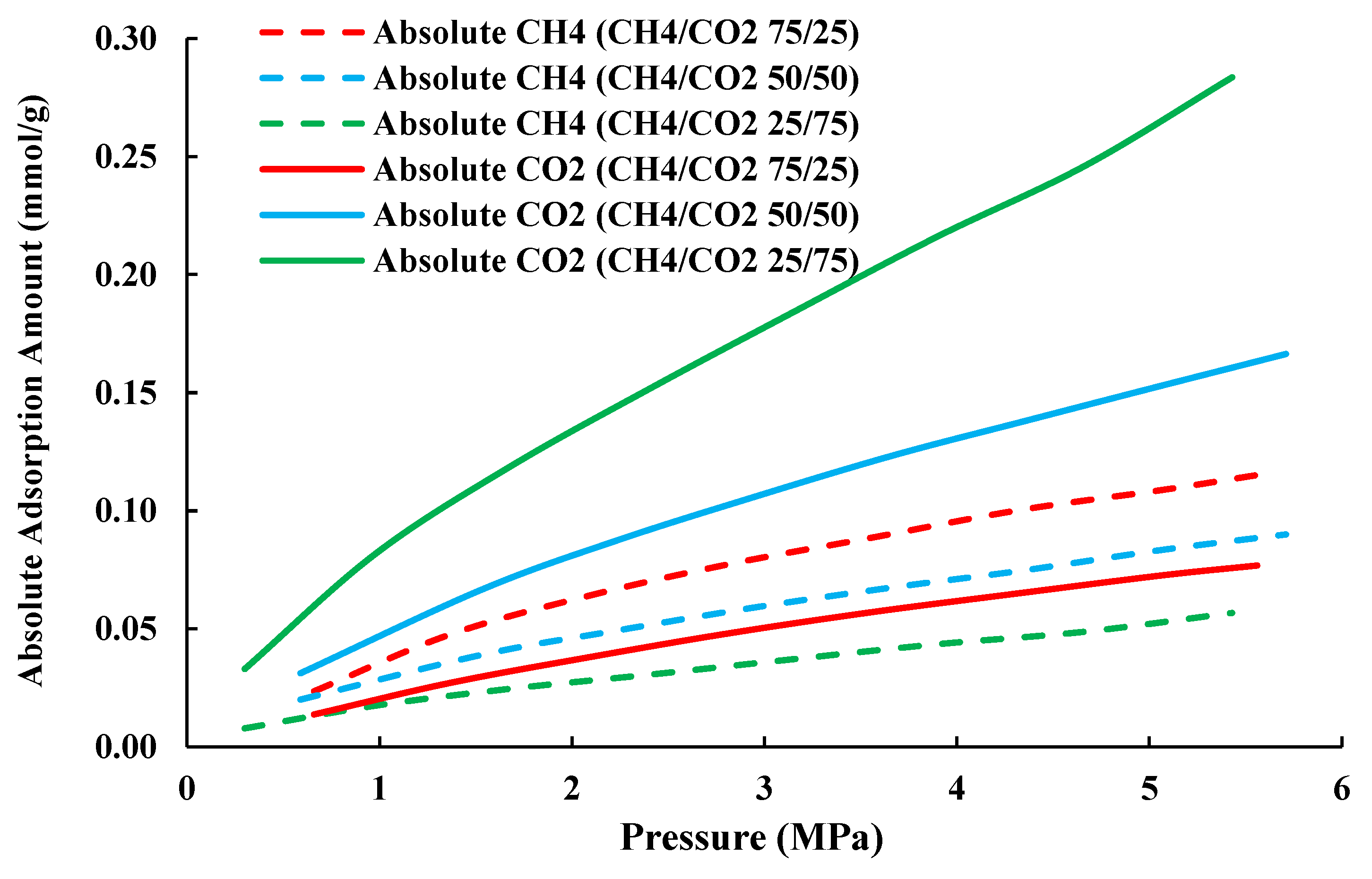
- The adsorption capacity of the gas component in a gas mixture is proportional to the initial feed-gas mole fraction. Accordingly, there will be a threshold of feed-gas mole fraction, above which the CO2 adsorption capacity would be larger than the CH4 adsorption capacity. Such a threshold is critical for CO2-EGR and CO2 sequestration operations in shale gas reservoirs.
- In this study, adsorption selectivity (SCO2/CH4) ranges approximately from 2.5 to 5, indicating the preferential adsorption of CO2 over CH4 in shale. The adsorption selectivity increases with the feed-gas mole fraction, demonstrating that the higher mole fraction of CO2 in feed-gas will weaken the selectivity of CO2 over CH4, despite that the higher mole fraction of CO2 in feed-gas will lead to the larger CO2 adsorption capacity. As a result, a proper mole fraction for feed-gas should be determined to ensure the high adsorption capacity and selectivity of CO2 over CH4 in the process of CO2-EGR and CO2 sequestration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, Z.; Clarens, A. Estimating the Carbon Sequestration Capacity of Shale Formations Using Methane Production Rates. Environ. Sci. Technol. 2013, 47, 11318–11325. [Google Scholar] [CrossRef] [PubMed]
- Khosrokhavar, R.; Griffiths, S.; Wolf, K.-H. Shale Gas Formations and Their Potential for Carbon Storage: Opportunities and Outlook. Environ. Process. 2014, 1, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Pei, P.; Ling, K.; He, J.; Liu, Z. Shale gas reservoir treatment by a CO2-based technology. J. Nat. Gas Sci. Eng. 2015, 26, 1595–1606. [Google Scholar] [CrossRef]
- Sun, H.; Yao, J.; Gao, S.-H.; Fan, D.-Y.; Wang, C.-C.; Sun, Z.-X. Numerical study of CO2 enhanced natural gas recovery and sequestration in shale gas reservoirs. Int. J. Greenh. Gas Control 2013, 19, 406–419. [Google Scholar] [CrossRef]
- Etminan, S.R.; Javadpour, F.; Maini, B.B.; Chen, Z. Measurement of gas storage processes in shale and of the molecular diffusion coefficient in kerogen. Int. J. Coal Geol. 2014, 123, 10–19. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, Y.; Soliman, M.Y.; Shen, Y. Experimental measurement and analytical estimation of methane absorption in shale kerogen. Fuel 2019, 240, 192–205. [Google Scholar] [CrossRef]
- Huo, P.; Zhang, D.; Yang, Z.; Li, W.; Zhang, J.; Jia, S. CO2 geological sequestration: Displacement behavior of shale gas methane by carbon dioxide injection. Int. J. Greenh. Gas Control 2017, 66, 48–59. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon Dioxide Storage Capacity of Organic-Rich Shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef] [Green Version]
- Charoensuppanimit, P.; Mohammad, S.A.; Gasem, K.A. Measurements and Modeling of Gas Adsorption on Shales. Energy Fuels 2016, 30, 2309–2319. [Google Scholar] [CrossRef]
- Klewiah, I.; Berawala, D.S.; Walker, H.C.A.; Andersen, P.Ø.; Nadeau, P.H. Review of experimental sorption studies of CO2 and CH4 in shales. J. Nat. Gas Sci. Eng. 2020, 73, 103045. [Google Scholar] [CrossRef]
- Liu, S.; Sun, B.; Xu, J.; Li, H.; Wang, X. Study on Competitive Adsorption and Displacing Properties of CO2 Enhanced Shale Gas Recovery: Advances and Challenges. Geofluids 2020, 2020, 6657995. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y.; Krooss, B. Methane and CO2 sorption and desorption measurements on dry Argonne premium coals: Pure components and mixtures. Int. J. Coal Geol. 2003, 55, 205–224. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Sheng, J.J.; Hao, Y.; Wang, W.; Lv, Y.; Zhao, Q.; Wang, H. Experimental and Numerical Study on CO2 Sweep Volume during CO2 Huff-n-Puff Enhanced Oil Recovery Process in Shale Oil Reservoirs. Energy Fuels 2019, 33, 4017–4032. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Zhang, W.; Cheng, Z.; Wu, X.; Qin, H. Effect of organic type and moisture on CO2/CH4 competitive adsorption in kerogen with implications for CO2 sequestration and enhanced CH4 recovery. Appl. Energy 2018, 210, 28–43. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Zhao, H.; Li, M. Molecular simulation of adsorption and thermodynamic properties on type II kerogen: Influence of maturity and moisture content. Fuel 2017, 190, 198–207. [Google Scholar] [CrossRef]
- Yu, W.; Al-Shalabi, E.W.; Sepehrnoori, K. A Sensitivity Study of Potential CO2 Injection for Enhanced Gas Recovery in Barnett Shale Reservoirs. In Proceedings of the SPE Unconventional Resources Conference, The Woodlands, TX, USA, 1–3 April 2014. [Google Scholar]
- Jiang, J.; Shao, Y.; Younis, R. Development of a Multi-Continuum Multi-Component Model for Enhanced Gas Recovery and CO2 Storage in Fractured Shale Gas Reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Zhang, R.-H.; Wu, J.-F.; Zhao, Y.-L.; He, X.; Wang, R.-H. Numerical simulation of the feasibility of supercritical CO2 storage and enhanced shale gas recovery considering complex fracture networks. J. Pet. Sci. Eng. 2021, 204, 108671. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Hu, W.; Cui, J.; Jiang, L.; Liu, Y.; Zhao, Y.; Li, W.; Song, Y. Asymmetric competitive adsorption of CO2/CH4 binary mixture in shale matrix with heterogeneous surfaces. Chem. Eng. J. 2021, 422, 130025. [Google Scholar] [CrossRef]
- Hu, X.; Deng, H.; Lu, C.; Tian, Y.; Jin, Z. Characterization of CO2/CH4 competitive adsorption in various clay minerals in relation to shale gas recovery from molecular simulation. Energy Fuels 2019, 33, 8202–8214. [Google Scholar] [CrossRef]
- Niu, Y.; Yue, C.; Li, S.; Ma, Y.; Xu, X. Influencing Factors and Selection of CH4 and CO2 Adsorption on Silurian Shale in Yibin, Sichuan Province of China. Energy Fuels 2018, 32, 3202–3210. [Google Scholar] [CrossRef]
- Luo, X.; Wang, S.; Wang, Z.; Jing, Z.; Lv, M.; Zhai, Z.; Han, T. Adsorption of methane, carbon dioxide and their binary mixtures on Jurassic shale from the Qaidam Basin in China. Int. J. Coal Geol. 2015, 150–151, 210–223. [Google Scholar] [CrossRef]
- Cancino, O.P.O.; Pérez, D.P.; Pozo, M.; Bessieres, D. Adsorption of pure CO2 and a CO2/CH4 mixture on a black shale sample: Manometry and microcalorimetry measurements. J. Pet. Sci. Eng. 2017, 159, 307–313. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Elsworth, D.; Gan, Q. CO2/CH4 competitive adsorption in shale: Implications for enhancement in gas production and reduction in carbon emissions. Environ. Sci. Technol. 2019, 53, 9328–9336. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, J.; Liu, D.; Chen, J.; Pan, Z. A new application of NMR in characterization of multiphase methane and adsorption capacity of shale. Int. J. Coal Geol. 2019, 201, 76–85. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin, Germany, 2012; Volume 16. [Google Scholar]
- Clarkson, C.R.; Haghshenas, B. Modeling of Supercritical Fluid Adsorption on Organic-Rich Shales and Coal. In Proceedings of the SPE Unconventional Resources Conference, The Woodlands, TX, USA, 10–12 April 2013. [Google Scholar]
- Pang, Y.; Soliman, M.Y.; Deng, H.; Emadi, H. Analysis of Effective Porosity and Effective Permeability in Shale-Gas Reservoirs with Consideration of Gas Adsorption and Stress Effects. SPE J. 2017, 22, 1739–1759. [Google Scholar] [CrossRef]
- Rangarajan, B.; Lira, C.T.; Subramanian, R. Simplified local density model for adsorption over large pressure ranges. AIChE J. 1995, 41, 838–845. [Google Scholar] [CrossRef]
- Pang, Y.; Hu, X.; Wang, S.; Chen, S.; Soliman, M.Y.; Deng, H. Characterization of adsorption isotherm and density profile in cylindrical nanopores: Modeling and measurement. Chem. Eng. J. 2020, 396, 125212. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Mohammad, S.; Robinson, R.L.; Gasem, K.A. Ono-Kondo lattice model for high-pressure adsorption: Pure gases. Fluid Phase Equilibria 2010, 299, 238–251. [Google Scholar] [CrossRef]
- Zhou, S.; Ning, Y.; Wang, H.; Liu, H.; Xue, H. Investigation of methane adsorption mechanism on Longmaxi shale by combining the micropore filling and monolayer coverage theories. Adv. Geo-Energy Res. 2018, 2, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Arri, L.; Yee, D.; Morgan, W.; Jeansonne, M. Modeling coalbed methane production with binary gas sorption. In Proceedings of the SPE Rocky Mountain Regional Meeting, Casper, WY, USA, 18–21 May 1992. [Google Scholar]
- Clarkson, C.; Bustin, R. Binary gas adsorption/desorption isotherms: Effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int. J. Coal Geol. 2000, 42, 241–271. [Google Scholar] [CrossRef]
- Walton, K.S.; Sholl, D.S. Predicting multicomponent adsorption: 50 years of the ideal adsorbed solution theory. AIChE J. 2015, 61, 2757–2762. [Google Scholar] [CrossRef]
- Duan, S.; Gu, M.; Du, X.; Xian, X. Adsorption Equilibrium of CO2 and CH4 and Their Mixture on Sichuan Basin Shale. Energy Fuels 2016, 30, 2248–2256. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Robinson, R.L.; Gasem, K.A. Modeling High-Pressure Adsorption of Gas Mixtures on Activated Carbon and Coal Using a Simplified Local-Density Model. Langmuir 2006, 22, 9610–9618. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Mohammad, S.A.; Robinson, R.L., Jr.; Gasem, K.A. Ono-Kondo Model for High-Pressure Mixed-Gas Adsorption on Activated Carbons and Coals. Energy Fuels 2011, 25, 3355–3367. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Pan, Z.; Fitzgerald, J.E.; Robinson, R.L.; Gasem, K.A. Adsorption of Methane, Nitrogen, Carbon Dioxide, and Their Binary Mixtures on Dry Activated Carbon at 318.2 K and Pressures up to 13.6 MPa. Langmuir 2003, 19, 5323–5331. [Google Scholar] [CrossRef]
- Mohammad, S.A.; Arumugam, A.; Robinson, R.L., Jr.; Gasem, K.A. High-Pressure Adsorption of Pure Gases on Coals and Activated Carbon: Measurements and Modeling. Energy Fuels 2012, 26, 536–548. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.; Robinson, R.L., Jr.; Gasem, K.A. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Lee, L.L. Molecular Thermodynamics of Nonideal Fluids; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Chen, J.H.; Wong, A.D.S.H.; Tan, C.S.; Subramanian, R.; Lira, A.C.T.; Orth, M. Adsorption and Desorption of Carbon Dioxide onto and from Activated Carbon at High Pressures. Ind. Eng. Chem. Res. 1997, 36, 2808–2815. [Google Scholar] [CrossRef]
- Gasem, K.; Gao, W.; Pan, Z.; Robinson, R., Jr. A modified temperature dependence for the Peng-Robinson equation of state. Fluid Phase Equilibria 2001, 181, 113–125. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Song, X.; Liu, Z.; Li, D.; Zhou, F.; Xie, Y.; Xie, C. Effect of supercritical CO2 extraction on CO2/CH4 competitive adsorption in Yanchang shale. Chem. Eng. J. 2021, 412, 128701. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Z.; Wang, H.; Yan, Y.; Liu, X. Molecular insights into competitive adsorption of CO2/CH4 mixture in shale nanopores. RSC Adv. 2018, 8, 33939–33946. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Ripepi, N.; Luxbacher, K.; Pitcher, E. Adsorption Models for Methane in Shales: Review, Comparison, and Application. Energy Fuels 2017, 31, 10787–10801. [Google Scholar] [CrossRef]
- Wang, S.; Yao, X.; Feng, Q.; Javadpour, F.; Yang, Y.; Xue, Q.; Li, X. Molecular insights into carbon dioxide enhanced multi-component shale gas recovery and its sequestration in realistic kerogen. Chem. Eng. J. 2021, 425, 130292. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Q.; Duan, W.; Hou, L.; Liu, K.; Qin, Q.; Liu, S.; Dai, J. Equilibrium and Kinetics Analysis of CO2 Adsorption on Waste Ion-exchange Resin-based Activated Carbon. J. Taiwan Inst. Chem. Eng. 2017, 77, 161–167. [Google Scholar] [CrossRef]

| Gas Molecule | Critical Pressure (MPa) | Critical Temperature (K) | Molecular Diameter (nm) | Acentric Factor |
|---|---|---|---|---|
| CH4 | 4.599 | 190.56 | 0.373 | 0.0113 |
| CO2 | 7.377 | 304.13 | 0.3648 | 0.225 |
| Gas Component | L (nm) | εfs/kB (K) | A (m2/g) | Ab | %AAD |
|---|---|---|---|---|---|
| CH4 | 8.6 | 37.81 | 16 | 0.1 | 3.76 |
| CO2 | 8.6 | 72.25 | 16 | −0.01 | 4.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Chen, S.; Wang, H. Predicting Adsorption of Methane and Carbon Dioxide Mixture in Shale Using Simplified Local-Density Model: Implications for Enhanced Gas Recovery and Carbon Dioxide Sequestration. Energies 2022, 15, 2548. https://doi.org/10.3390/en15072548
Pang Y, Chen S, Wang H. Predicting Adsorption of Methane and Carbon Dioxide Mixture in Shale Using Simplified Local-Density Model: Implications for Enhanced Gas Recovery and Carbon Dioxide Sequestration. Energies. 2022; 15(7):2548. https://doi.org/10.3390/en15072548
Chicago/Turabian StylePang, Yu, Shengnan Chen, and Hai Wang. 2022. "Predicting Adsorption of Methane and Carbon Dioxide Mixture in Shale Using Simplified Local-Density Model: Implications for Enhanced Gas Recovery and Carbon Dioxide Sequestration" Energies 15, no. 7: 2548. https://doi.org/10.3390/en15072548






