Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms
Abstract
:1. Introduction
1.1. Background
1.2. Hydrogen Generation Methods
1.3. Scope of the Review
2. Methane Dehydrogenation Mechanism
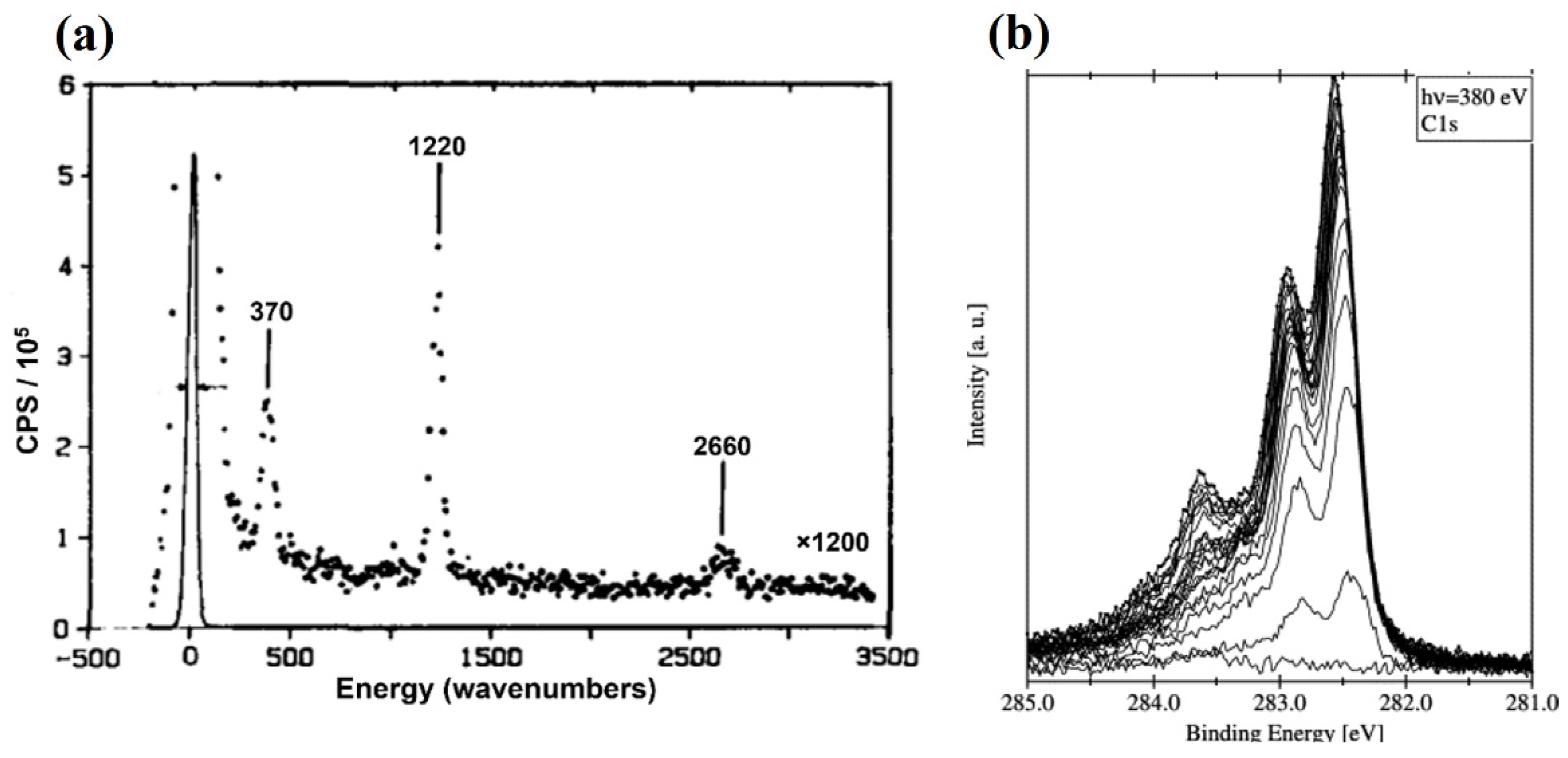
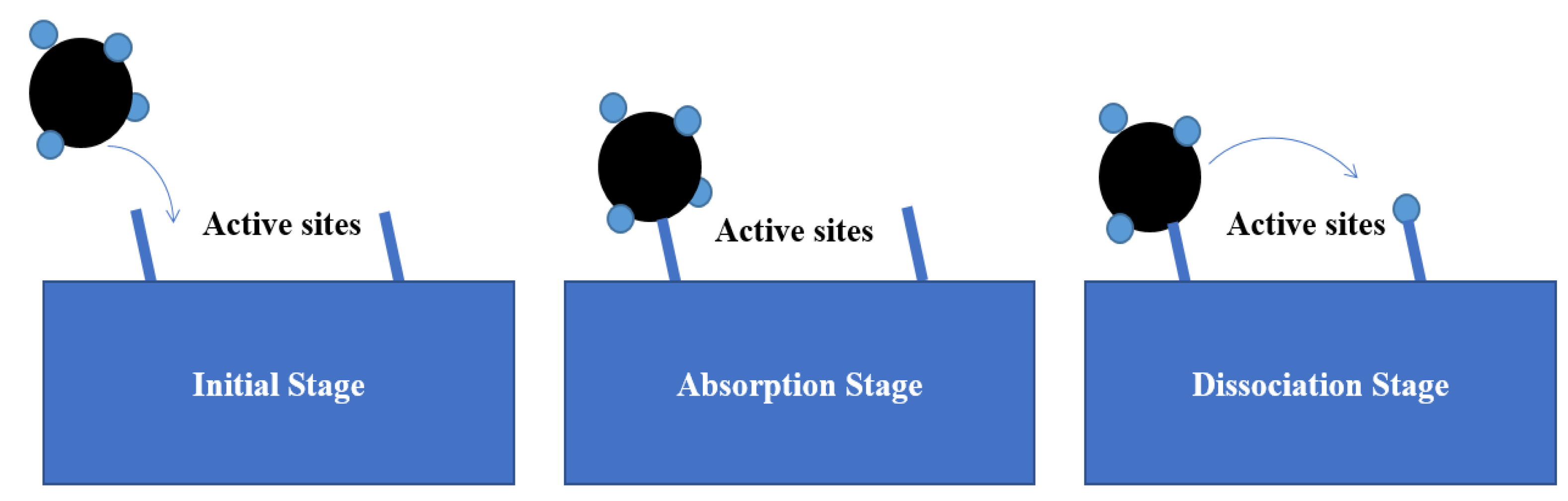
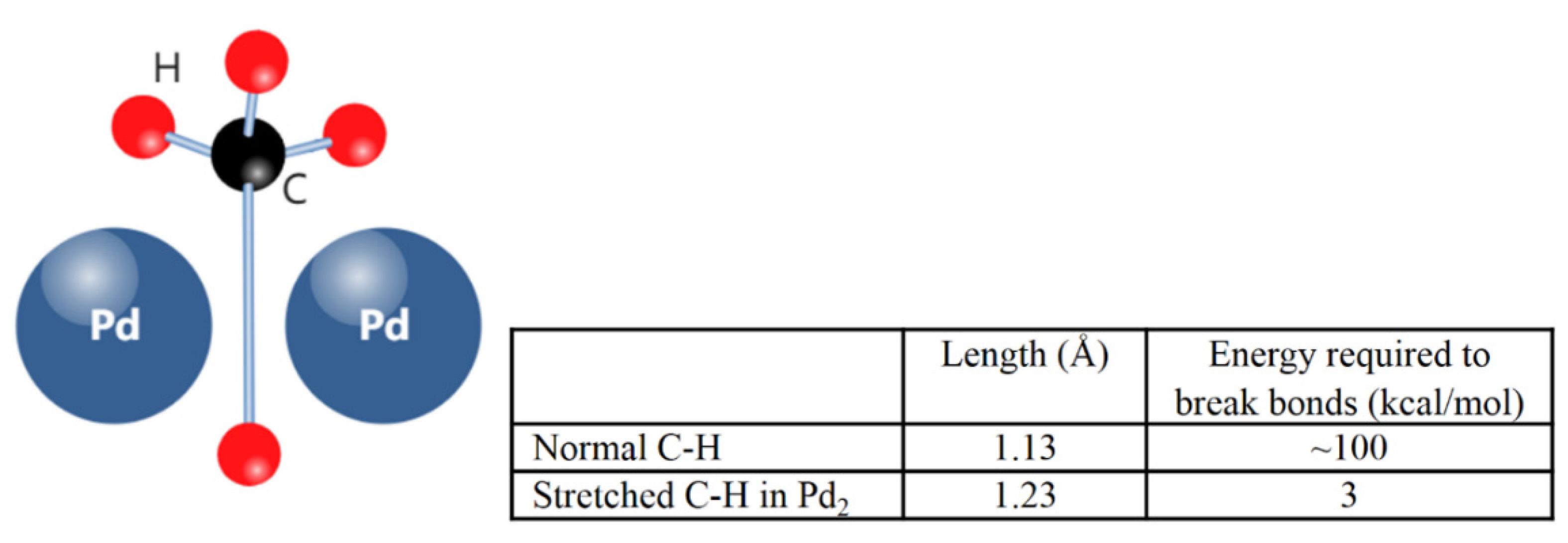
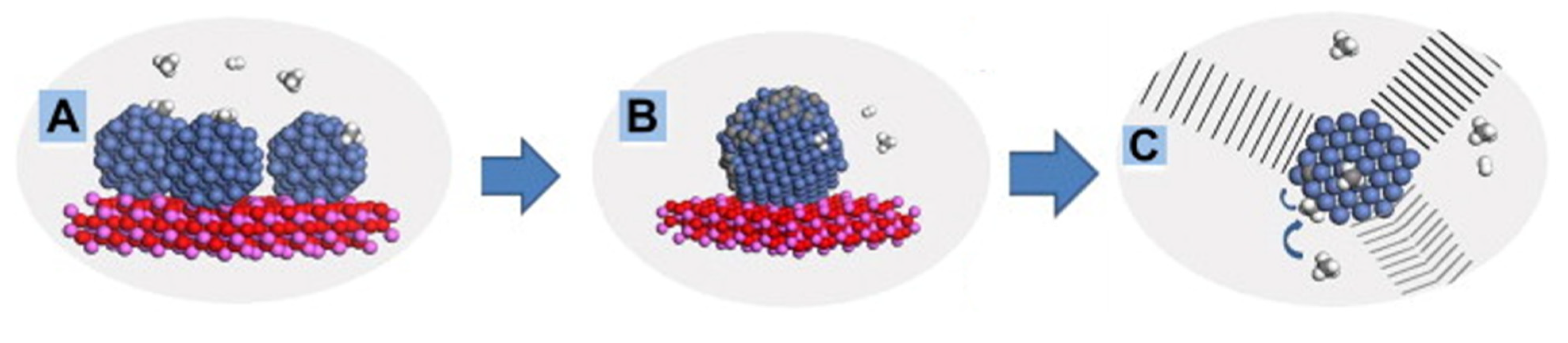
2.1. Dissociative Methane Adsorption
2.2. Non-Dissociative Methane Adsorption
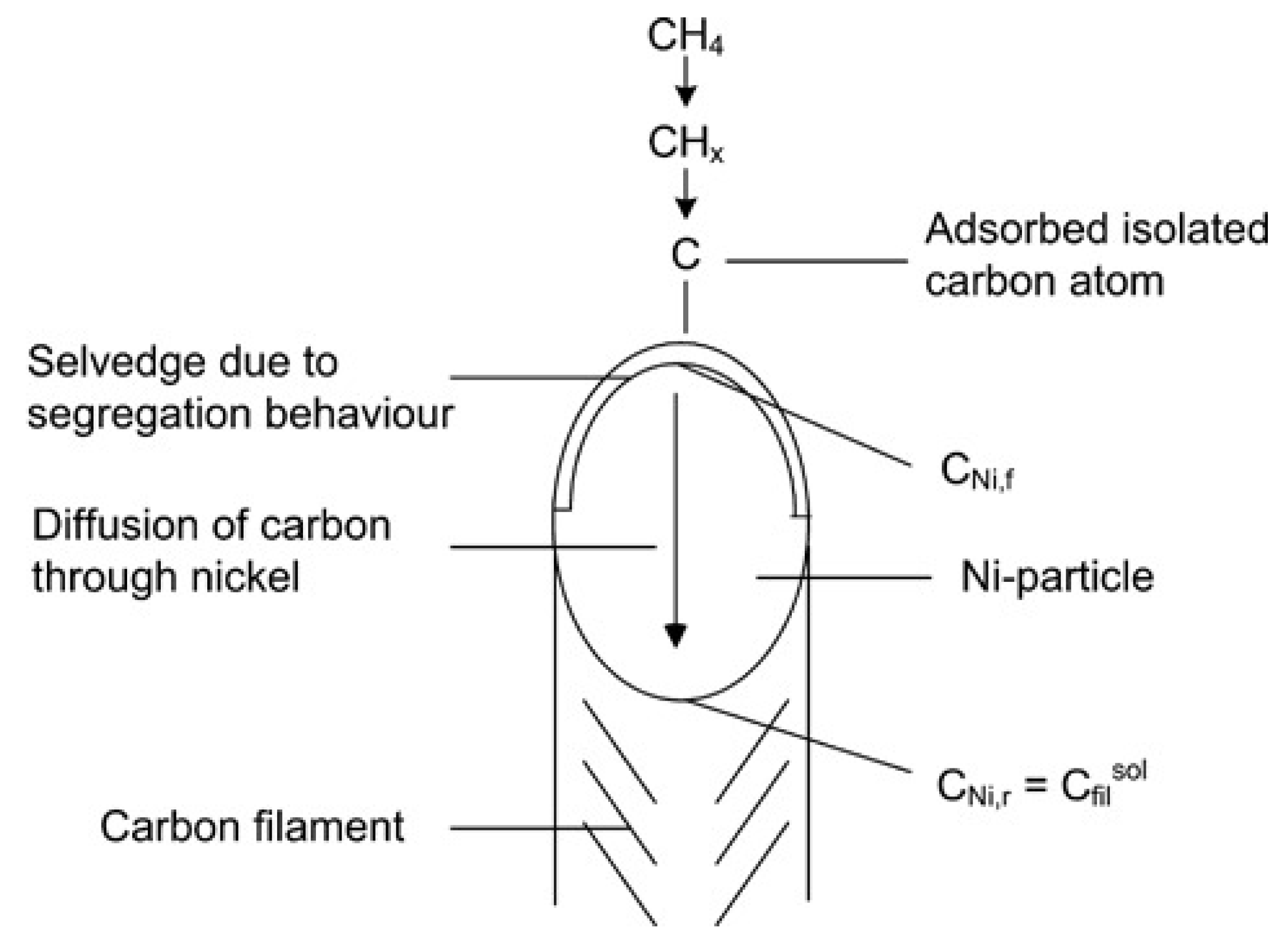
3. Carbon Formation Mechanism
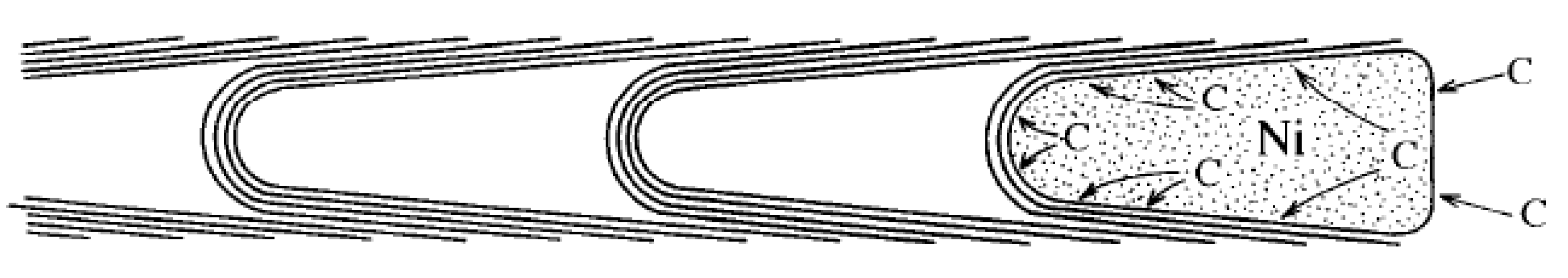
3.1. “Tip Growth” Mechanism
3.2. “Base Growth” Mechanism
4. Special Carbon Depositions in CDM Reaction
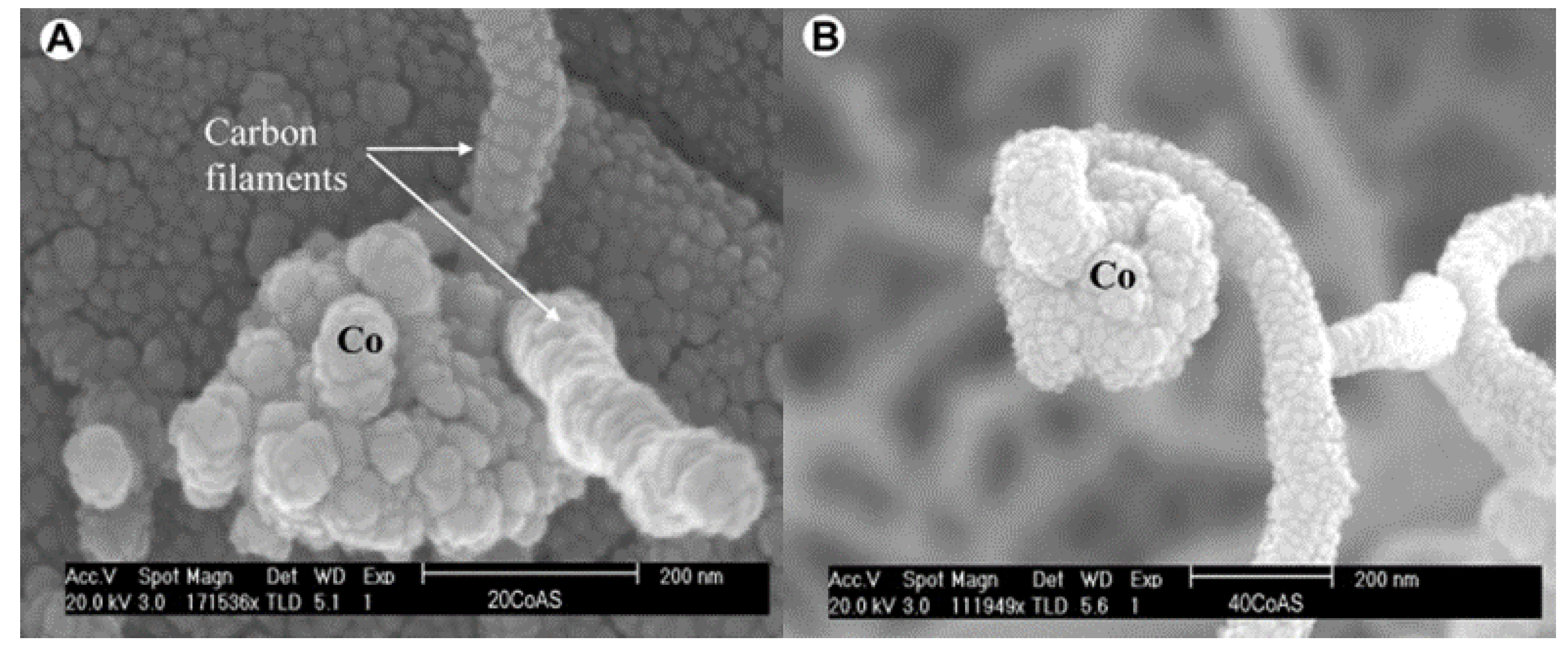
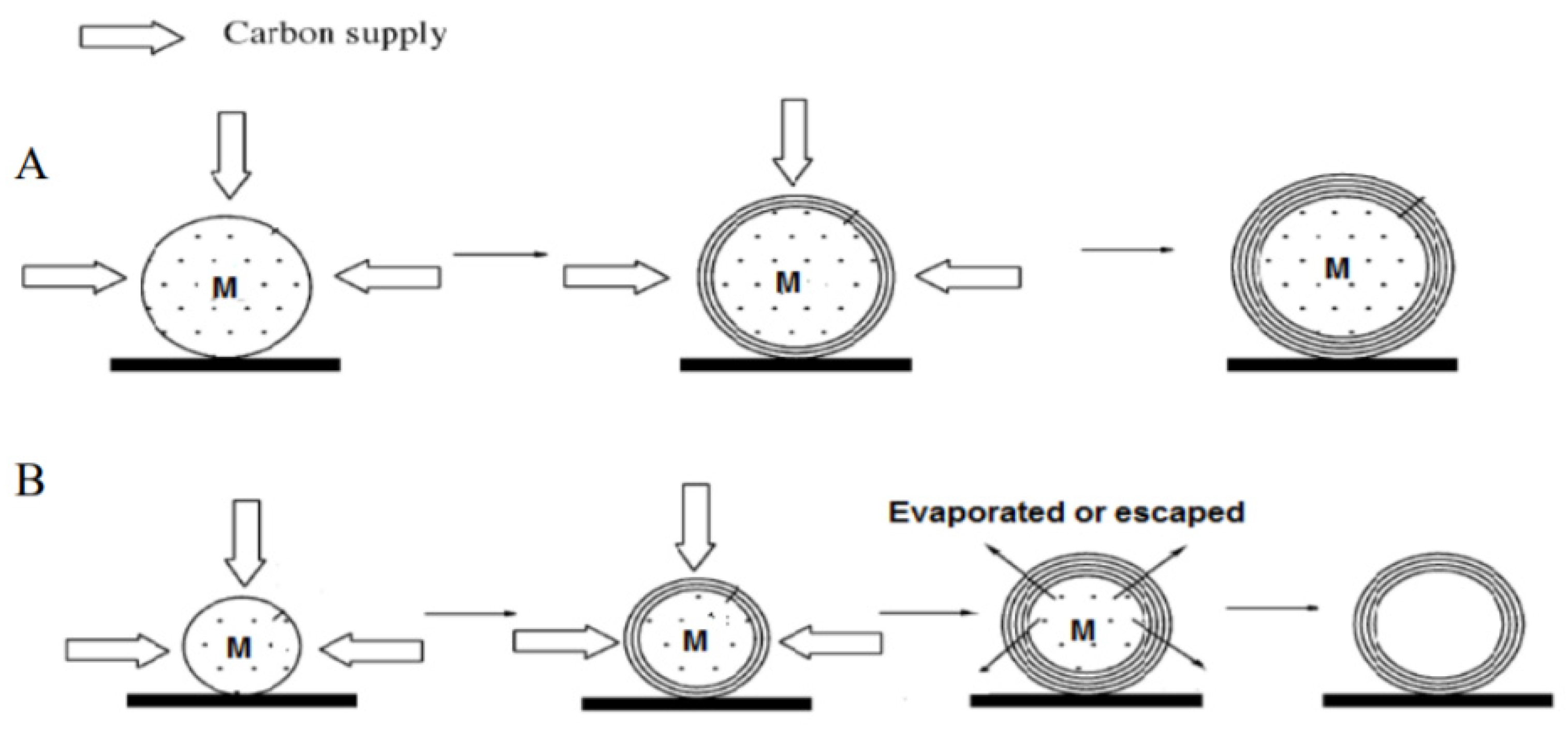
4.1. Bamboo-Shaped Carbon Nanotubes (BCNTs)
4.1.1. Formation Mechanism of BCNTs
4.1.2. Influencing Factors and Related Experiments of BCNTs
4.2. Octopus-Shaped Carbon Nanofibers (OCNFs)
4.2.1. Formation Mechanism of OCNFs
4.2.2. Influencing Factors and Related Experiments of OCNFs
4.3. Carbon Nano-Onions (CNOs)
4.3.1. Formation Mechanism of CNOs
4.3.2. Influencing Factors and Related Experiments of CNOs
5. Catalyst Recovery Methods
5.1. Separation Methods
5.2. Regeneration Methods
6. Challenges and Perspectives of CDM Hydrogen Generation Method
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDM | Catalyst decomposition of methane |
| SMR | Steam gas reforming |
| CCS | Carbon capture and sequestration technology |
| WGS | Water-gas shifting units |
| HREELS | High-resolution electron energy loss spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| DFT | Density-functional theory |
| LEED | Low energy electron diffraction |
| AES | Auger electron spectroscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| TPR | Temperature-programmed reduction |
| CNTs | Carbon nanotubes |
| BCNTs | Bamboo-shaped carbon nanotubes |
| OCNFs | Octopus-shaped carbon nanofibers |
| CNOs | Carbon nano-onions |
Appendix A. Parametric Study between Various Hydrogen Generation Methods
| Steam Methane Reforming (SMR) | Catalytic Decomposition of Methane (CDM) | Autothermal Reforming (ATR) | Dry Reforming of Methane (DRM) | Partial Oxidation (POX) | Coal Gasification | Water Electrolysis | Photovoltaics-Based Electrolysis | |
|---|---|---|---|---|---|---|---|---|
| Technical mature | Industrialized | Lab stage | Industrialized | Lab stage | Industrialized | Industrialized | Lab stage | Lab stage |
| Energy efficiency | 65–75% [105] | ~55% | 40–45% [106] | ~60% [107] | 46.8% [108] | 70.4–87.1% (Dep on coal type) [109] | ~80% [110] | 42% (theoretical max); 8–14% (currently) [111] |
| Energy required for per mole hydrogen (kJ/mol) | 41.21 | 37.4 | −18 to 68.72 [112] | 123.5 | −5.65 | Complex steps [113] | 286 [114] | 286 [114] |
| Reaction temperature (°C) | 700–1000 | 500–800 | 900–1100 | 600–950 [115] | 550–1258 (non-catalysts); 300–800 (with catalysts) [116] | ≥700 | Room temperature or 700–1000 (for steam) | 25–65 [117] |
| Reaction Pressure (bar) | 3–25 | 1.013 | 30–80 [118] | ~1.013 | 8 [119] | 5–14 | 1.013 or high pressure (120–200) | 1.013 |
| By-products | CO, CO2 & Sulfide (trace) | Solid carbon, CO & CO2 (trace) | CO & CO2 | Solid carbon, CO & CO2 (trace) | Solid carbon, CO & CO2 (trace) | coke, coal tar, sulfur and ammonia | None | None |
| CO2 emission (kg CO2/kg H2) | 7 | Can be negligible | ~3 [120] | 2.34 [121] | 18–20 [122] | Can be negligible | Can be negligible | |
| Catalyst lifetime | Stable | Regeneration cycle required | Stable | Unstable | Unstable | - | - | - |
| Cost ($/kg) | 2.08–2.27 [123] | Depend on catalysts | 1.48 [124] | Depend on catalysts | Expensive (due to pure oxygen) [108] | 1.34–1.63 [123] | 4 | 10.36 [123] |
References
- Delbeke, J.; Runge-Metzger, A.; Slingenberg, Y.; Werksman, J. The paris agreement. In Towards a Climate-Neutral Europe Curbing Trend; Routledge: London, UK, 2019; pp. 24–45. Available online: http://hdl.handle.net/1814/65859 (accessed on 28 February 2022).
- Nationally Determined Contributions (NDCs). United Nations Climate Change 2020. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/nationally-determined-contributions-ndcs/nationally-determined-contributions-ndcs (accessed on 21 December 2021).
- World Energy Council. World Energy Trilemma Index. 2021. Available online: https://www.worldenergy.org/transition-toolkit/world-energy-trilemma-index (accessed on 28 February 2022).
- World Energy Council. Energy Trilemma Index for Singapore. 2020. Available online: https://trilemma.worldenergy.org/#!/country-profile?country=Singapore&year=2021 (accessed on 21 December 2021).
- IEA. The Future of Hydrogen: Seizing Today’s Opportunities; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe—A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2020, 228, 113649. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- FSR. Between Green and Blue: A debate on Turquoise Hydrogen. In Florence School of Regulation; EUI: Florence, Italy, 2021; Available online: https://fsr.eui.eu/between-green-and-blue-a-debate-on-turquoise-hydrogen/ (accessed on 28 February 2022).
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Simpson, A.P.; Lutz, A.E. Exergy analysis of hydrogen production via steam methane reforming. Int. J. Hydrogen Energy 2007, 32, 4811–4820. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Kansha, Y.; Tsutsumi, A. Optimization of steam methane reforming coupled with pressure swing adsorption hydrogen production process by heat integration. Appl. Energy 2015, 154, 392–401. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for CO2-Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, B.B.; Koyama, S.; Ng, K.C.; Yoon, S.H. Thermodynamic trends in the uptake capacity of porous adsorbents on methane and hydrogen. Appl. Phys. Lett. 2008, 92, 90–93. [Google Scholar] [CrossRef]
- Banu, A.; Bicer, Y. Review on COx-free hydrogen from methane cracking: Catalysts, solar energy integration and applications. Energy Convers. Manag. X 2021, 12, 100117. [Google Scholar] [CrossRef]
- Maag, G.; Zanganeh, G.; Steinfeld, A. Solar thermal cracking of methane in a particle-flow reactor for the co-production of hydrogen and carbon. Int. J. Hydrogen Energy 2009, 34, 7676–7685. [Google Scholar] [CrossRef]
- Lumbers, B.; Agar, D.W.; Gebel, J.; Platte, F. Mathematical modelling and simulation of the thermo-catalytic decomposition of methane for economically improved hydrogen production. Int. J. Hydrogen Energy 2022, 47, 4265–4283. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Shen, T.; Lu, Y.; Chen, K.; Tu, Z.; Lu, S.; Wang, D. A Low-Temperature Carbon Encapsulation Strategy for Stable and Poisoning-Tolerant Electrocatalysts. Small Methods 2021, 5, 2100937. [Google Scholar] [CrossRef] [PubMed]
- Msheik, M.; Rodat, S.; Abanades, S. Methane cracking for hydrogen production: A review of catalytic and molten media pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Soltani, R.; Rosen, M.A.; Dincer, I. Assessment of CO2 capture options from various points in steam methane reforming for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 20266–20275. [Google Scholar] [CrossRef]
- Lee, M.B.; Yang, Q.Y.; Ceyer, S.T. Dynamics of the activated dissociative chemisorption of CH4 and implication for the pressure gap in catalysis: A molecular beam-high resolution electron energy loss study. J. Chem. Phys. 1987, 87, 2724–2741. [Google Scholar] [CrossRef]
- Fuhrmann, T.; Kinne, M.; Whelan, C.M.; Zhu, J.F.; Denecke, R.; Steinrück, H.P. Vibrationally resolved in situ XPS study of activated adsorption of methane on Pt(111). Chem. Phys. Lett. 2004, 390, 208–213. [Google Scholar] [CrossRef]
- Oakes, D.J.; Newell, H.E.; Rutten, F.J.M.; McCoustra, M.R.S.; Chesters, M.A. The chemistry of simple alkyl species on Pt(111) generated by hyperthermal collisions. J. Vac. Sci. Technol. A Vac. Surf. Film. 1996, 14, 1439–1447. [Google Scholar] [CrossRef]
- Salam, M.A.; Abdullah, B. Catalysis mechanism of Pd-promoted γ-alumina in the thermal decomposition of methane to hydrogen: A density functional theory study. Mater. Chem. Phys. 2017, 188, 18–23. [Google Scholar] [CrossRef]
- Zavarukhin, S.G.; Kuvshinov, G.G. The kinetic model of formation of nanofibrous carbon from CH4-H2 mixture over a high-loaded nickel catalyst with consideration for the catalyst deactivation. Appl. Catal. A Gen. 2004, 272, 219–227. [Google Scholar] [CrossRef]
- Borghei, M.; Karimzadeh, R.; Rashidi, A.; Izadi, N. Kinetics of methane decomposition to COx-free hydrogen and carbon nanofiber over Ni-Cu/MgO catalyst. Int. J. Hydrogen Energy 2010, 35, 9479–9488. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Daud, W.M.A.W.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef] [Green Version]
- Grabke, H.J. Evidence on the surface concentration of carbon on gamma iron from the kinetics of the carburization in CH4-H2. Metall. Trans. 1970, 1, 2972–2975. [Google Scholar] [CrossRef]
- Grabke, H.J. Die Kinetik der Entkohlung und Aufkohlung. Ber. Bunsenges. Phys. Chem. 1965, 69, 409–414. [Google Scholar] [CrossRef]
- Tait, S.L.; Dohnálek, Z.; Campbell, C.T.; Kay, B.D. Methane adsorption and dissociation and oxygen adsorption and reaction with CO on Pd nanoparticles on MgO(1 0 0) and on Pd(1 1 1). Surf. Sci. 2005, 591, 90–107. [Google Scholar] [CrossRef]
- Hamza, A.V.; Steinruck, H.P.; Madix, R.J. The dynamics of the dissociative adsorption of methane on Pt(533). J. Chem. Phys. 1987, 118, 3334–3341. [Google Scholar] [CrossRef]
- Mullins, C.B.; Weinberg, W.H. Dynamics of the interaction of ethane with Ir(110)-(1×2). J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 2458–2462. [Google Scholar] [CrossRef]
- Broclawik, E.; Haber, J.; Endou, A.; Stirling, A.; Yamauchi, R.; Kubo, M.; Miyamoto, A. Electronic structure and adsorption properties of precious metals and their oxides: Density functional calculations. J. Mol. Catal. A Chem. 1997, 119, 35–44. [Google Scholar] [CrossRef]
- Snoeck, J.W.; Froment, G.F.; Fowles, M. Kinetic study of the carbon filament formation by methane cracking on a nickel catalyst. J. Catal. 1997, 169, 250–262. [Google Scholar] [CrossRef]
- Demicheli, M.C.; Ponzi, E.N.; Ferretti, O.A.; Yeramian, A.A. Kinetics of carbon formation from CH4-H2 mixtures on nickel-alumina catalyst. Chem. Eng. J. 1991, 46, 129–136. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.; Ismagilov, Z.; Pushkarev, V.; Cherepanova, S.; Chuvilin, A.; Likholobov, V. Catalytic filamentous carbon: Structural and textural properties. Carbon N. Y. 2003, 41, 1605–1615. [Google Scholar] [CrossRef]
- Zhang, T.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl. Catal. A Gen. 1998, 167, 161–172. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Synthesis of Ni and Ni-Cu supported on carbon nanotubes for hydrogen and carbon production by catalytic decomposition of methane. Appl. Catal. B Environ. 2015, 164, 61–69. [Google Scholar] [CrossRef]
- Takenaka, S.; Serizawa, M.; Otsuka, K. Formation of filamentous carbons over supported Fe catalysts through methane decomposition. J. Catal. 2004, 222, 520–531. [Google Scholar] [CrossRef]
- Takenaka, S.; Ishida, M.; Serizawa, M.; Tanabe, E.; Otsuka, K. Formation of carbon nanofibers and carbon nanotubes through methane decomposition over supported cobalt catalysts. J. Phys. Chem. B 2004, 108, 11464–11472. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Methane decomposition over Ni, Co and Fe based monometallic catalysts supported on sol gel derived SiO2 microflakes. Chem. Eng. J. 2015, 262, 1009–1021. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Seshan, K.; al Otaibi, R.L.; Al-Fatesh, A.S. Hydrogen production via catalytic methane decomposition over alumina supported iron catalyst. Arab. J. Chem. 2018, 11, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, L.B.; Goncharova, O.; Kochubey, D.; Zaikovskii, V.; Plyasova, L.; Novgorodov, B.; Shaikhutdinov, S. Coprecipitated Ni-alumina and Ni-Cu-alumina catalysts of methane decomposition and carbon deposition. II. Evolution of the catalysts in reaction. Appl. Catal. A Gen. 1996, 141, 117–129. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, D.; Kuai, P. Catalytic decomposition of methane over Ni/Al2O3 catalysts: Effect of plasma treatment on carbon formation. Energy Fuels 2008, 22, 1480–1484. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Li, K.; Wang, W. Active Nb2O5-supported nickel and nickel-copper catalysts for methane decomposition to hydrogen and filamentous carbon. J. Mol. Catal. A Chem. 2004, 221, 105–112. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Kuvshinov, G.G.; Plyasova, L.M. New nickel catalysts for the formation of filamentous carbon in the reaction of methane decomposition. J. Catal. 1999, 187, 77–84. [Google Scholar] [CrossRef]
- Takenaka, S.; Kobayashi, S.; Ogihara, H.; Otsuka, K. Ni/SiO2 catalyst effective for methane decomposition into hydrogen and carbon nanofiber. J. Catal. 2003, 217, 79–87. [Google Scholar] [CrossRef]
- Takenaka, S.; Ogihara, H.; Yamanaka, I.; Otsuka, K. Decomposition of methane over supported-Ni catalysts: Effects of the supports on the catalytic lifetime. Appl. Catal. A Gen. 2001, 217, 101–110. [Google Scholar] [CrossRef]
- Avdeeva, L.B.; Kochubey, D.I.; Shaikhutdinov, S.K. Cobalt catalysts of methane decomposition: Accumulation of the filamentous carbon. Appl. Catal. A Gen. 1999, 177, 43–51. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Daud, W.M.A.W. Probing the differential methane decomposition behaviors of n-Ni/SiO2, n-Fe/SiO2 and n-Co/SiO2 catalysts prepared by co-precipitation cum modified Stöber method. RSC Adv. 2015, 5, 67227–67241. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Saih, Y.; Loptain, S.; Gary, D.; Del-Gallo, P.; Basset, J.-M. Catalytic Methane Decomposition over Fe-Al2O3. ChemSusChem 2016, 9, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, M.A.; Ermakov, D.Y. Ni/SiO2 and Fe/SiO2 catalysts for production of hydrogen and filamentous carbon via methane decomposition. Catal. Today 2002, 77, 225–235. [Google Scholar] [CrossRef]
- Kumar, M. Carbon Nanotube Synthesis and Growth Mechanism. Intech 2016, i, 13. [Google Scholar]
- Frusteri, F.; Italiano, G.; Espro, C.; Cannilla, C.; Bonura, G. H2 production by methane decomposition: Catalytic and technological aspects. Int. J. Hydrogen Energy 2012, 37, 16367–16374. [Google Scholar] [CrossRef]
- Italiano, G.; Delia, A.; Espro, C.; Bonura, G.; Frusteri, F. Methane decomposition over Co thin layer supported catalysts to produce hydrogen for fuel cell. Int. J. Hydrogen Energy 2010, 35, 11568–11575. [Google Scholar] [CrossRef]
- Wang, I.W.; Kutteri, D.A.; Gao, B.; Tian, H.; Hu, J. Methane Pyrolysis for Carbon Nanotubes and COx-Free H2 over Transition-Metal Catalysts. Energy Fuels 2019, 33, 197–205. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gedanken, A. Electrochemical properties of bamboo-shaped multiwalled carbon nanotubes generated by solid state pyrolysis. Electrochem. Commun. 2006, 8, 1099–1105. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Ma, Y.; Qin, Y.; Chang, L. Formation of bamboo-shaped carbon filaments and dependence of their morphology on catalyst composition and reaction conditions. Carbon N. Y. 2001, 39, 1467–1475. [Google Scholar] [CrossRef]
- Saito, Y. Nanoparticles and filled nanocapsules. Carbon N. Y. 1995, 33, 979–988. [Google Scholar] [CrossRef]
- Saito, Y.; Yoshikawa, T. Bamboo-shaped carbon tube filled partially with nickel. J. Cryst. Growth 1993, 134, 154–156. [Google Scholar] [CrossRef]
- Zhao, N.Q.; He, C.; Ding, J.; Zou, T.; Qiao, Z.; Shi, C.; Du, X.; Li, J.; Li, Y. Bamboo-shaped carbon nanotubes produced by catalytic decomposition of methane over nickel nanoparticles supported on aluminum. J. Alloys Compd. 2007, 428, 79–83. [Google Scholar] [CrossRef]
- Lin, M.; Tan, J.P.Y.; Boothroyd, C.; Loh, K.P.; Tok, E.S.; Foo, Y.L. Dynamical observation of bamboo-like carbon nanotube growth. Nano Lett. 2007, 7, 2234–2238. [Google Scholar] [CrossRef]
- Lv, R.; Zou, L.; Gui, X.; Kang, F.; Zhu, Y.; Zhu, H.; Wei, J.; Gu, J.; Wang, K.; Wu, D. High-yield bamboo-shaped carbon nanotubes from cresol for electrochemical application. Chem. Commun. 2008, 17, 2046–2048. [Google Scholar] [CrossRef]
- González, I.; de Jesus, J.; Cañizales, E. Bamboo-shaped carbon nanotubes generated by methane thermal decomposition using Ni nanoparticles synthesized in water-oil emulsions. Micron 2011, 42, 819–825. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Pant, K.K. Synthesis of hydrogen and carbon nanotubes over copper promoted Ni/SiO2 catalyst by thermocatalytic decomposition of methane. J. Nat. Gas Sci. Eng. 2013, 13, 52–59. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Xie, X.; Liu, J.; Xu, Y.; Shen, W. Novel Ni catalysts for methane decomposition to hydrogen and carbon nanofibers. J. Catal. 2006, 238, 412–424. [Google Scholar] [CrossRef]
- Li, J.; Dong, L.; Xiong, L.; Yang, Y.; Du, Y.; Zhao, L.; Wang, H.; Peng, S. High-loaded Ni[sbnd]Cu[sbnd]SiO2 catalysts for methane decomposition to prepare hydrogen and carbon filaments. Int. J. Hydrogen Energy 2016, 41, 12038–12048. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Z.; Zhang, X. Production of COx-free hydrogen and nanocarbon by direct decomposition of undiluted methane on Ni-Cu-alumina catalysts. Appl. Catal. A Gen. 2004, 269, 179–186. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Tapia, A.A.; Ould-Chikh, S.; Hazemann, J.-L.; Li, J.; Wei, N.; Gary, D.; et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl. Catal. B Environ. 2017, 208, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Harb, M.; Enakonda, L.R.; Almana, N.; Hedhili, M.N.; Basset, J.M. Reverse microemulsion prepared Ni-Pt catalysts for methane cracking to produce CO:X-free hydrogen. RSC Adv. 2017, 7, 43546–43550. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Kou, K.; Qin, M.; Wu, H.; Puleo, F.; Liotta, L.F. Controllable and large-scale synthesis of carbon nanostructures: A review on bamboo-like Nanotubes. Catalysts 2017, 7, 256. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, M.A.; Ermakov, D.Y.; Cherepanova, S.V.; Plyasova, L.M. Synthesis of ultradispersed nickel particles by reduction of high-loaded NiO-SiO2 systems prepared by heterophase sol-gel method. J. Phys. Chem. B 2002, 106, 11922–11928. [Google Scholar] [CrossRef]
- Monthioux, M.; Noé, L.; Dussault, L.; Dupin, J.-C.; Latorre, N.; Ubieto, T.; Romeo, E.; Royo, C.; Monzón, A.; Guimon, C. Texturising and structurising mechanisms of carbon nanofilaments during growth. J. Mater. Chem. 2007, 17, 4611–4618. [Google Scholar] [CrossRef]
- Muradov, N.; Chen, Z.; Smith, F. Fossil hydrogen with reduced CO2 emission: Modeling thermocatalytic decomposition of methane in a fluidized bed of carbon particles. Int. J. Hydrogen Energy 2005, 30, 1149–1158. [Google Scholar] [CrossRef]
- Alstrup, I. A new model explaining carbon filament growth on nickel, iron, and NiCu alloy catalysts. J. Catal. 1988, 109, 241–251. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Dupuis, A.C. The catalyst in the CCVD of carbon nanotubes-a review. Prog. Mater. Sci. 2005, 50, 929–961. [Google Scholar] [CrossRef]
- Schouten, F.C.; Gijzeman, O.L.J.; Bootsma, G.A. Interaction of methane with Ni(111) and Ni(100); diffusion of carbon into nickel through the (100) surface; An aes-leed study. Surf. Sci. 1979, 87, 1–12. [Google Scholar] [CrossRef]
- Schouten, F.C.; Kaleveld, E.W.; Bootsma, G.A. AES-LEED-ellipsometry study of the kinetics of the interaction of methane with Ni(110). Surf. Sci. 1977, 63, 460–474. [Google Scholar] [CrossRef]
- Ponec, G.B.V. Catalysis by Metals and Alloys, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1995; Available online: https://www.elsevier.com/books/catalysis-by-metals-and-alloys/ponec/978-0-444-89796-1 (accessed on 28 February 2022).
- Li, D.; Chen, J.; Li, Y. Evidence of composition deviation of metal particles of a Ni-Cu/Al2O3 catalyst during methane decomposition to COx-free hydrogen. Int. J. Hydrogen Energy 2009, 34, 299–307. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Vieira, R.; Louis, B.; Carvalho, A.; Amadou, J.; Dintzer, T.; LeDoux, M.J. About the octopus-like growth mechanism of carbon nanofibers over graphite supported nickel catalyst. J. Catal. 2006, 240, 194–202. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Chuvilin, A.L.; Ushakov, V.A. Carbon capacious Ni-Cu-Al2O3 catalysts for high-temperature methane decomposition. Appl. Catal. A Gen. 2003, 247, 51–63. [Google Scholar] [CrossRef]
- Takenaka, S.; Shigeta, Y.; Tanabe, E.; Otsuka, K. Methane decomposition into hydrogen and carbon nanofibers over supported Pd-Ni catalysts. J. Catal. 2003, 220, 468–477. [Google Scholar] [CrossRef]
- Wang, H.; Baker, R.T.K. Decomposition of methane over a Ni-Cu-MgO catalyst to produce hydrogen and carbon nanofibers. J. Phys. Chem. B 2004, 108, 20273–20277. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Chuvilin, A.L.; Kuvshinov, G.G. Decomposition of methane over iron catalysts at the range of moderate temperatures: The influence of structure of the catalytic systems and the reaction conditions on the yield of carbon and morphology of carbon filaments. J. Catal. 2001, 201, 183–197. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Buyanov, R.A.; Mishakov, I.V.; Zaikovskii, V.I. Effect of zinc added to the Co/Al2O3 catalyst on the formation of carbon nanofilaments from methane and butadiene-1,3. Kinet. Catal. 2006, 47, 445–450. [Google Scholar] [CrossRef]
- Boretti, A. A perspective on the production of hydrogen from solar-driven thermal decomposition of methane. Int. J. Hydrogen Energy 2021, 46, 34509–34514. [Google Scholar] [CrossRef]
- Wang, M.J.; Gray, C.A.; Reznek, S.R.; Mahmud, K.; Kutsovsky, Y. Carbon Black. Transport 2004, 4, 21–24. [Google Scholar]
- Iijima, S. Direct Observation of the Tetrahedral Bonding in Graphitized Carbon Black By. J. Cryst. Growth 1980, 50, 675–683. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef]
- He, C.; Zhao, N.; Shi, C.; Du, X.; Li, J. Carbon nanotubes and onions from methane decomposition using Ni/Al catalysts. Mater. Chem. Phys. 2006, 97, 109–115. [Google Scholar] [CrossRef]
- Kang, J.L.; Li, J.; Du, X.; Shi, C.; Zhao, N.; Cui, L.; Nash, P. Synthesis and growth mechanism of metal filled carbon nanostructures by CVD using Ni/Y catalyst supported on copper. J. Alloys Compd. 2008, 456, 290–296. [Google Scholar] [CrossRef]
- Keller, M.; Matsumura, A.; Sharma, A. Spray-dried Fe/Al2O3 as a carbon carrier for COx-free hydrogen production via methane cracking in a fluidized bed process. Chem. Eng. J. 2020, 398, 125612. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, L.; Zhang, M.; Cui, J.; Li, Y.; Gao, L.; Zhang, S. Methane catalytic cracking to make hydrogen and graphitic nano carbons (Nanotubes, Microfibers, Microballs, Onions) with zero emission. Synth. React. Inorg. Met. Nano-Met. Chem. 2014, 44, 1166–1174. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the Effect of Nickel Particle Size Decomposition Reaction. Catalysts 2020, 10, 890. [Google Scholar] [CrossRef]
- Cornejo, A.; Zhang, W.; Gao, L.; Varsani, R.R.; Saunders, M.; Iyer, K.S.; Raston, C.L.; Chua, H.T. Generating hydrogen gas from methane with carbon captured as pure spheroidal nanomaterials. Chem.-A Eur. J. 2011, 17, 9188–9192. [Google Scholar] [CrossRef]
- Ahlawat, J.; Asil, S.M.; Barroso, G.G.; Nurunnabi, M.; Narayan, M. Application of carbon nano onions in the biomedical field: Recent advances and challenges. Biomater. Sci. 2021, 9, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Myung, Y.S.; Das, G.S.; Bhatnagar, A.; Park, J.-W.; Tripathi, K.M.; Kim, T. Carbon nano-onions from waste oil for application in energy storage devices. New J. Chem. 2020, 44, 7369–7375. [Google Scholar] [CrossRef]
- Postels, S.; Abánades, A.; von der Assen, N.; Rathnam, R.K.; Stückrad, S.; Bardow, A. Life cycle assessment of hydrogen production by thermal cracking of methane based on liquid-metal technology. Int. J. Hydrogen Energy 2016, 41, 23204–23212. [Google Scholar] [CrossRef]
- Steinberg, M.; Dong, Y. Method for Convertng Natural Gas and Carbon Doxde to Methanoland Reducing Co, Emissions. U.S. Patent 5,767,165, 16 June 1998. Available online: https://patents.google.com/patent/US5767165A/en (accessed on 28 February 2022).
- Rahman, M.S.; Croiset, E.; Hudgins, R.R. Catalytic decomposition of methane for hydrogen production. Top. Catal. 2006, 37, 137–145. [Google Scholar] [CrossRef]
- Otsuka, K.; Takenaka, S.; Ohtsuki, H. Production of pure hydrogen by cyclic decomposition of methane and oxidative elimination of carbon nanofibers on supported-Ni-based catalysts. Appl. Catal. A Gen. 2004, 273, 113–124. [Google Scholar] [CrossRef]
- Aiello, R.; Fiscus, J.E.; Loye, H.C.Z.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over Ni/SiO2: Catalyst deactivation and regeneration. Appl. Catal. A Gen. 2000, 192, 227–234. [Google Scholar] [CrossRef]
- Cai, L.; He, T.; Xiang, Y.; Guan, Y. Study on the reaction pathways of steam methane reforming for H2 production. Energy 2020, 207, 118296. [Google Scholar] [CrossRef]
- De Smet, C.R.H.; Berger, R.J.; Slaa, J.C.; Marin, G.B. Kinetic modelling of the partial oxidation of methane to syn-gas at high temperatures. Stud. Surf. Sci. Catal. 1998, 119, 825–829. [Google Scholar] [CrossRef]
- Snoeckx, R.; Zeng, Y.X.; Tu, X.; Bogaerts, A. Plasma-based dry reforming: Improving the conversion and energy efficiency in a dielectric barrier discharge. RSC Adv. 2015, 5, 29799–29808. [Google Scholar] [CrossRef]
- Dias, P.; Lopes, T.; Andrade, L.; Mendes, A. Temperature effect on water splitting using a Si-doped hematite photoanode. J. Power Sources 2014, 272, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.; Lee, C.H. Evaluation of the energy efficiency of the shell coal gasification process by coal type. Energy Convers. Manag. 2017, 143, 123–136. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Tasic, G.S.; Maksic, A.D.; Saponjic, D.P.; Miulovic, S.M.; Kaninski, M.P.M. Raising efficiency of hydrogen generation from alkaline water electrolysis—Energy saving. Int. J. Hydrogen Energy 2010, 35, 12369–12373. [Google Scholar] [CrossRef]
- Hydrogen and Fuel Cell Technologies Office DOE Technical Targets for Hydrogen Production from Electrolysis. In Energy Efficiency & Renewable Energy; EERE: Washington, DC, USA, 2020. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-production-electrolysis (accessed on 28 February 2022).
- Voitic, G.; Pichler, B.; Basile, A.; Malli, K.; Bock, S.; Hacker, V. Chapter 10—Hydrogen Production. 2018. Available online: https://www.sciencedirect.com/science/article/pii/B9780128114599000104?via%3Dihub#! (accessed on 28 February 2022).
- Mishra, A.; Gautam, S.; Sharma, T. Effect of operating parameters on coal gasification. Int. J. Coal Sci. Technol. 2018, 5, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, W.; Liu, T.; Liu, X.; Hu, X. Thermodynamic analysis and optimization of photovoltaic/thermal hybrid hydrogen generation system based on complementary combination of photovoltaic cells and proton exchange membrane electrolyzer. Energy Convers. Manag. 2019, 183, 97–108. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E. Carbon dioxide reforming of methane to synthesis gas over Ni/La2O3 catalysts. Appl. Catal. A Gen. 1996, 138, 109–133. [Google Scholar] [CrossRef]
- Konnov, A.A.; Zhu, J.N.; Bromly, J.H.; Zhang, D.K. Noncatalytic partial oxidation of methane into syngas over a wide temperature range. Combust. Sci. Technol. 2004, 176, 1093–1116. [Google Scholar] [CrossRef]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-precious electrodes for practical alkaline water electrolysis. Materials 2019, 12, 1336. [Google Scholar] [CrossRef] [Green Version]
- Copeland, R.; Gershanovich, Y.; Windecker, B. High Efficiency Syngas Generation; 2005; pp. 1–30. Available online: http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=840258 (accessed on 28 February 2022).
- Mantripragada, H.C.; Veser, G. Chemical Looping Partial Oxidation of Methane for Co-Production of Syngas and Electricity: Process Modeling and Systems Analysis. Energy Technol. 2020, 8, 1900580. [Google Scholar] [CrossRef]
- Salemme, L.; Menna, L.; Simeone, M. Analysis of the energy efficiency of innovative ATR-based PEM fuel cell system with hydrogen membrane separation. Int. J. Hydrogen Energy 2009, 34, 6384–6392. [Google Scholar] [CrossRef]
- Er-rbib, H.; Bouallou, C.; Werkoff, F. Dry reforming of methane—Review of feasibility studies. Chem. Eng. Trans. 2012, 29, 163–168. [Google Scholar] [CrossRef]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Solar Hydrogen Production | ScienceDirect. 2019. Available online: https://www.sciencedirect.com/book/9780128148532/solar-hydrogen-production (accessed on 28 February 2022).
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef] [Green Version]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, S.; Miao, B.; Zhang, L.; Chan, S.H. Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms. Energies 2022, 15, 2573. https://doi.org/10.3390/en15072573
Tong S, Miao B, Zhang L, Chan SH. Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms. Energies. 2022; 15(7):2573. https://doi.org/10.3390/en15072573
Chicago/Turabian StyleTong, Sirui, Bin Miao, Lan Zhang, and Siew Hwa Chan. 2022. "Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms" Energies 15, no. 7: 2573. https://doi.org/10.3390/en15072573
APA StyleTong, S., Miao, B., Zhang, L., & Chan, S. H. (2022). Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms. Energies, 15(7), 2573. https://doi.org/10.3390/en15072573






