High Solid and Low Cellulase Enzymatic Hydrolysis of Cardoon Stems Pretreated by Acidified γ-Valerolactone/Water Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Experiment Approach
2.3. Microwave-Assisted OV Pretreatment
2.4. Enzymatic Hydrolysis
2.5. Laboratory Analytical Procedure
3. Results and Discussion
3.1. Characterization of CP and Aqueous Fraction Obtained from OV PreTreatment
3.2. Optimization of Cellulose Recovery and Enrichment
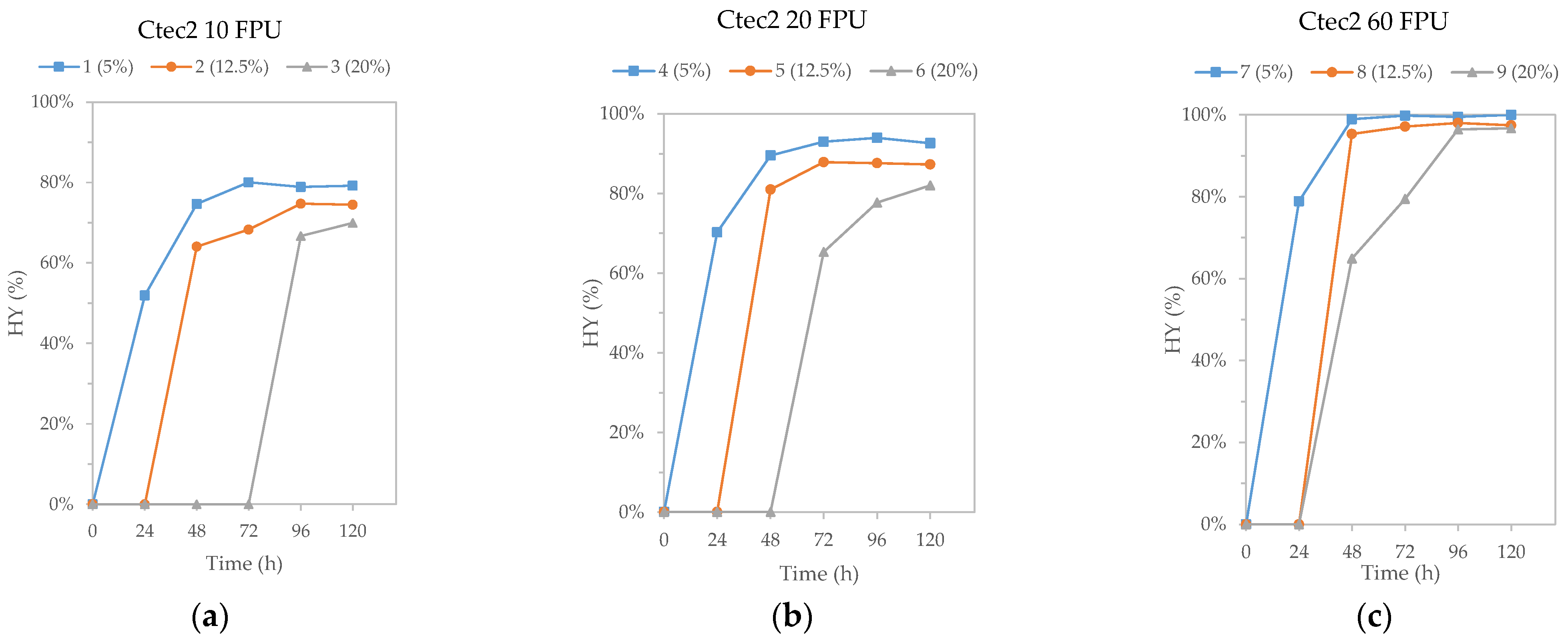
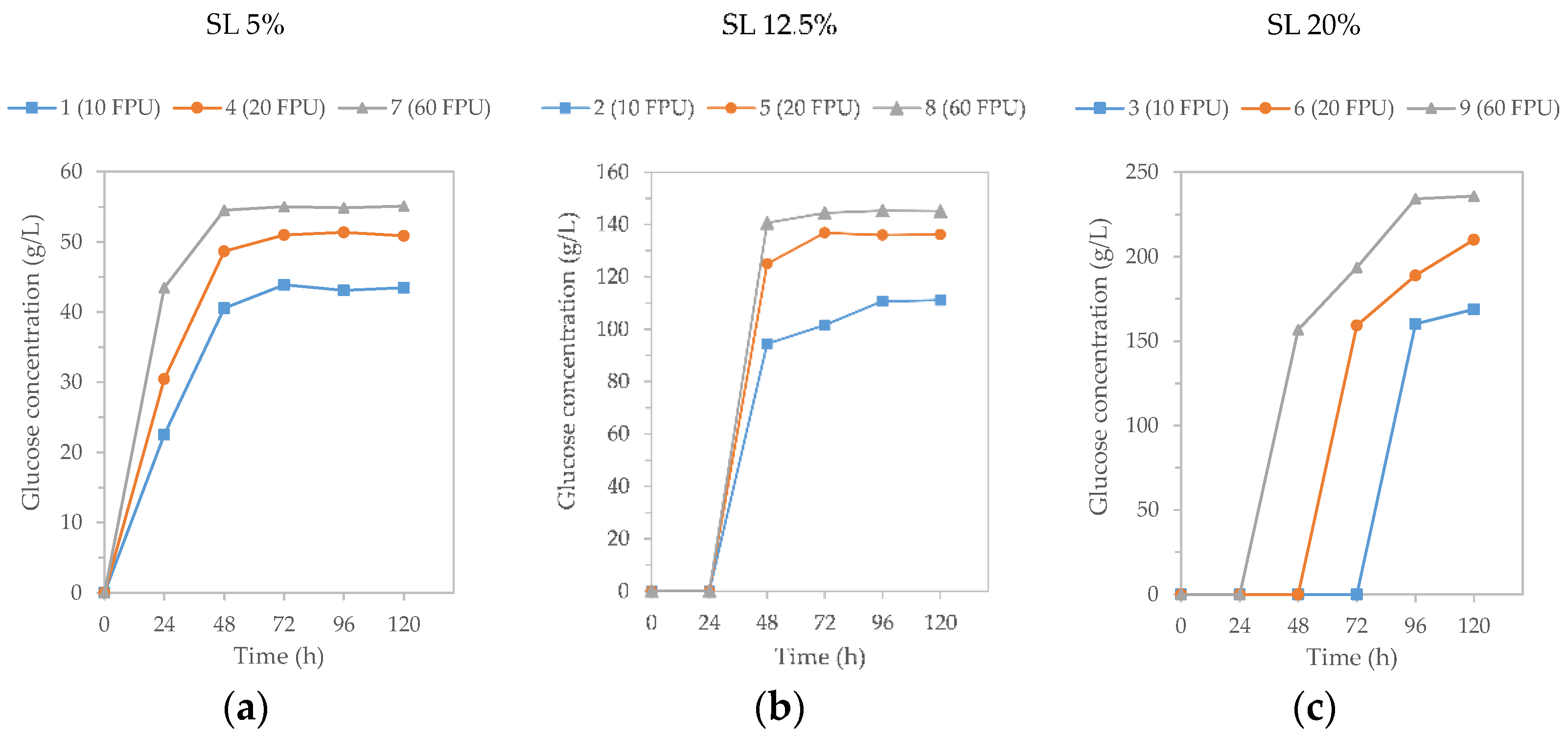
3.3. High Solid and Low Cellulase Enzymatic Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Nattrass, L.; Alberts, G.; Robson, P.; Chudziak, C.; Bauen, A.; Libelli, I.; Lotti, G.; Prussi, M.; Nistri, R. From the Sugar Platform to Biofuels and Biochemicals: Final Report for the European Commission Directorate-General Energy. E4tech/Re-CORD/Wageningen UR 2015, 183, 492297. [Google Scholar]
- Lange, J.P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Biorefin. Innov. A Sustain. Econ. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Brethauer, S.; Studer, M.H. Biochemical conversion processes of lignocellulosic biomass to fuels and chemicals—A review. CHIMIA Int. J. Chem. 2015, 69, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Mauromicale, G.; Sortino, O.; Pesce, G.R.; Agnello, M.; Mauro, R.P. Suitability of cultivated and wild cardoon as a sustainable bioenergy crop for low input cultivation in low quality Mediterranean soils. Ind. Crops Prod. 2014, 57, 82–89. [Google Scholar] [CrossRef]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a sustainable crop for biomass and bioactive compounds production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef]
- Shatalov, A.; Pereira, H. Biorefinery of energy crop cardoon (Cynara cardunculus L.)-hydrolytic xylose production as entry point to complex fractionation scheme. J. Chem. Eng. Process Technol. 2011, 2, 18–20. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Dissolving grade eco-clean cellulose pulps by integrated fractionation of cardoon (Cynara cardunculus L.) stalk biomass. Chem. Eng. Res. Des. 2014, 92, 2640–2648. [Google Scholar] [CrossRef]
- Van Dyk, J.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef] [Green Version]
- Raj, K.; Krishnan, C. Improved high solid loading enzymatic hydrolysis of low-temperature aqueous ammonia soaked sugarcane bagasse using laccase-mediator system and high concentration ethanol production. Ind. Crops Prod. 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Wyman, C.; Dale, B. Producing biofuels via the sugar platform. Chem. Eng. Prog. 2015, 111, 45–57. [Google Scholar]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, P.; Liu, H.; Wu, K.; Xiao, W.; Gong, Y.; Lin, J.; Liu, Z. Ethanol production from xylan-removed sugarcane bagasse using low loading of commercial cellulase. Bioresour. Technol. 2014, 163, 390–394. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Azarpira, A.; Motagamwala, A.H.; Lu, F.; Ralph, J.; Dumesic, J.A. Lignin monomer production integrated into the γ-valerolactone sugar platform. Energy Environ. Sci. 2015, 8, 2657–2663. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Zhao, X.; Sun, R.-c.; Mu, T. Biomass-derived γ-valerolactone-based solvent systems for highly efficient dissolution of various lignins: Dissolution behavior and mechanism study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Banu, R.; Yoon, J.-J.; Kumar, G.; Kim, S.-H. Synthesis of γ-valerolactone (GVL) and their applications for lignocellulosic deconstruction for sustainable green biorefineries. Fuel 2021, 303, 121333. [Google Scholar] [CrossRef]
- Gelosia, M.; Bertini, A.; Barbanera, M.; Giannoni, T.; Nicolini, A.; Cotana, F.; Cavalaglio, G. Acid-Assisted Organosolv Pre-Treatment and Enzymatic Hydrolysis of Cynara cardunculus L. for Glucose Production. Energies 2020, 13, 4195. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Gelosia, M.; Giannoni, T.; Barros Lovate Temporim, R.; Nicolini, A.; Cotana, F.; Bertini, A. Acid-catalyzed steam explosion for high enzymatic saccharification and low inhibitor release from lignocellulosic cardoon stalks. Biochem. Eng. J. 2021, 174, 108121. [Google Scholar] [CrossRef]
- Ballesteros, I.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Oliva, J.M.; Sáez, F. Dilute sulfuric acid pretreatment of cardoon for ethanol production. Biochem. Eng. J. 2008, 42, 84–91. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.; Chaves, H.T.; Evtuguin, D.V.; Xavier, A.M. Comparative study on hydrolysis and bioethanol production from cardoon and rockrose pretreated by dilute acid hydrolysis. Ind. Crops Prod. 2018, 111, 633–641. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.; Mendes, J.A.; Gravitis, J.; Evtuguin, D.V.; Xavier, A.M. Enzymatic saccharification and bioethanol production from Cynara cardunculus pretreated by steam explosion. Bioresour. Technol. 2015, 186, 309–315. [Google Scholar] [CrossRef]
- Vergara, P.; Ladero, M.; García-Ochoa, F.; Villar, J.C. Pre-treatment of corn stover, Cynara cardunculus L. stems and wheat straw by ethanol-water and diluted sulfuric acid: Comparison under different energy input conditions. Bioresour. Technol. 2018, 270, 449–456. [Google Scholar] [CrossRef]
- Giannoni, T.; Gelosia, M.; Bertini, A.; Fabbrizi, G.; Nicolini, A.; Coccia, V.; Iodice, P.; Cavalaglio, G. Fractionation of Cynara cardunculus L. by Acidified Organosolv Treatment for the Extraction of Highly Digestible Cellulose and Technical Lignin. Sustainability 2021, 13, 8714. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2010, 1617, 1–16. [Google Scholar]
- Chotirotsukon, C.; Raita, M.; Yamada, M.; Nishimura, H.; Watanabe, T.; Laosiripojana, N.; Champreda, V. Sequential fractionation of sugarcane bagasse using liquid hot water and formic acid-catalyzed glycerol-based organosolv with solvent recycling. BioEnergy Res. 2021, 14, 135–152. [Google Scholar] [CrossRef]
- Sánchez, O.; Sierra, R.; Alméciga-Díaz, C.J. Delignification process of agro-industrial wastes an alternative to obtain fermentable carbohydrates for producing fuel. Altern. Fuel 2011, 7, 111–154. [Google Scholar]
- Ruiz, H.A.; Conrad, M.; Sun, S.-N.; Sanchez, A.; Rocha, G.J.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crops Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Du, J.; Cao, Y.; Liu, G.; Zhao, J.; Li, X.; Qu, Y. Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour. Technol. 2017, 229, 88–95. [Google Scholar] [CrossRef]
- da Silva, A.S.A.; Espinheira, R.P.; Teixeira, R.S.S.; de Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P.S. Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: A critical review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 2014, 343, 277–280. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Guo, H.; Huang, C.; Xiong, L.; Chen, X. Kinetic study on the liquefaction of wood and its three cell wall component in polyhydric alcohols. Appl. Energy 2014, 113, 1596–1600. [Google Scholar] [CrossRef]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I. Characterization of the promising poly (3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef]
- Zhang, X.; Wilson, K.; Lee, A.F. Heterogeneously catalyzed hydrothermal processing of C5–C6 sugars. Chem. Rev. 2016, 116, 12328–12368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A short overview on the hydrogen production via aqueous phase reforming (APR) of cellulose, C6-C5 sugars and polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhang, S.; Hu, H.; Duan, C.; He, Z.; Ni, Y. Separation of hemicellulose and cellulose from wood pulp using a γ-valerolactone (GVL)/water mixture. Sep. Purif. Technol. 2020, 248, 117071. [Google Scholar] [CrossRef]
- Trevorah, R.M.; Huynh, T.; Brkljača, R.; Othman, M.Z. Structural and Morphological Analysis of Cellulose Pulp Produced from the Fractionation of Eucalyptus obliqua Sawdust Using γ-Valerolactone. ACS Omega 2021, 6, 4126–4136. [Google Scholar] [CrossRef]
- Smith, M.D.; Cheng, X.; Petridis, L.; Mostofian, B.; Smith, J.C. Organosolv-water cosolvent phase separation on cellulose and its influence on the physical deconstruction of cellulose: A molecular dynamics analysis. Sci. Rep. 2017, 7, 14494. [Google Scholar] [CrossRef] [Green Version]
- Wojtusik, M.; Zurita, M.; Villar, J.C.; Ladero, M.; Garcia-Ochoa, F. Influence of fluid dynamic conditions on enzymatic hydrolysis of lignocellulosic biomass: Effect of mass transfer rate. Bioresour. Technol. 2016, 216, 28–35. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Sui, W.; Liu, X.; Sun, H.; Li, C.; Parvez, A.M.; Wang, G. Improved high-solid loading enzymatic hydrolysis of steam exploded corn stalk using rapid room temperature γ-valerolactone delignification. Ind. Crops Prod. 2021, 165, 113389. [Google Scholar] [CrossRef]

| Factor | −1 | 0 | +1 |
|---|---|---|---|
| Temperature | 140 | 150 | 160 |
| GVL/water (w/w) | 0.55 | 0.65 | 0.75 |
| Cellulose (g/100 g RM) | Xylan (g/100 g RM) | Acetyls (g/100 g RM) | Lignin (g/100 g RM) | Extractives (g/100 g RM) | Ash (g/100 g RM) |
|---|---|---|---|---|---|
| 37.28 ± 0.36 | 15.33 ± 0.09 | 2.38 ± 0.03 | 22.63 ± 2.00 | 9.47 ± 0.36 | 6.42 ± 0.21 |
| T | GVL/Water (w/w) | CW | XW | [Xylose] g/L | [Glucose] g/L |
|---|---|---|---|---|---|
| 150 | 0.65 | 13.99% | 19.80% | 10.06 | 16.90 |
| 160 | 0.75 | 1.96% | 2.85% | 2.00 | 3.28 |
| 150 | 0.55 | 5.33% | 57.22% | 22.77 | 5.04 |
| 150 | 0.65 | 11.75% | 27.27% | 13.81 | 14.16 |
| 140 | 0.55 | 4.56% | 53.16% | 21.01 | 4.29 |
| 150 | 0.65 | 8.05% | 37.14% | 18.96 | 9.77 |
| 140 | 0.65 | 6.54% | 41.98% | 21.33 | 7.90 |
| 160 | 0.55 | 5.63% | 59.22% | 23.34 | 5.28 |
| 140 | 0.75 | 14.14% | 11.94% | 8.44 | 23.78 |
| 150 | 0.75 | 14.35% | 11.80% | 8.44 | 24.42 |
| 150 | 0.65 | 11.77% | 33.66% | 16.93 | 14.09 |
| 150 | 0.65 | 8.07% | 41.92% | 21.27 | 9.74 |
| 160 | 0.65 | 7.59% | 38.25% | 19.20 | 9.06 |
| Factor Settings | Response | Composite Desirability | Fit | SE Fit | 95% CI |
|---|---|---|---|---|---|
| 140 °C; 0.6 GVL/water (w/w) | CY | 0.97 | 0.83 | 0.037 | (0.74; 0.91) |
| C% | 0.93 | 0.0069 | (0.91; 0.94) |
| Sample | CY | C% | HY | CW | XW | [Xylose] g/L | [Glucose] g/L |
|---|---|---|---|---|---|---|---|
| CSopt | 86.04% ± 3.29 | 91.49% ± 2.38 | 100% ± 1.55 | 5.05% ± 0.45 | 51.88% ± 2.08 | 26.16 ± 0.65 | 6.19 ± 0.44 |
| Csopt Sample Number | Solid Loadings (%) | Cellulase Loading (FPU/g Cellulose) | OY (g Glucose/100 G RM) |
|---|---|---|---|
| 1 | 5 | 10 | 30.44 |
| 2 | 12.5 | 10 | 28.63 |
| 3 | 20 | 10 | 26.89 |
| 4 | 5 | 20 | 35.60 |
| 5 | 12.5 | 20 | 33.55 |
| 6 | 20 | 20 | 31.52 |
| 7 | 5 | 60 | 38.43 |
| 8 | 12.5 | 60 | 37.46 |
| 9 | 20 | 60 | 37.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbrizi, G.; Giannoni, T.; Lorenzi, L.; Nicolini, A.; Iodice, P.; Coccia, V.; Cavalaglio, G.; Gelosia, M. High Solid and Low Cellulase Enzymatic Hydrolysis of Cardoon Stems Pretreated by Acidified γ-Valerolactone/Water Solution. Energies 2022, 15, 2600. https://doi.org/10.3390/en15072600
Fabbrizi G, Giannoni T, Lorenzi L, Nicolini A, Iodice P, Coccia V, Cavalaglio G, Gelosia M. High Solid and Low Cellulase Enzymatic Hydrolysis of Cardoon Stems Pretreated by Acidified γ-Valerolactone/Water Solution. Energies. 2022; 15(7):2600. https://doi.org/10.3390/en15072600
Chicago/Turabian StyleFabbrizi, Giacomo, Tommaso Giannoni, Leonardo Lorenzi, Andrea Nicolini, Paola Iodice, Valentina Coccia, Gianluca Cavalaglio, and Mattia Gelosia. 2022. "High Solid and Low Cellulase Enzymatic Hydrolysis of Cardoon Stems Pretreated by Acidified γ-Valerolactone/Water Solution" Energies 15, no. 7: 2600. https://doi.org/10.3390/en15072600








