Emission of Methane and Carbon Dioxide during Soil Freezing without Permafrost
Abstract
:1. Introduction
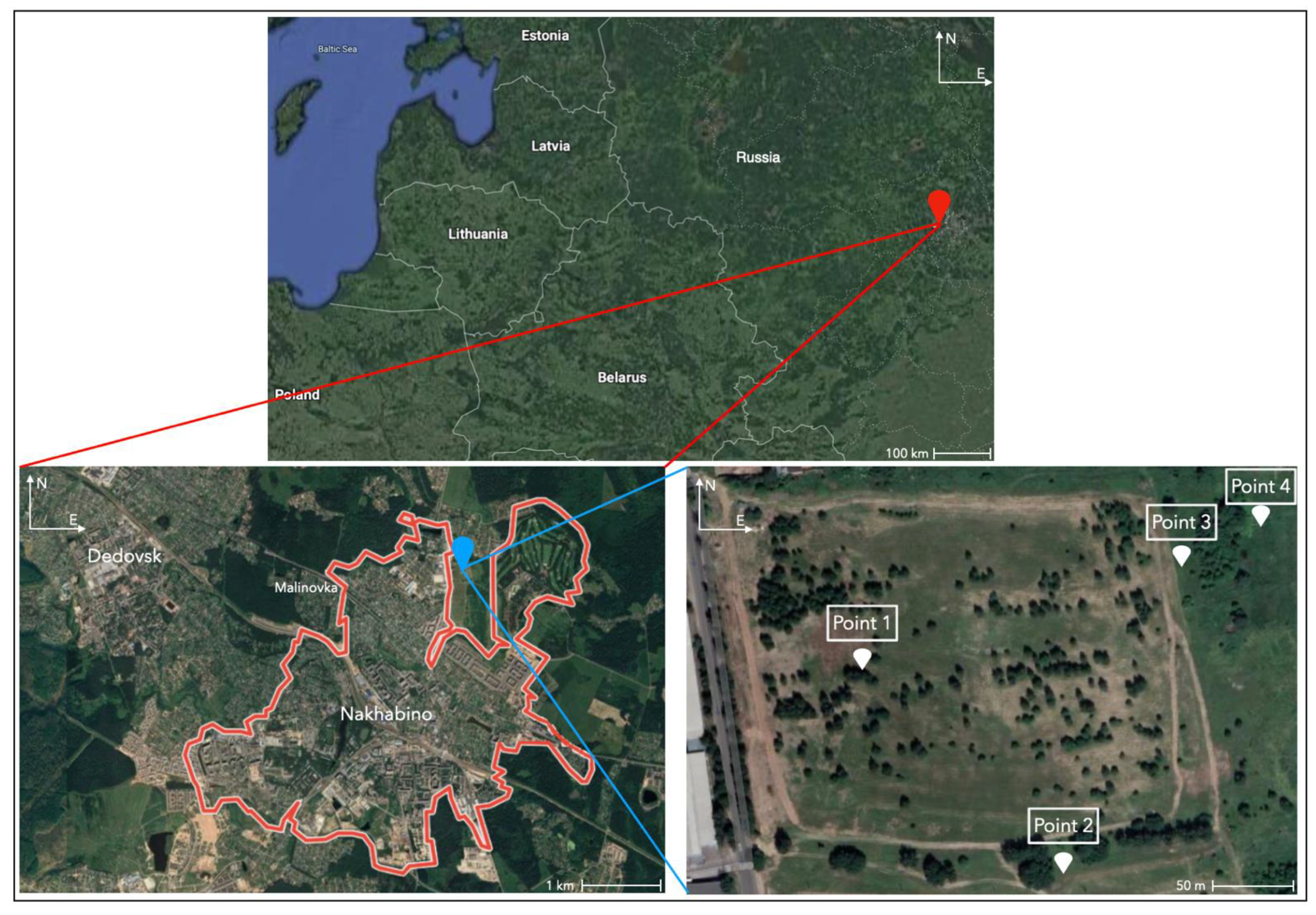
2. Experimental Area and Conditions
3. Experimental Instruments and Methods
4. Research Results
5. Discussion
6. Conclusions
- (1)
- Rainfall was conducive to the release of methane and carbon dioxide in soil.
- (2)
- Much methane and carbon dioxide were released from the soil during the freezing process, and especially methane. With the emergence of the permafrost table and the formation of snow, in a short period of 16 days, emissions per square meter could reach even more than 750 mg/m3. Although the survey area was not in the permafrost area, there were already many greenhouse gases. In permafrost regions, with the increase in temperature, permafrost melts. If scientists take adequate measures to capture methane and carbon dioxide in permafrost and reuse them, their values are even more surprising. If proper measures are not taken, on the other hand, large amounts of methane and carbon dioxide are released into the air, increasing the greenhouse effect.
- (3)
- The formation of frozen layers and snow cover was critical in accumulating methane and carbon dioxide in the soil. After forming the frozen layer and snow on the soil surface, the methane concentration was 57.6 times higher than that without snow. This result provides strong evidence that permafrost is the most important natural source of greenhouse gases.
- (4)
- With the progress of freezing, the concentration of carbon dioxide in the soil surface and soil increased, while the concentration of carbon dioxide in the air first decreased and then sharply increased until it stabilized with the decrease in temperature.
- (5)
- The trend of methane change showed that, as the freezing process continued, methane content in the soil first stabilized, increased sharply, and lastly decreased, tending to be stable. However, methane content in the air and soil surface at first increased, then decreased, and lastly stabilized.
- (6)
- The gas cycle and gas emissions in frozen soil were mainly related to the upper frozen soil layer and the thickness of snow cover.
- (7)
- The formation of snow would restrain the release of methane and carbon dioxide in soil. Snow cover is a significant factor that should not be ignored while studying the mechanism of greenhouse gas emissions in permafrost.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.; Romanovsky, V.E. Report from the International Permafrost Association: State of permafrost in the first decade of the 21st century. Permafr. Periglac. Process. 2008, 19, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Claussen, E.; Cochran, V.A.; Davis, D.P. Climate Change: Science, Strategies, & Solutions; Brill: Leiden, The Netherlands; Boston, MA, USA; Cologne, Germany, 2001; 373p. [Google Scholar]
- Commane, R.; Lindaas, J.; Benmergui, J.; Luus, K.A.; Chang, R.; Daube, B.C.; Euskirchen, E.S.; Henderson, J.M.; Karion, A.; Miller, J.; et al. Carbon dioxide sources from Alaska driven by increasing early winter respiration from Arctic tundra. Proc. Natl. Acad. Sci. USA 2017, 114, 5361–5366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.A.G.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D.; et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef] [Green Version]
- Koven, C.D.; Riley, W.J.; Stern, A. Analysis of Permafrost Thermal Dynamics and Response to Climate Change in the CMIP5 Earth System Models. J. Clim. 2013, 26, 1877–1900. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.H. Global Climate Change in the Human Perspective. In Geosphere-Biosphere Interactions and Climate; Bengtsson, L.O., Hammer, C.U., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 90–91. [Google Scholar]
- Tubiello, F.N.; Salvatore, M.; Cóndor Golec, R.D.; Ferrara, A.; Rossi, S.; Biancalani, R.; Federici, S.; Jacobs, H.; Flammini, A. Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks: 1990–2011 Analysis; FAO: Rome, Italy, 2014. [Google Scholar] [CrossRef]
- Romanovsky, V.E.; Drozdov, D.S.; Oberman, N.G.; Malkova, G.V.; Kholodov, A.L.; Marchenko, S.S.; Moskalenko, N.G.; Sergeev, D.O.; Ukraintseva, N.G.; Abramov, A.A.; et al. Thermal state of permafrost in Russia. Permafr. Periglac. Process. 2010, 21, 136–155. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Bockheim, J.; Canadell, J.G.; Euskirchen, E.; Field, C.B.; Goryachkin, S.V.; Hagemann, S.; Kuhry, P.; LaFleur, P.M.; Lee, H.; et al. Vulnerability of Permafrost Carbon to Climate Change: Implications for the Global Carbon Cycle. Bioscience 2008, 58, 701–714. [Google Scholar] [CrossRef]
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S.A. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycle 2009, 23, 1–11. [Google Scholar] [CrossRef]
- Zimov, S.A.; Schuur, E.A.G.; Chapin, F.S. Permafrost and the Global Carbon Budget. Science 2006, 312, 1612–1613. [Google Scholar] [CrossRef]
- Avis, C.A.; Weaver, A.J.; Meissner, K.J. Reduction in areal extent of high-latitude wetlands in response to permafrost thaw. Nat. Geosci. 2011, 4, 444–448. [Google Scholar] [CrossRef]
- Hertwich, E. The Carbon Footprint of Material Production Rises to 23% of Global Greenhouse Gas Emissions; Center for Open Science: Charlottesville, VA, USA, 2019. [Google Scholar]
- Turetsky, M.R.; Abbott, B.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Koven, C.; McGuire, A.D.; Grosse, G.; Kuhry, P.; et al. Permafrost collapse is accelerating carbon release. Nature 2019, 569, 32–34. [Google Scholar] [CrossRef] [Green Version]
- MacDougall, A.H.; Avis, C.A.; Weaver, A.J. Significant contribution to climate warming from the permafrost carbon feedback. Nat. Geosci. 2012, 5, 719–721. [Google Scholar] [CrossRef]
- Kurganova, I.; Teepe, R.; Loftfield, N. Influence of freeze-thaw events on carbon dioxide emission from soils at different moisture and land use. Carbon Balance Manag. 2007, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natali, S.M.; Watts, J.D.; Rogers, B.M.; Potter, S.; Ludwig, S.M.; Selbmann, A.K.; Sullivan, P.F.; Abbott, B.W.; Arndt, K.A.; Birch, L.; et al. Large loss of CO2 in winter observed across the northern permafrost region. J. Nat. Clim. Chang. 2019, 9, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.A.; Lipson, D.A.; Hashemi, J.; Oechel, W.C.; Zona, D. Snow melt stimulates ecosystem respiration in Arctic ecosystems. Glob. Chang. Biol. 2020, 26, 5042–5051. [Google Scholar] [CrossRef]
- Bing, H.; He, P.; Zhang, Y. Cyclic freeze–thaw as a mechanism for water and salt migration in soil. Environ. Earth Sci. 2015, 74, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Mooney, H.A.; Canadell, J.; Chapin, F.S.; Ehleringer, J.E.; Korner, C.H.; Mcmurtie, R.E.; Parton, W.J.; Pitelka, L.F.; Schulze, E.-D. Ecosystem physiology responses to global change. In The Terrestrial Biosphere and Global Change; Walker, B.H., Steffen, W.L., Canadell, J., Ingram, J.S.I., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 141–189. [Google Scholar]
- Briggs, M.A.; Walvoord, M.; McKenzie, J.M.; Voss, C.I.; Day-Lewis, F.; Lane, J.W. New permafrost is forming around shrinking Arctic lakes, but will it last? Geophys. Res. Lett. 2014, 41, 1585–1592. [Google Scholar] [CrossRef]
- Burke, S.A.; Wik, M.; Lang, A.; Contosta, A.R.; Palace, M.; Crill, P.M.; Varner, R.K. Long-Term Measurements of Methane Ebullition from Thaw Ponds. J. Geophys. Res. Biogeosci. 2019, 124, 2208–2221. [Google Scholar] [CrossRef]
- Jorgenson, M.T.; Romanovsky, V.; Harden, J.; Shur, Y.; O’Donnell, J.; Schuur, E.A.G.; Kanevskiy, M.; Marchenko, S. Resilience and vulnerability of permafrost to climate change. Can. J. For. Res.-Rev. Can. Rech. For. 2010, 40, 1219–1236. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.V.; Froese, D.G.; Jensen, B.J. Permafrost response to last interglacial warming: Field evidence from non-glaciated Yukon and Alaska. Quat. Sci. Rev. 2010, 29, 3256–3274. [Google Scholar] [CrossRef]
- Zamolodchikov, D.G.; Karelin, D. An empirical model of carbon fluxes in Russian tundra. Glob. Chang. Biol. 2001, 7, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Ye, W.; Rahardjo, H.; Satyanaga, A.; Dai, G.; Zhao, X. Theoretical method for the estimation of vapour conductivity for unsaturated soil. Eng. Geol. 2021, 295, 106447. [Google Scholar] [CrossRef]
- Friborg, T.; Christensen, T.R.; Hansen, B.U.; Nordstroem, C.; Soegaard, H. Trace gas exchange in a high-arctic valley 2. Landscape CH4 fluxes measured and modeled using eddy correlation data. Glob. Biogeochem. Cycles 2000, 14, 715–724. [Google Scholar] [CrossRef]
- Olefeldt, D.; Turetsky, M.R.; Crill, P.M.; McGuire, A.D. Environmental and physical controls on northern terrestrial methane emissions across permafrost zones. Glob. Chang. Biol. 2013, 19, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Mastepanov, M.; Sigsgaard, C.; Tagesson, T.; Ström, L.; Tamstorf, M.P.; Lund, M.; Christensen, T.R. Revisiting factors controlling methane emissions from high-Arctic tundra. Biogeosciences 2013, 10, 5139–5158. [Google Scholar] [CrossRef] [Green Version]
- Amthor, J.S. Terrestrial higher-plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Glob. Chang. Biol. 1995, 1, 243–274. [Google Scholar] [CrossRef]
- Pirk, N.; Santos, T.; Gustafson, C.; Johansson, A.J.; Tufvesson, F.; Parmentier, F.W.; Mastepanov, M.; Christensen, T.R. Methane emission bursts from permafrost environments during autumn freeze-in: New insights from ground-penetrating radar. Geophys. Res. Lett. 2015, 42, 6732–6738. [Google Scholar] [CrossRef]
- Wille, C.; Kutzbach, L.; Sachs, T.; Wagner, D.; Pfeiffer, E.-M. Methane emission from Siberian arctic polygonal tundra: Eddy covariance measurements and modeling. Glob. Chang. Biol. 2008, 14, 1395–1408. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.J.; Fowler, D.; Pitcairn, C.E.R.; Aurela, M. Annual methane emission from Finnish mires estimated from eddy covariance campaign measurements. Theor. Appl. Climatol. 2001, 70, 203–213. [Google Scholar] [CrossRef]
- Bao, T.; Xu, X.; Jia, G.; Billesbach, D.P.; Sullivan, R.C. Much stronger tundra methane emissions during autumn freeze than spring thaw. Glob. Chang. Biol. 2020, 27, 376–387. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Xue, D.; Zhu, D.; Zhan, W.; Li, W.; Wu, N.; Yang, G. Methane emissions during different freezing-thawing periods from a fen on the Qinghai-Tibetan Plateau: Four years of measurements. Agric. For. Meteorol. 2021, 297, 108279. [Google Scholar] [CrossRef]
- Mastepanov, M.; Sigsgaard, C.; Dlugokencky, E.J.; Houweling, S.; Ström, L.; Tamstorf, M.; Christensen, T.R. Large tundra methane burst during onset of freezing. Nature 2008, 456, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagesson, T.; Mölder, M.; Mastepanov, M.; Sigsgaard, C.; Taster, M.P.; Lund, M.; Falk, J.M.; Lindroth, A.; Christensen, T.R.; Ström, L. Land-atmosphere exchange of methane from soil thawing to soil freezing in a high-Arctic wet tundra ecosystem. Glob. Chang. Biol. 2012, 18, 1928–1940. [Google Scholar] [CrossRef] [Green Version]



| Cooling Process—11 November 2020 (Soil is Not Frozen) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Point Number | CO2 Concentration,% (Dry-Air) | CO2 Concentration,% (Dry-Surface) | CO2 Concentration,% (Dry-Ground) | CH4 Concentration, mg/m3 (Dry-Air) | CH4 Concentration, mg/m3 (Dry-Surface) | CH4 Concentration, mg/m3 (Dry-Ground) | CO2 Concentration,% (Wet-Air) | CO2 Concentration,% (Wet-Surface) | CO2 Concentration,% (Wet-Ground) | CH4 Concentration, mg/m3 (Wet-Air) | CH4 Concentration, mg/m3 (Wet-Surface) | CH4 Concentration, mg/m3 (Wet-Ground) |
| 1 | 0.02 | 0.03 | 0.31 | 1.83 | 2 | 6 | — | — | — | — | — | — |
| 2 | 0.03 | 0.03 | 0.06 | 1.67 | 1.83 | 13 | — | — | — | — | — | — |
| 3 | 0.02 | 0.04 | 0.06 | 1.67 | 1.83 | 19.75 | 0.02 | 0.05 | 0.11 | 1.92 | 3.25 | 17.17 |
| 4 | 0.02 | 0.05 | 0.11 | 1.75 | 6.25 | 4.5 | 0.02 | 0.09 | 0.17 | 1.83 | 4.25 | 6.17 |
| Freezing Process—28 November 2020 (Soil Begins to Freeze; Depth of Freezing, 3 cm; Snow Thickness, 2–4 cm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Point Number | CO2 Concentration,% (Air) | CO2 Concentration,% (Surface) | CO2 Concentration,% (Ground) | CH4 Concentration, mg/m3 (Air) | CH4 Concentration, mg/m3 (Surface) | CH4 Concentration, mg/m3 (Ground) | CO2 Concentration,% (in Bottle) | CH4 Concentration, mg/m3 (in Bottle) |
| 1 | 0.02 | 0.04 | 0.23 | 2.33 | 3.75 | 25.42 | — | — |
| 2 | 0.02 | 0.04 | 0.43 | 2.42 | 2.58 | 61.17 | — | — |
| 3 | 0.02 | 0.05 | 0.23 | 2.17 | 2.42 | 34.42 | 0.17 | 78.42 |
| 4 | 0.02 | 0.11 | 0.13 | 2.08 | 2.58 | 7.75 | 0.12 | 750.00 |
| Process | Point Number | CO2 Concentration,% (Air) | CO2 Concentration,% (Surface) | CO2 Concentration,% (Ground) | CH4 Concentration, mg/m3 (Air) | CH4 Concentration, mg/m3 (Surface) | CH4 Concentration, mg/m3 (Ground) |
|---|---|---|---|---|---|---|---|
| Process was stronger than freezing—10 December 2020 (depth of soil freezing 3–5 cm, snow thickness 7–10 cm) | 1 | 0.02 | 0.05 | 0.09 | 1.75 | 2.25 | 113 |
| 2 | 0.03 | 0.06 | 0.52 | 1.92 | 2.42 | 750 | |
| 3 | 0.03 | 0.06 | 0.24 | 1.67 | 2.33 | 540.33 | |
| 4 | 0.04 | 0.06 | 0.11 | 1.92 | 2.08 | 24.33 | |
| Freezing state—8 January 2021 (depth of soil freezing 0–4 cm, snow thickness 24–31 cm) | 1 | 0.04 | 0.86 | 0.75 | 1.42 | 1.75 | 73.67 |
| 2 | 0.03 | 0.06 | 0.53 | 1.67 | 1.83 | 477.25 | |
| 3 | 0.03 | 1.10 | 0.37 | 1.67 | 2.42 | 457 | |
| 4 | 0.04 | 0.06 | 0.16 | 1.83 | 2.25 | 19.08 | |
| Freezing state—22 February 2021 (depth of soil freezing 0 cm, snow thickness 41–80 cm) | 1 | 0.03 | 1.02 | 0.23 | 1.08 | 1.58 | 44.75 |
| 2 | 0.03 | 0.05 | 0.15 | 2.08 | 6.42 | 283.25 | |
| 3 | 0.02 | 0.05 | 0.09 | 2.25 | 2.92 | 242.33 | |
| 4 | 0.02 | 0.06 | 0.13 | 2.08 | 2.67 | 3.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Brouchkov, A.V.; Cheverev, V.G.; Sokolov, A.V.; Li, K. Emission of Methane and Carbon Dioxide during Soil Freezing without Permafrost. Energies 2022, 15, 2693. https://doi.org/10.3390/en15072693
Li C, Brouchkov AV, Cheverev VG, Sokolov AV, Li K. Emission of Methane and Carbon Dioxide during Soil Freezing without Permafrost. Energies. 2022; 15(7):2693. https://doi.org/10.3390/en15072693
Chicago/Turabian StyleLi, Chenzheng, Anatoly V. Brouchkov, Viktor G. Cheverev, Andrey V. Sokolov, and Kunyang Li. 2022. "Emission of Methane and Carbon Dioxide during Soil Freezing without Permafrost" Energies 15, no. 7: 2693. https://doi.org/10.3390/en15072693
APA StyleLi, C., Brouchkov, A. V., Cheverev, V. G., Sokolov, A. V., & Li, K. (2022). Emission of Methane and Carbon Dioxide during Soil Freezing without Permafrost. Energies, 15(7), 2693. https://doi.org/10.3390/en15072693






