Influence of Natural Convection and Volume Change on Numerical Simulation of Phase Change Materials for Latent Heat Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. PCM Selection
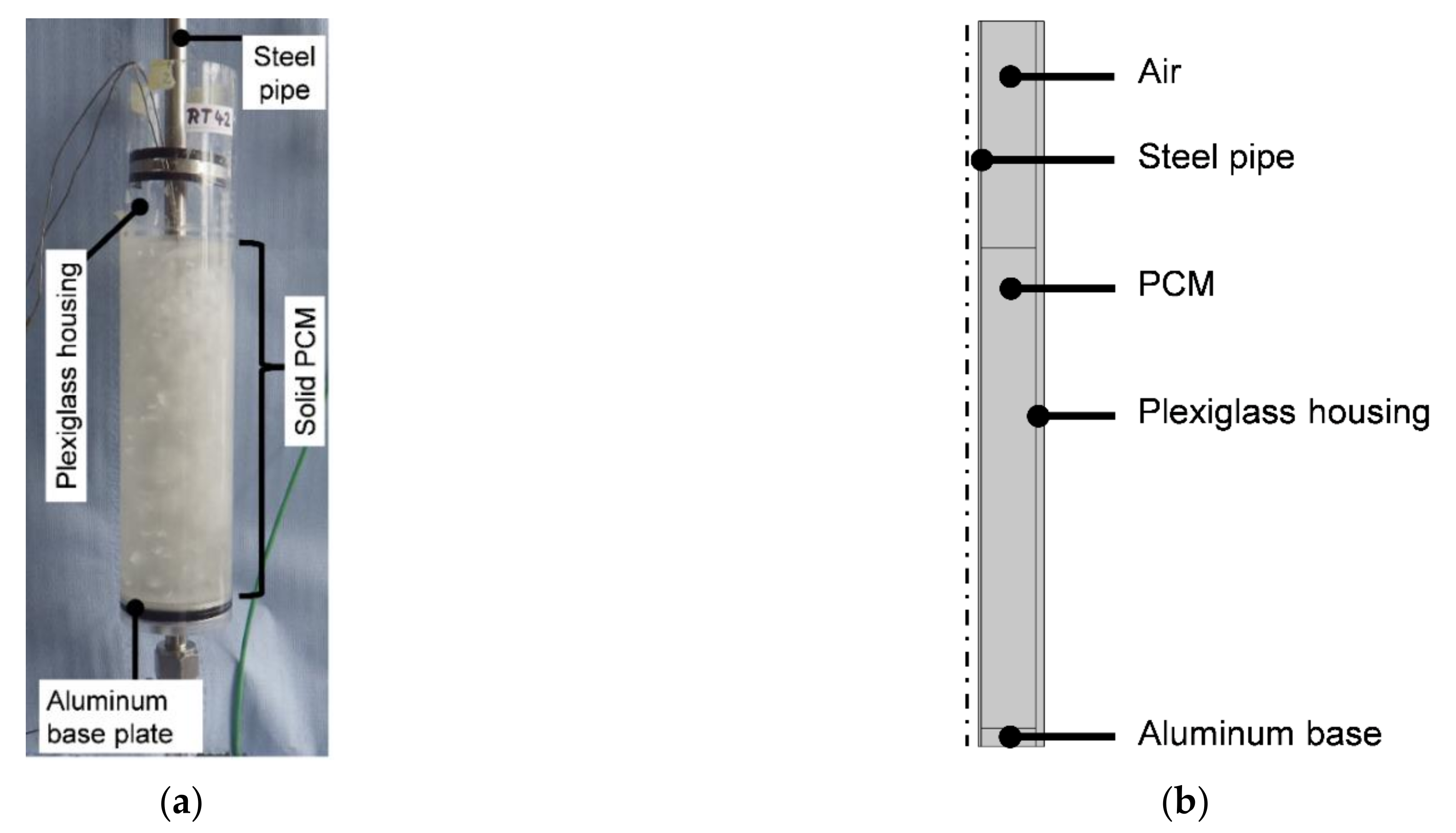
2.2. Experimental Setup
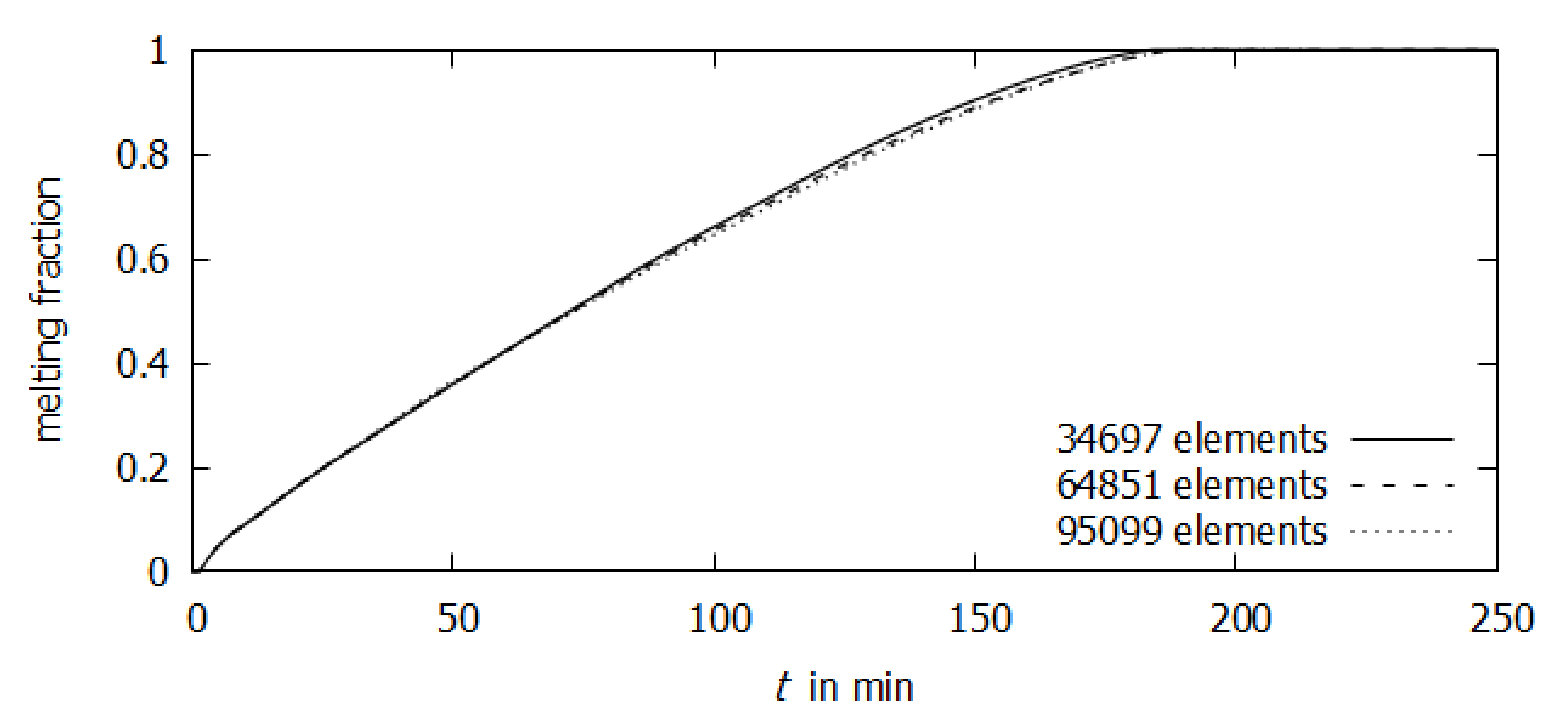
2.3. Numerical Model
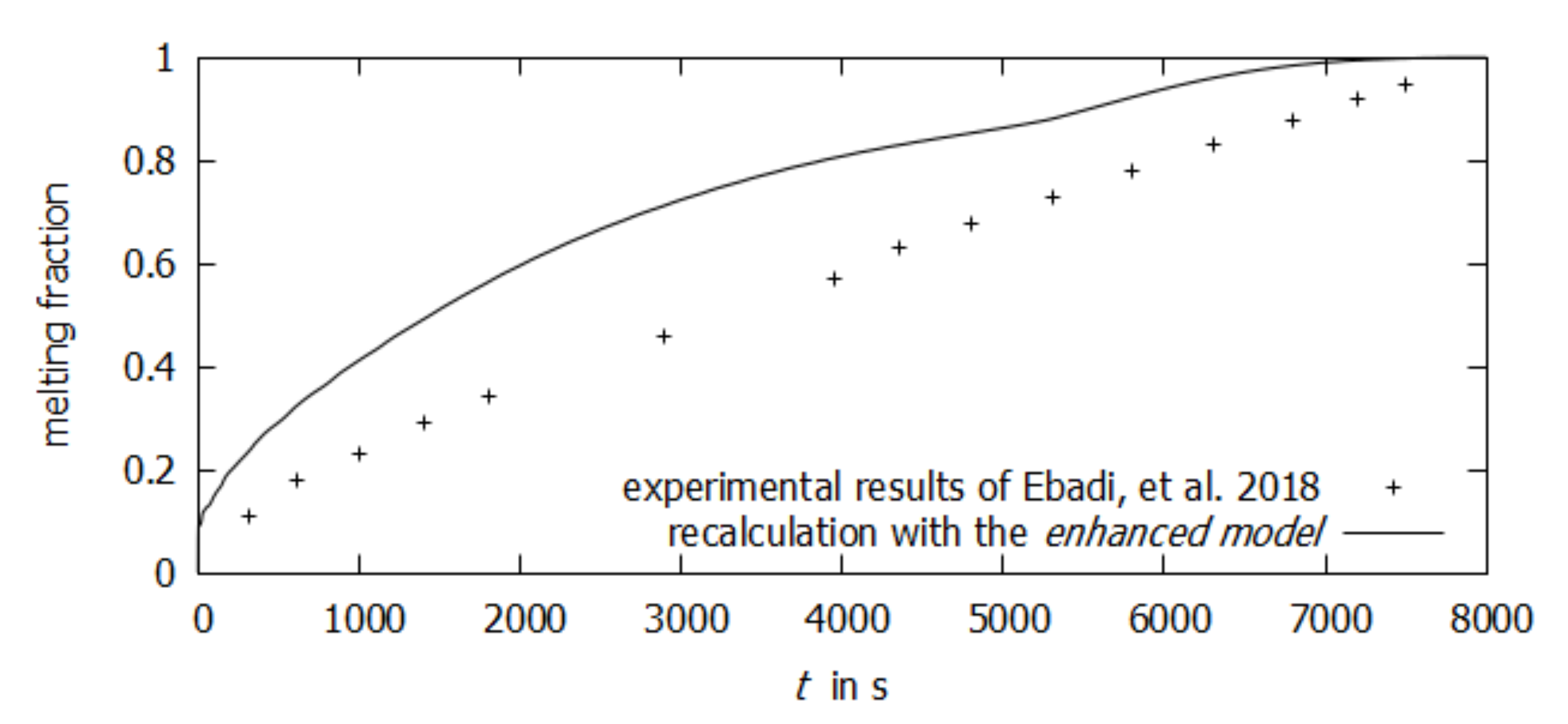
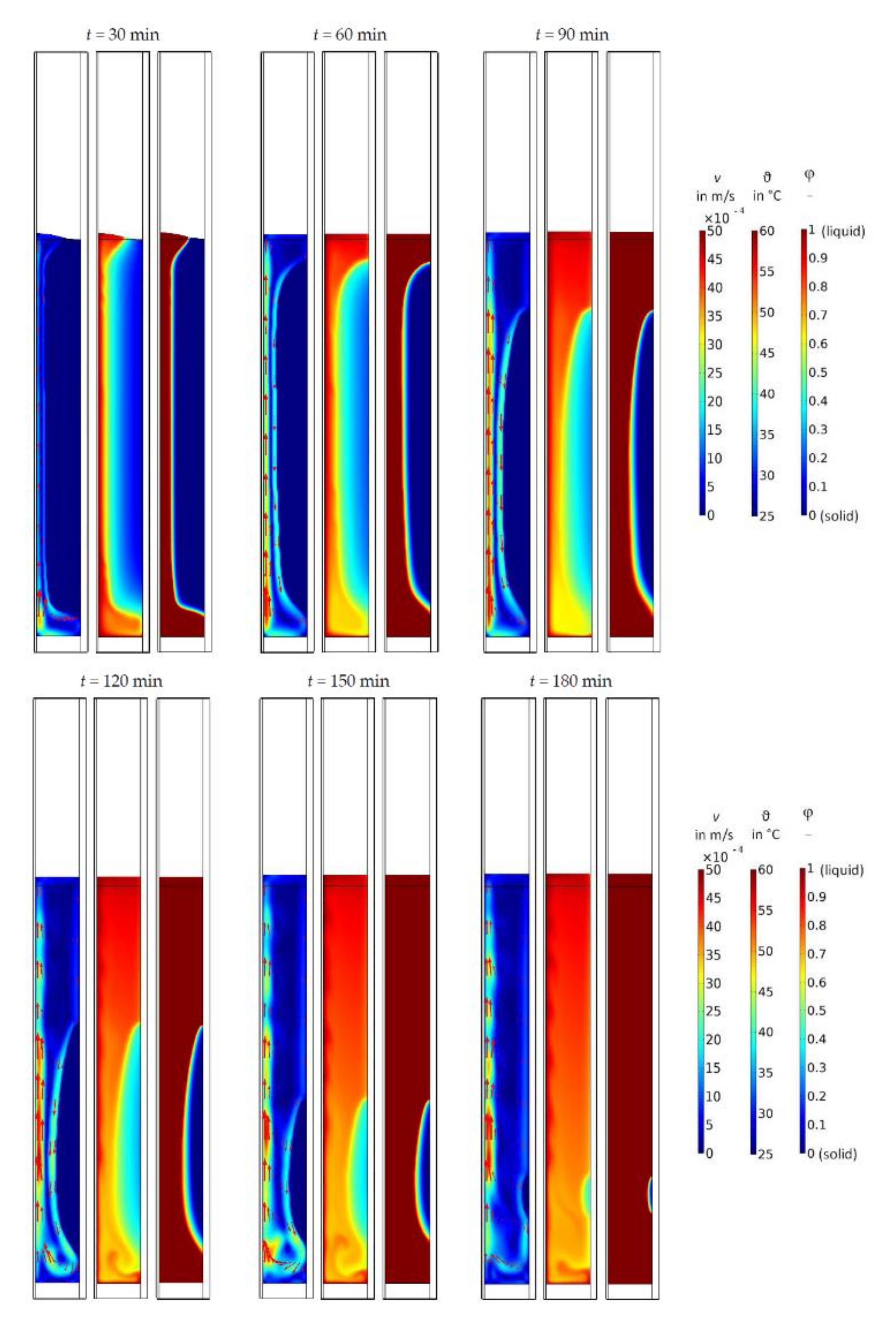
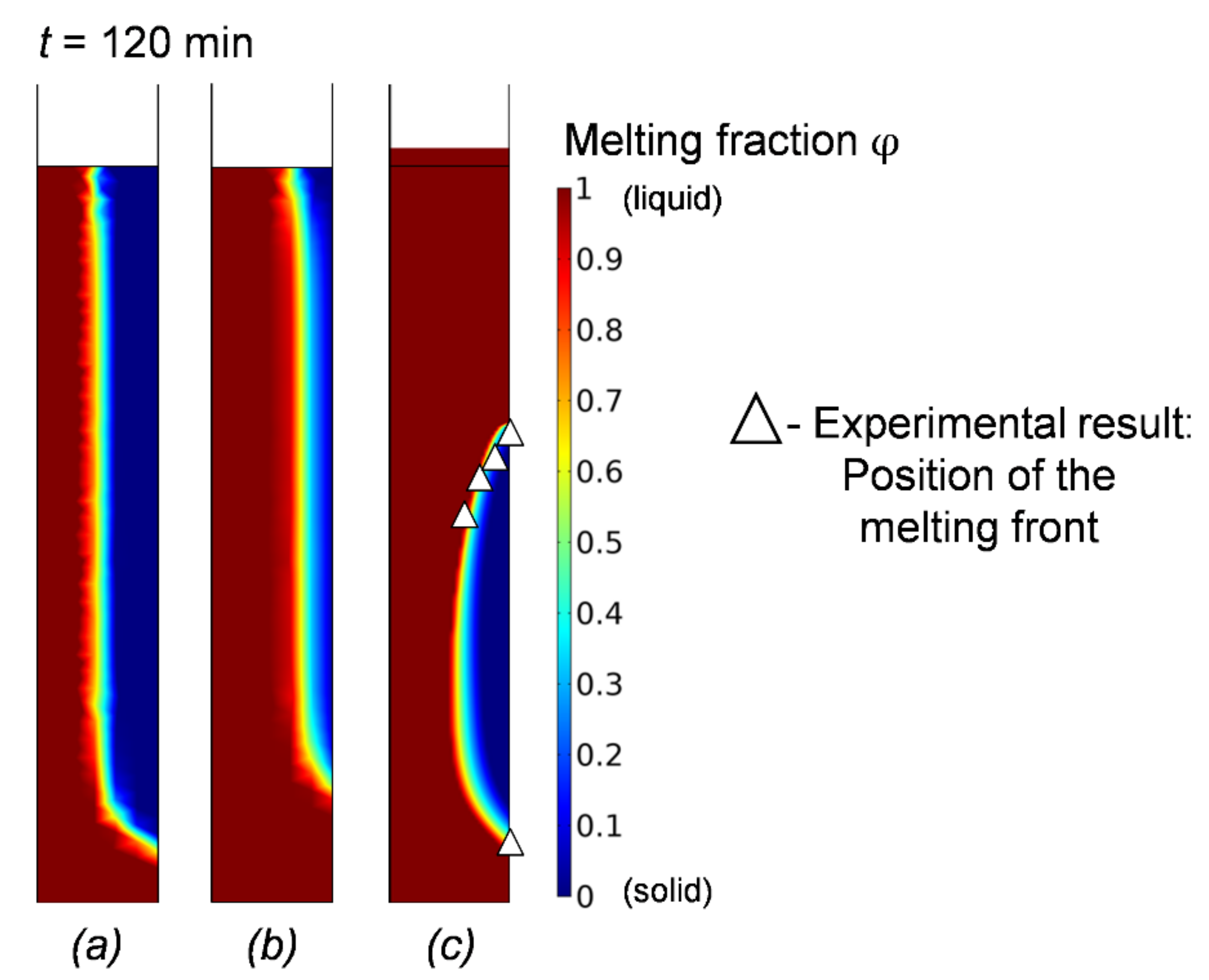
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Specific heat capacity in J/(kg·K) | |
| Acceleration of gravity in m/s2 | |
| Pressure in Pa | |
| Time in s | |
| Velocity in m/s | |
| Mushy zone factor in kg/(m3·s) | |
| Gauß function in 1/K | |
| Volume force in N/m3 | |
| Unit vector | |
| Latent heat in J/kg | |
| Source term in kg/(m3·s) | |
| Temperature in K | |
| V | Volume in m3 |
| Greek Symbols | |
| Difference | |
| Heat transfer coefficient in W/(m²·K) | |
| Thermal volume expansion coefficient in 1/K | |
| Constant | |
| Temperature in °C | |
| Thermal conductivity in W/(m·K) | |
| Dynamic viscosity in Pa·s | |
| Constant in m² | |
| Density in kg/m³ | |
| Surface tension in N/m | |
| Melting fraction | |
| Abbreviations and Indexes | |
| PCM | Phase change material |
| Buoyancy | |
| Liquid phase | |
| Phase change | |
| Solid phase | |
| Vector quantity | |
References
- Hübner, S. Techno-Ökonomische Optimierung Eines Hochtemperatur-Latentwärmespeichers. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2018. [Google Scholar]
- Kötter, T. Hochleistungsdichte Phasenwechsel-Komposit-Materialien für das Thermische Management Elektrotechnischer Systeme. Ph.D. Thesis, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany, 2017. [Google Scholar]
- Vogel, J. Influence of Natural Convection on Melting of Phase Change Materials. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2019. [Google Scholar]
- Sedeh, M.; Khodadadi, J. Solidification of phase change materials infiltrated in porous media in presence of voids. J. Heat Transf. 2014, 136, 112603. [Google Scholar] [CrossRef]
- Voller, V.R. An enthalpy method for convection/diffusion phase change. Int. J. Numer. Methods Eng. 1987, 24, 271–284. [Google Scholar] [CrossRef]
- Samara, F.; Groulx, D.; Biwole, P.H. Natural convection driven melting of phase change material: Comparison of two methods. In Proceedings of the COMSOL Conference in Boston, Newton, MA, USA, 3–5 October 2012. [Google Scholar]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Coulson, R. Numerical Modeling of a Lead Melting Front under the Influence of Natural Convection. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, 2013. [Google Scholar]
- Goyal, P.; Dutta, A.; Verma, V.; Thangamani, I.; Singh, R.K. Enthalpy porosity method for CFD simulation of natural convection phenomenon for phase change problems in the molten pool and its importance during melting of solids. In Proceedings of the Comsol Conference in Bangalore, Bangalore, India, 18 October 2013. [Google Scholar]
- Groulx, D.; Biwole, P.H. Solar PV passive temperature control using phase change materials. In Proceedings of the 15th International Heat Transfer Conference, Kyoto, Japan, 10–15 August 2014. [Google Scholar]
- Kheirabadi, A.C.; Groulx, D. Simulating phase change heat transfer using COMSOL and FLUENT: Effekt of the mushy-zone constant. Comput. Therm. Sci. 2015, 7, 427–440. [Google Scholar] [CrossRef]
- Kheirabadi, A.C.; Groulx, D. The effekt of the mushy-zone constant on simulated phase change heat transfer. In Proceedings of the ICHMT International Symposium on Advances in Computational Heat Transfer, New Brunswick, NJ, USA, 25–29 May 2015. [Google Scholar]
- Rubinetti, D.; Weiss, D.A. Modeling approach to facilitate thermal energy management in buildings with phase change materials. In Proceedings of the COMSOL Conference in Lausanne, Lausanne, Switzerland, 22–24 October 2018. [Google Scholar]
- Material Data Sheet, PCM RT42 (Rubitherm GmbH). Available online: https://www.rubitherm.eu/index.php/produktkategorie/organische-pcm-rt (accessed on 1 February 2022).
- Sathe, T.M.; Dhoble, A.S. Thermal management of low concentrated photovoltaic systems using extended surfaces in phase change materials. J. Renew. Sustain. Energy 2018, 10, 043704. [Google Scholar] [CrossRef]
- COMSOL Multiphysics 5.5: Microfluids Module User’s Guide. Available online: https://doc.comsol.com/5.5/doc/com.comsol.help.mfl/MicrofluidicsModuleUsersGuide.pdf (accessed on 1 February 2022).
- Kneer, A.; Nestler, B.; Bantle, B.; Kotitschke, T. Entwicklung und Erprobung eines auf Metallschaum basierenden Systems (Demonstrator) zur regenerativen Nutzung und Speicherung von Abwärme aus Energiegewinnungsprozessen. 2012. Available online: https://pudi.lubw.de/detailseite/-/publication/22381 (accessed on 1 February 2022).
- COMSOL Multiphysics 5.5: Reference Manual. Available online: https://doc.comsol.com/5.5/doc/com.comsol.help.comsol/COMSOL_ReferenceManual.pdf (accessed on 1 February 2022).
- Oertel, H. (Ed.) Prandtl—Führer durch Die Strömungslehre: Grundlagen und Phänomene; Springer Vieweg: Wiesbaden, Germany, 2002. [Google Scholar]
- Ebadi, S.; Al-Jethelah, M.; Tasnim, S.H.; Mahmud, S. An investigation of the melting process of RT-35 filled circular thermal energy storage system. Open Phys. 2018, 16, 574–580. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM; Springer-Verlag GmbH: Berlin/Heidelberg, Germany, 2008. [Google Scholar]


| Phase change temperature | 42 °C | |
| Latent heat | 144 kJ/kg | |
| Specific heat capacity | 2 kJ/(kg·K) | |
| Density, solid phase (15 °C) | 880 kg/m³ | |
| Density, liquid phase (80 °C) | 760 kg/m³ | |
| Volume expansion | 13.6 % | |
| Thermal conductivity | 0.2 W/(m·K) | |
| Phase change temperature range | 6 K | |
| Dynamic viscosity | 1.8 mPa·s | |
| Thermal volume expansion coefficient | 0.0001 1/K |
| Parameter | Parameter Change of −20% | Parameter Change of +20% | ||
|---|---|---|---|---|
| +29.7 % | +54.8 min | −17.0 % | −31.3 min | |
| −8.1 % | −15.0 min | +16.5 % | +30.6 min | |
| −14.4 % | −26.5 min | +15.8 % | +29.1 min | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moench, S.; Dittrich, R. Influence of Natural Convection and Volume Change on Numerical Simulation of Phase Change Materials for Latent Heat Storage. Energies 2022, 15, 2746. https://doi.org/10.3390/en15082746
Moench S, Dittrich R. Influence of Natural Convection and Volume Change on Numerical Simulation of Phase Change Materials for Latent Heat Storage. Energies. 2022; 15(8):2746. https://doi.org/10.3390/en15082746
Chicago/Turabian StyleMoench, Sabine, and Robert Dittrich. 2022. "Influence of Natural Convection and Volume Change on Numerical Simulation of Phase Change Materials for Latent Heat Storage" Energies 15, no. 8: 2746. https://doi.org/10.3390/en15082746






