Biomass and Coal Modification to Prepare Activated Coke for Desulfurization and Denitrification
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Experimental Samples
2.2. Experiment Procedure
2.3. Material Characterization
2.4. Performance Evaluation Method
3. Results and Discussion
3.1. Study on the Desulfurization and Denitrification Performance of NH3-Modified Activated Coke
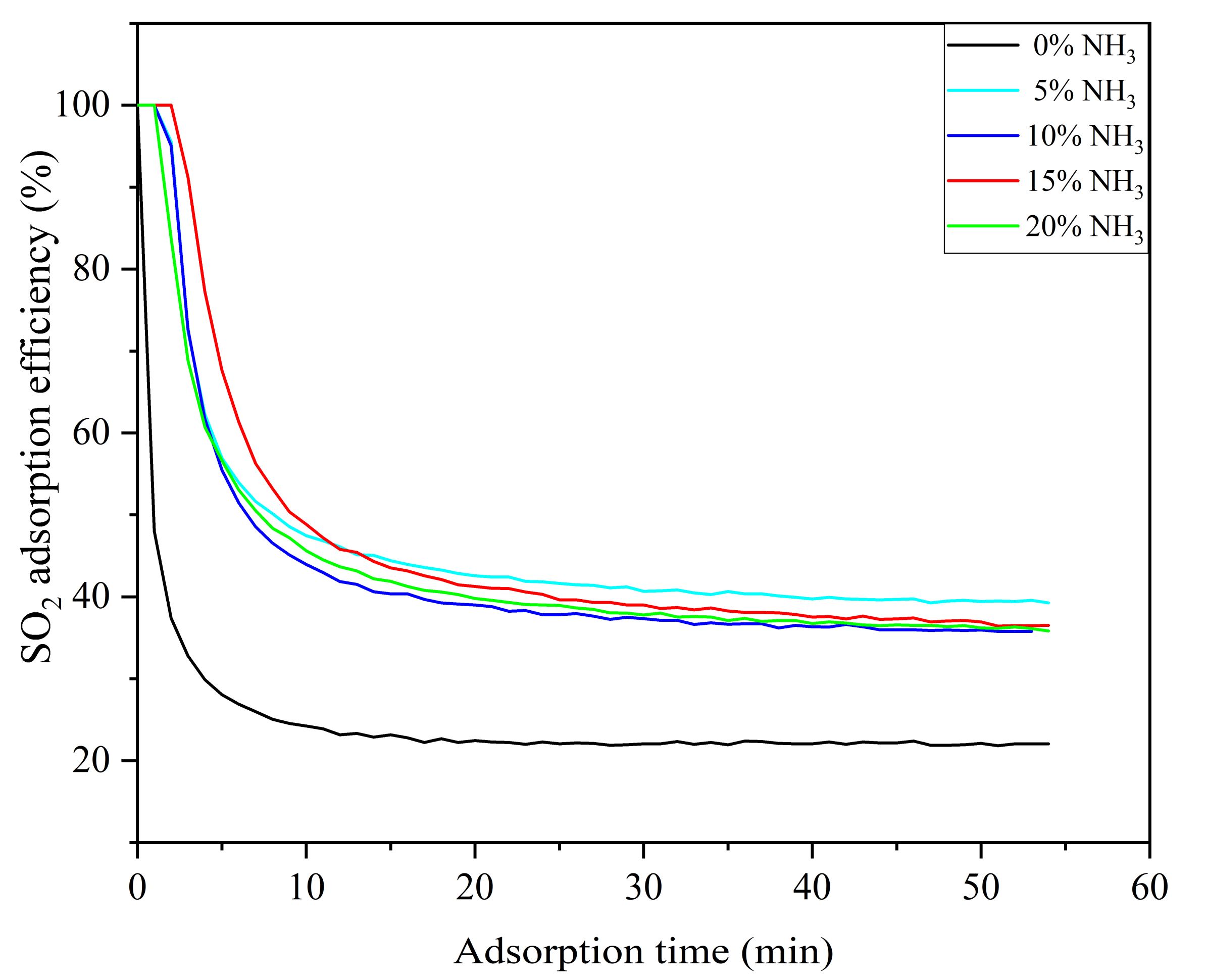
3.1.1. Effect of Modified Activated Coke with Different NH3 Concentrations on SO2 Adsorption Efficiency
3.1.2. Effect of Modified Activated Coke with Different NH3 Concentrations on NO Adsorption Efficiency
3.1.3. SO2/NO Adsorption Capacity per Unit Mass of Activated Coke after Modification with Different NH3 Concentrations
3.2. Study on the Desulfurization and Denitrification Performance of K2CO3-Modified Activated Coke
3.2.1. Effect of Modified Activated Coke with Different K2CO3 Concentrations on SO2 Adsorption Efficiency
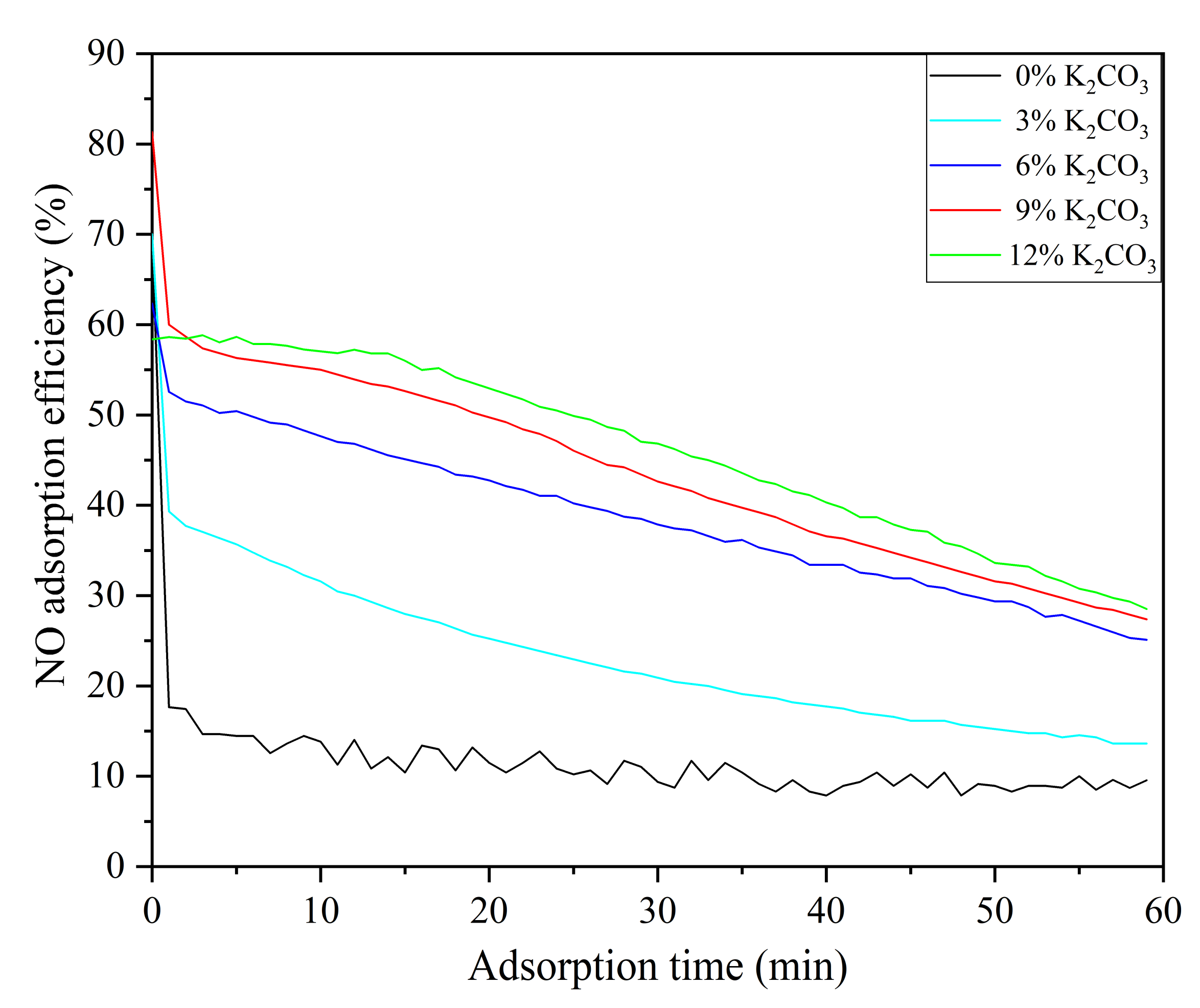
3.2.2. Effect of Modified Activated Coke with Different K2CO3 Concentrations on NO Adsorption Efficiency
3.2.3. SO2/NO Adsorption Capacity per Unit Mass of Activated Coke after Modification with Different K2CO3 Concentrations
3.3. Study on the Solo/Synergistic Performance of Modified Activated Coke for Desulfurization and Denitrification
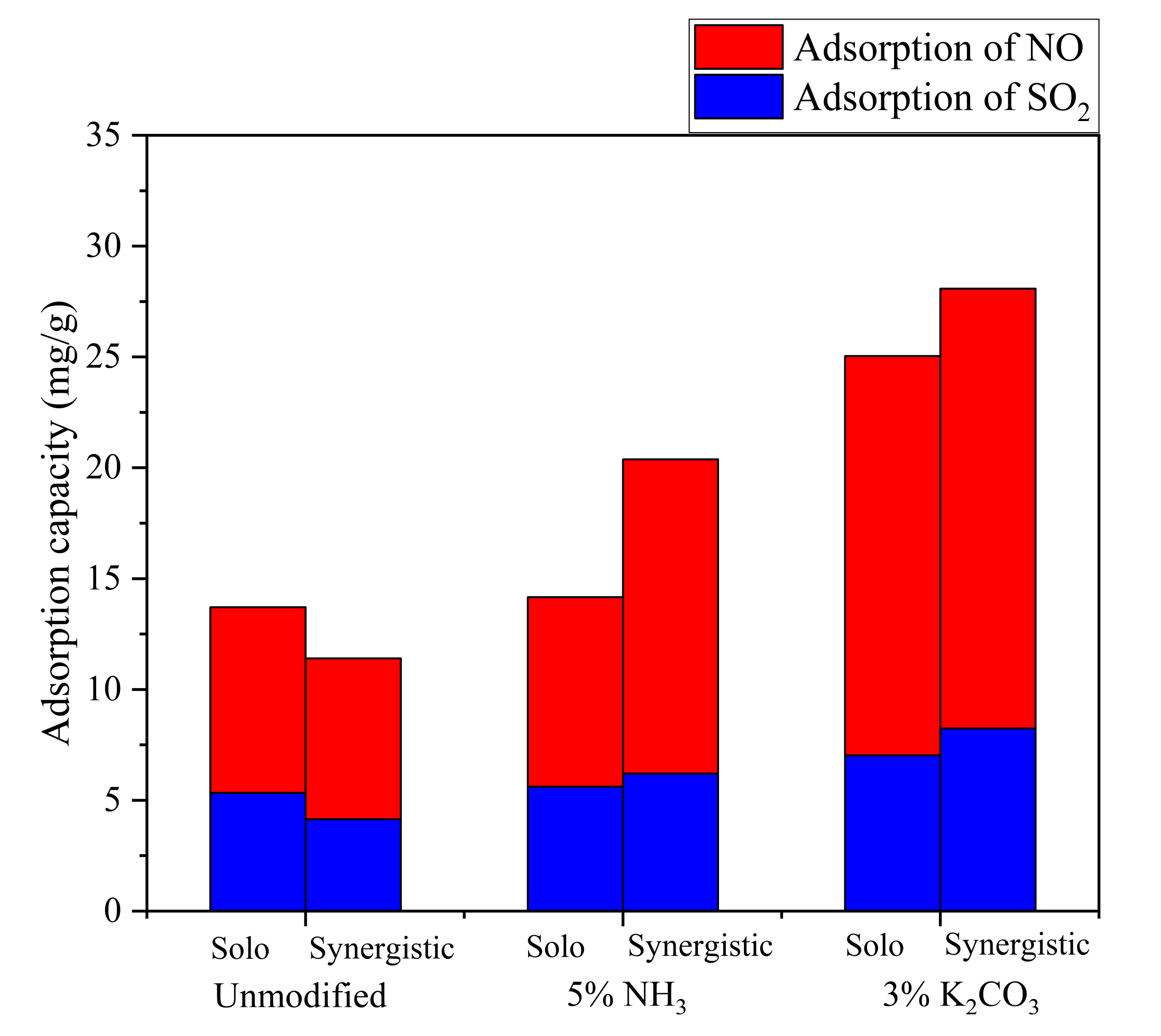
3.3.1. Solo and Synergistic Adsorption EFFICIENCIES of Activated Coke
3.3.2. Capacity of Activated Coke per Unit Mass to Adsorb SO2 and NO Solo/Synergistically
3.4. Changes of Surface Microstructure before and after Adsorption of Modified Activated Coke
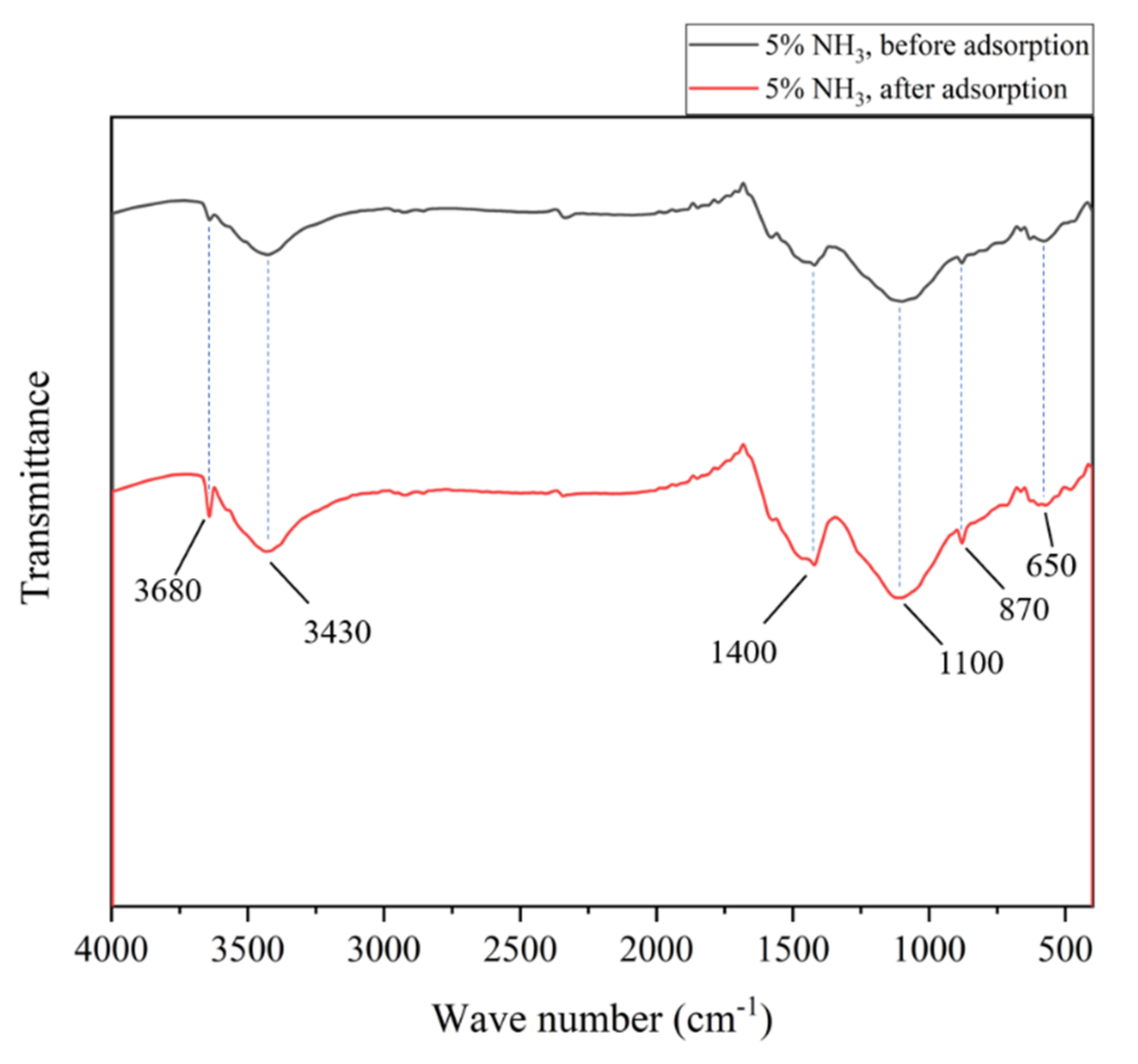
3.4.1. Study on the Microstructure Change of Activated Coke Modified with 5% NH3
3.4.2. Study on the Microstructure Change of Activated Coke Modified with 3% K2CO3
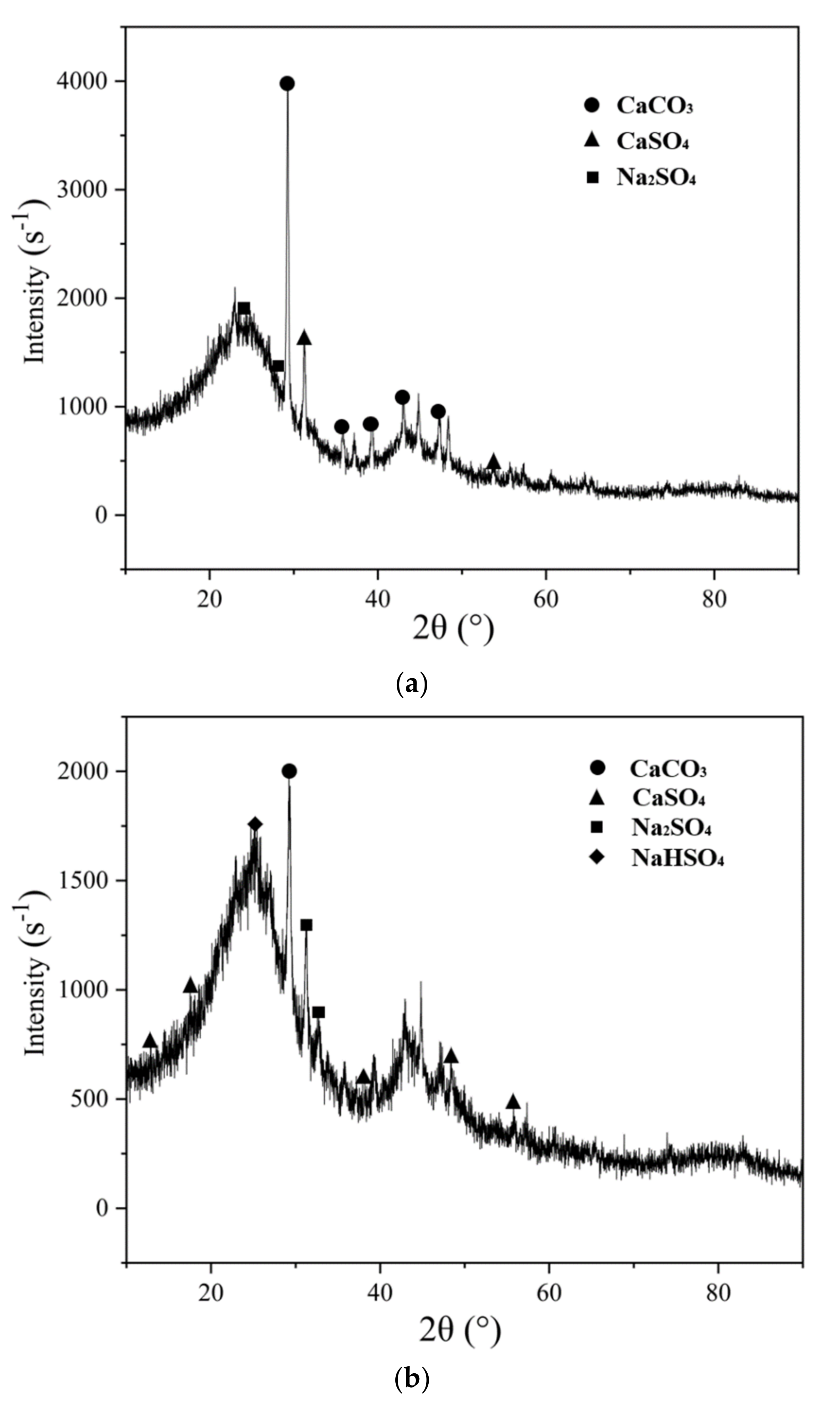
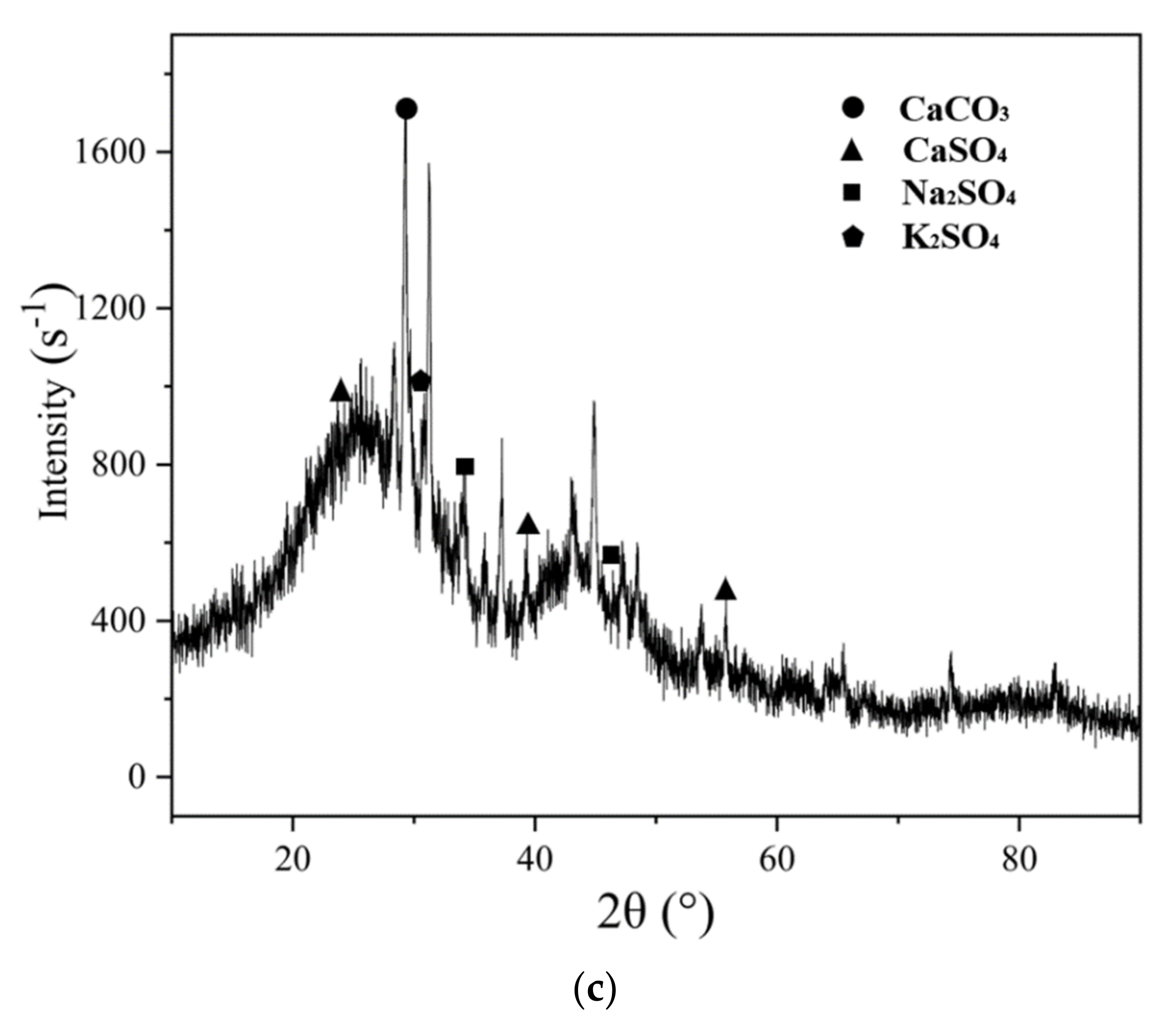
3.5. Phase Analysis of Activated Coke after Adsorption
4. Conclusions
- NH3 modification has a promoting effect on the adsorption of SO2 and NO in activated coke. With the continuous increase of NH3 concentration, the promoting effect is continuously enhanced, but the increase is not large. The presence of NH3 causes NO to undergo a reduction reaction to generate N2. A large number of crystalline particles appeared in the adsorbed activated coke. Combined with the FTIR spectrum after adsorption, SO2 was oxidized and combined with steam to form sulfate, which existed on the surface of activated coke in the form of crystals.
- K2CO3 solution modification greatly improves the desulfurization performance of activated coke. The fast adsorption stage of activated coke modified with 9% and 12% K2CO3 solutions was as long as 60 min, and the adsorption efficiency decreased slowly. The desulfurization efficiency of activated coke modified with 3% and 6% K2CO3 concentrations is approximately the same, and both are stable at about 60%. SO2 and NO are oxidized and combined with steam to form H2SO4 (adsorbed state) and HNO3 (adsorbed state), which regenerate activated sites. Through FTIR analysis, KNO3 was formed on the surface of the adsorbed activated coke.
- The K2CO3 solution modification did not change the surface functional groups of the activated coke, but the developed microporous structure and the loading of metal oxides enhanced the SO2 and NO adsorption capacity of the activated coke. When the unmodified activated coke synergistically adsorbs SO2 and NO, there is a competitive adsorption between NO and SO2; however, when the activated coke treated with K2CO3 solution and NH3 synergistically adsorbs SO2 and NO, both have a promoting effect. The vibration of different bonds will lead to the generation of absorption peaks in the same wavenumber range. With the adsorption of SO2 and NO on the activated coke, the functional groups on the surface of the activated coke will be reacted to generate SO42− and NO3−. The XRD patterns showed that the intensity of the diffraction peaks of the three decreased in turn, and the degree of graphitization decreased, indicating that the microporous structure was more developed. CaSO4 and Na2SO4 crystals appeared after the adsorption of NH3-modified activated coke, and K2SO4 existed after the adsorption of K2CO3 solution-modified activated coke. After SO2 is adsorbed, it combines with the substances in the activated coke in the form of SO42− to form sulfate minerals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Wu, Y.; Xu, Z.; Lu, S.; Li, X. Study on characteristics of organic components in condensable particulate matter before and after wet flue gas desulfurization system of coal-fired power plants. Chemosphere 2022, 294, 133668. [Google Scholar] [CrossRef] [PubMed]
- Iii, P.; Arden, C. Lung cancer, cardiopulmonary mortality, and long-term exposure to fineparticulate air pollution. Jama 2002, 287, 1132–1141. [Google Scholar]
- Wang, L.; Lu, L.; Li, M.; Liu, Y.; Levendis, Y. Effects of carbonization on the co-activation of sludge and biomass to produce activated coke. J. Energy Resour. Technol. 2021, 143, 102305. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Hao, M.; Liu, G.; Xu, S.; Chen, J.; Ren, X.; Levendis, Y.A. Effects of activation conditions on the properties of sludge-based activated coke. ACS Omega 2021, 6, 22020–22032. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, M.; Li, Y.; Ren, X.; Zhu, W.; Levendis, Y. Preparation of activated coke by one-step activation method, ammonization, and K2CO3 modification of coal and biomass. J. Energy Resour. Technol. 2022, 144, 012303. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Shuang, L.; Xu, H.; Levendis, Y.; Pyrolysis, A. Activated coke preparation by physical activation of coal and biomass co-carbonized chars. J. Anal. Appl. Pyrolysis 2021, 156, 105137. [Google Scholar] [CrossRef]
- Wey, M.; Fu, C.; Tseng, H.; Chen, K. Catalytic oxidization of SO2 from incineration flue gas over bimetallic Cu–Ce catalysts supported on pre-oxidized activated carbon. Fuel 2003, 82, 2285–2290. [Google Scholar] [CrossRef]
- Davini, P. Influence of surface properties and iron addition on the SO2 adsorption capacity of activated carbons. Carbon 2002, 40, 729–734. [Google Scholar] [CrossRef]
- Gaur, V.; Asthana, R.; Verma, N. Removal of SO2 by activated carbon fibers in the presence of O2 and H2O. Carbon 2006, 44, 46–60. [Google Scholar] [CrossRef]
- Mochida, I.; Korai, Y.; Shirahama, M.; Kawano, S.; Hada, T.; Seo, Y.; Yoshikawa, M.; Yasutake, A. Removal of SOx and NOx over activated carbon fibers. Carbon 2000, 38, 227–239. [Google Scholar] [CrossRef]
- Lizzio, A.A.; DeBarr, J.A.J.E. Mechanism of SO2 removal by carbon. Energy Fuels 1997, 11, 284–291. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Cazorla-Amoros, D.; Linares-Solano, A. Temperature programmed desorption study on the mechanism of SO2 oxidation by activated carbon and activated carbon fibres. Carbon 2001, 39, 231–242. [Google Scholar] [CrossRef]
- Bagreev, A.; Bashkova, S.; Bandosz, T.J. Adsorption of SO2 on activated carbons: The effect of nitrogen functionality and pore sizes. Langmuir 2002, 18, 1257–1264. [Google Scholar] [CrossRef]
- Martin, C.; Perrard, A.; Joly, J.; Gaillard, F.; Delecroix, V. Dynamic adsorption on activated carbons of SO2 traces in air: I. Adsorption capacities. Carbon 2002, 40, 2235–2246. [Google Scholar] [CrossRef]
- Wu, W.; Han, B.; Gao, H.; Liu, Z.; Jiang, T.; Huang, J. Desulfurization of flue gas: SO2 absorption by an ionic liquid. Angew. Chem. Int. Ed. 2004, 43, 2415–2417. [Google Scholar] [CrossRef] [PubMed]
- Mochida, I.; Kuroda, K.; Miyamoto, S.; Sotowa, C.; Korai, Y.; Kawano, S.; Sakanishi, K.; Yasutake, A.; Yoshikawa, M. Remarkable catalytic activity of calcined pitch based activated carbon fiber for oxidative removal of SO2 as aqueous H2SO4. Energy Fuels 1997, 11, 272–276. [Google Scholar] [CrossRef]
- Rubio, B.; Izquierdo, M.T. Low cost adsorbents for low temperature cleaning of flue gases. Fuel 1998, 77, 631–637. [Google Scholar] [CrossRef]
- Tamura, Z.; Hishinuma, Y.; Hisamura, T. Desulfurization process of flue gas by active carbons. Hitachi Hyoron 1968, 17, 1–9. [Google Scholar]
- Davini, P. Desulphurization properties of active carbons obtained from petroleum pitch pyrolysis. Carbon 1999, 37, 1363–1371. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A. The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon 2003, 41, 1925–1932. [Google Scholar] [CrossRef]
- Shirahama, N.; Moon, S.; Choi, K.-H.; Enjoji, T.; Kawano, S.; Korai, Y.; Tanoura, M.; Mochida, I. Mechanistic study on adsorption and reduction of NO2 over activated carbon fibers. Carbon 2002, 40, 2605–2611. [Google Scholar] [CrossRef]
- Boudou, J.-P.; Chehimi, M.; Broniek, E.; Siemieniewska, T.; Bimer, J. Adsorption of H2S or SO2 on an activated carbon cloth modified by ammonia treatment. Carbon 2003, 41, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-W.; Kim, H.-J.; Park, J.-W.; Choi, B.-U.; Choi, D.-K.; Park, J.-W. Adsorption and reaction behavior for the simultaneous adsorption of NO–NO2 and SO2 on activated carbon impregnated with KOH. Carbon 2003, 41, 1881–1888. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Blasiak, W.; Ponzio, A. Volumetric combustion of biomass for CO2 and NOx reduction in coal-fired boilers. Fuel 2012, 102, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Rokni, E.; Liu, Y.; Ren, X.; Levendis, Y. Nitrogen-bearing emissions from burning corn straw in a fixed-bed reactor: Effects of fuel moisture, torrefaction, and air flowrate. J. Energy Resour. Technol. 2019, 141, 082202. [Google Scholar] [CrossRef]
- Caliskan Sarikaya, A.; Haykiri Acma, H.; Yaman, S. Synergistic interactions during cocombustion of lignite, biomass, and their chars. J. Energy Resour. Technol. 2019, 141, 122203. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R. Surface modification and characterisation of a coal-based activated carbon. Carbon 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Chattopadhyaya, G.; Macdonald, D.G.; Bakhshi, N.N.; Mohammadzadeh, J.S.S.; Dalai, A.K. Preparation and characterization of chars and activated carbons from Saskatchewan lignite. Fuel Processing Technol. 2006, 87, 997–1006. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Q.; Liu, J.; Yang, M.; Yao, X. Technology. Role of Ni (NO3) 2 in the preparation of a magnetic coal-based activated carbon. Min. Sci. Technol. 2011, 21, 599–603. [Google Scholar]
- Teng, H.; Lin, H.C. Activated carbon production from low ash subbituminous coal with CO2 activation. AIChE J. 1998, 44, 1170–1177. [Google Scholar] [CrossRef]
- Teng, H.; Yeh, T.-S.; Hsu, L.-Y. Preparation of activated carbon from bituminous coal with phosphoric acid activation. Carbon 1998, 36, 1387–1395. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Teng, H. Influence of different chemical reagents on the preparation of activated carbons from bituminous coal. Fuel Processing Technol. 2000, 64, 155–166. [Google Scholar] [CrossRef]
- Wang, L.; Sha, L.; Zhang, S.; Cao, F.; Ren, X.; Levendis, Y. Preparation of activated coke by carbonization, activation, ammonization and thermal treatment of sewage sludge and waste biomass for SO2 absorption applications. Fuel Processing Technol. 2022, 231, 107233. [Google Scholar] [CrossRef]
- Chang, L.C.; Xiao, J.; Zhang, H.; Shen, L.H.; Yi-Qian, X.U. Experimental Study on Denitrification by Modified Activated Coke at Low Temperature. J. Taiyuan Univ. Technol. 2010, 5, 151–155. [Google Scholar]
- Zhang, X.J.; Bo, S.Y. Study on Modified Activated Coke and its Desulphurization Performance. Environ. Prot. Chem. Ind. 2001, 3, 52–55. [Google Scholar]
- Li, X.F.; Sun, Z.C.; Guo, Z.; Liang, D.M.; Xu, Z.G. Intrinsic kinetics of flue gas denitration by activated coke. J. China Coal Soc. 2010, 35, 1193–1196. [Google Scholar]
- Liang, J.; Hua, J.; Yin, H. Activated carbon-supported catalysts for denitrification of flue gas at low temperature. Sichuan Environ. 2006, 25, 99–104. [Google Scholar]
- Kong, Y.; Cha, C. NOx adsorption on char in presence of oxygen and moisture. Carbon 1996, 34, 1027–1033. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Yang, Q.Y.; Xu, L.S.; Zeng, H.C.; Zhang, L. Experimental study on adsorption and oxidation of activated carbon fiber to NO at low temperature. Electr. Power Environ. Prot. 2004, 20, 25–28. [Google Scholar]
- Lee, D.Y.; Cho, J.E.; Cho, N.I.; Lee, M.H.; Lee, S.J.; Kim, B.Y. Characterization of electrospun aluminum-doped zinc oxide nanofibers. Thin Solid Film. 2008, 517, 1262–1267. [Google Scholar] [CrossRef]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Characterization of the chemical bonding between Al2O3 and nanotube in MWCNT/Al2O3 nanocomposite. Curr. Nanosci. 2012, 8, 739–743. [Google Scholar] [CrossRef]
- Yang, D.J.; Ma, X.; Lv, H.; Li, B.; Zhang, C.M. NO adsorption and temperature programmed desorption on K2CO3 modified activated carbons. J. Cent. South Univ. 2018, 25, 2339–2348. [Google Scholar] [CrossRef]
- Zhai, Y.; Pang, D.; Chen, H.; Xiang, B.; Chen, J.; Li, C.; Zeng, G.; Qiu, L. Effects of ammonization on the surface physico-chemical properties of sludge-based activated carbon. Appl. Surf. Sci. 2013, 280, 590–597. [Google Scholar] [CrossRef]







| Modification Reagent | Total Surface Area (m2/g) | Micropore Specific Surface Area (m2/g) | Average Hole Diameter (nm) | Micropore Ratio (%) |
|---|---|---|---|---|
| unmodified | 585 | 334 | 3.40 | 57.1 |
| 5% NH3 | 620 | 325 | 3.18 | 52.5 |
| 10% NH3 | 626 | 328 | 3.28 | 52.4 |
| 15% NH3 | 637 | 332 | 2.86 | 52.0 |
| 20% NH3 | 652 | 330 | 3.38 | 50.6 |
| 3% K2CO3 | 627 | 517 | 2.67 | 82.4 |
| 6% K2CO3 | 602 | 540 | 1.94 | 89.8 |
| 9% K2CO3 | 484 | 469 | 1.94 | 96.9 |
| 12%K2CO3 | 438 | 427 | 2.20 | 97.5 |
| Sample | SO2 Adsorption Capacity (mg/g) | NO Adsorption Capacity (mg/g) |
|---|---|---|
| unmodified | 7.25 | 4.15 |
| 5% NH3 modified | 14.2 | 6.21 |
| 10% NH3 modified | 13.3 | 5.08 |
| 15% NH3 modified | 14.4 | 6.58 |
| 20% NH3 modified | 14.1 | 6.33 |
| 3% K2CO3 modified | 19.8 | 8.24 |
| 6% K2CO3 modified | 18.8 | 13.2 |
| 9% K2CO3 modified | 36.6 | 16.1 |
| 12% K2CO3 modified | 68.0 | 18.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Wang, L.; Li, Y.; Ren, X. Biomass and Coal Modification to Prepare Activated Coke for Desulfurization and Denitrification. Energies 2022, 15, 2904. https://doi.org/10.3390/en15082904
Liu G, Wang L, Li Y, Ren X. Biomass and Coal Modification to Prepare Activated Coke for Desulfurization and Denitrification. Energies. 2022; 15(8):2904. https://doi.org/10.3390/en15082904
Chicago/Turabian StyleLiu, Guangkui, Liwei Wang, Yukun Li, and Xiaohan Ren. 2022. "Biomass and Coal Modification to Prepare Activated Coke for Desulfurization and Denitrification" Energies 15, no. 8: 2904. https://doi.org/10.3390/en15082904
APA StyleLiu, G., Wang, L., Li, Y., & Ren, X. (2022). Biomass and Coal Modification to Prepare Activated Coke for Desulfurization and Denitrification. Energies, 15(8), 2904. https://doi.org/10.3390/en15082904








