Application of Slow Pyrolysis to Convert Waste Plastics from a Compost-Reject Stream into Py-Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstocks
2.2. Identification and Quantification
2.3. Carbonization Experiments
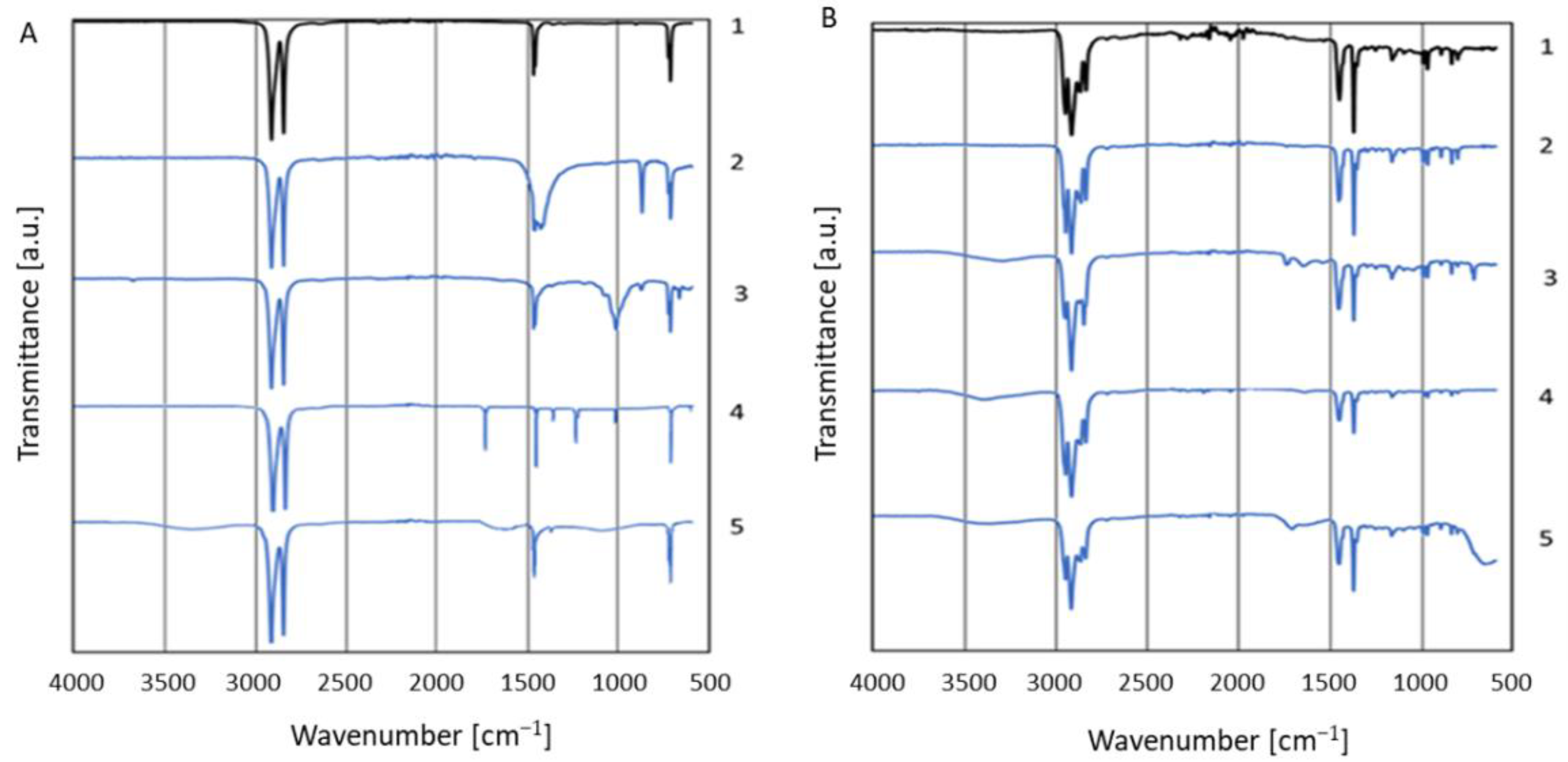
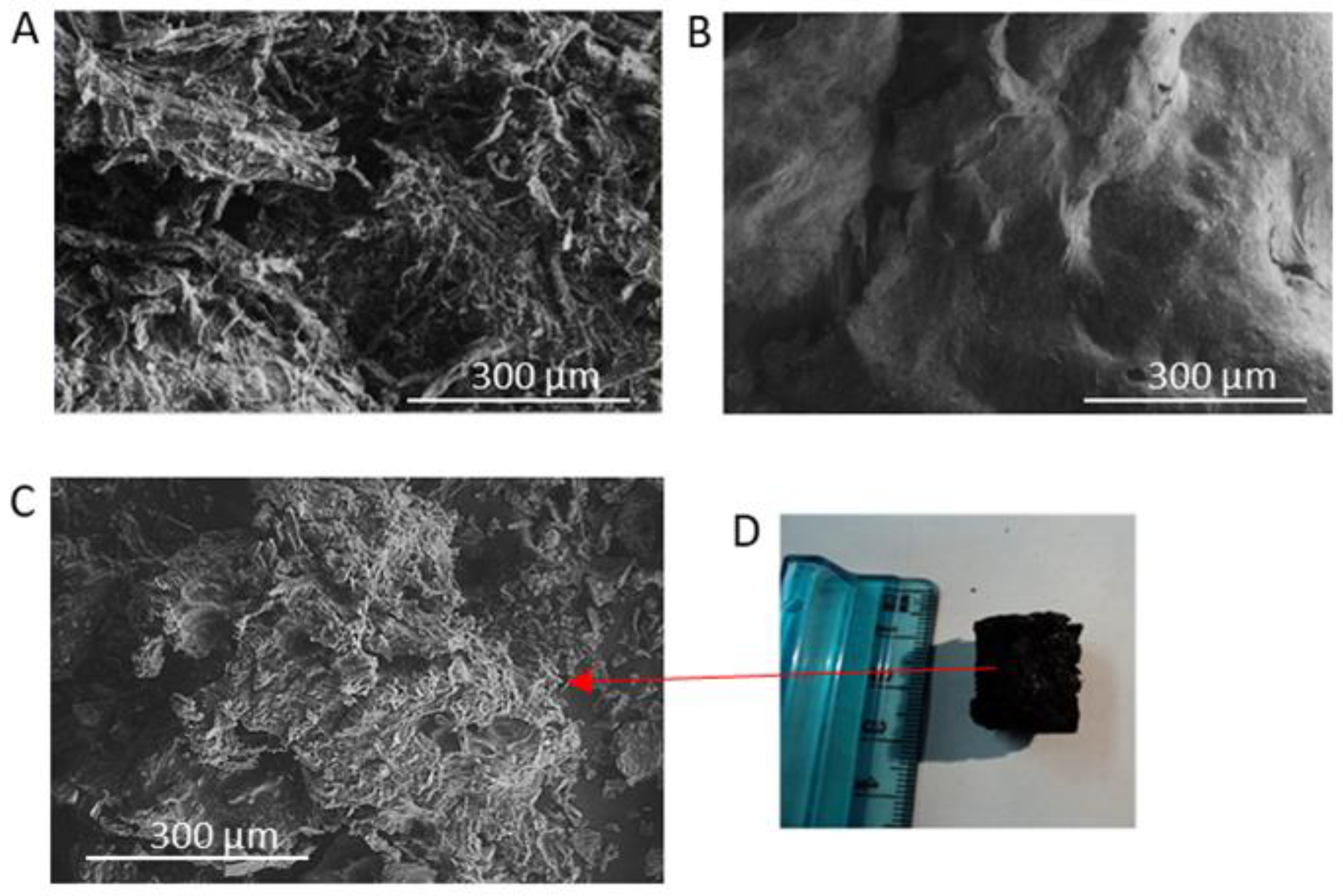
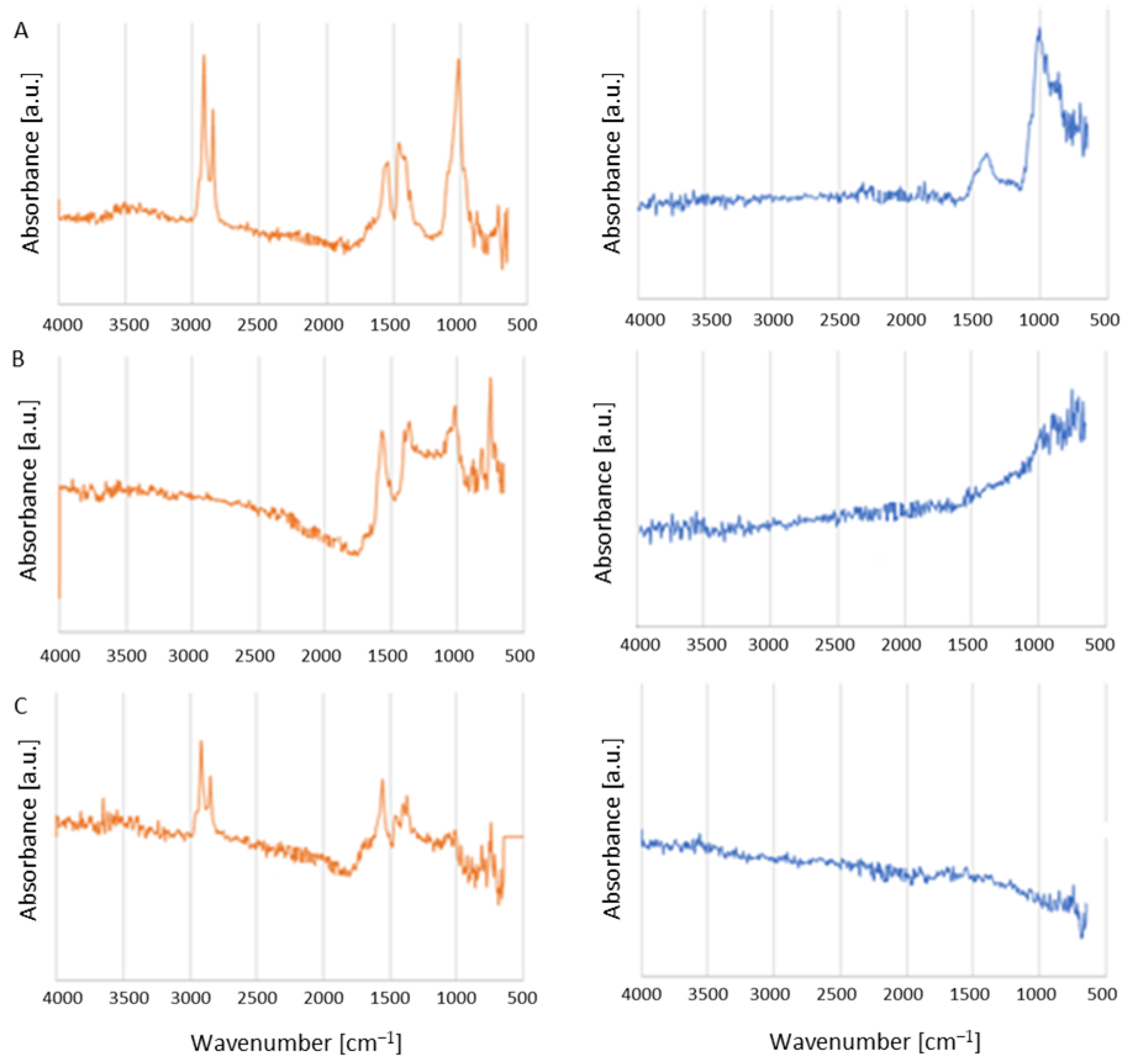
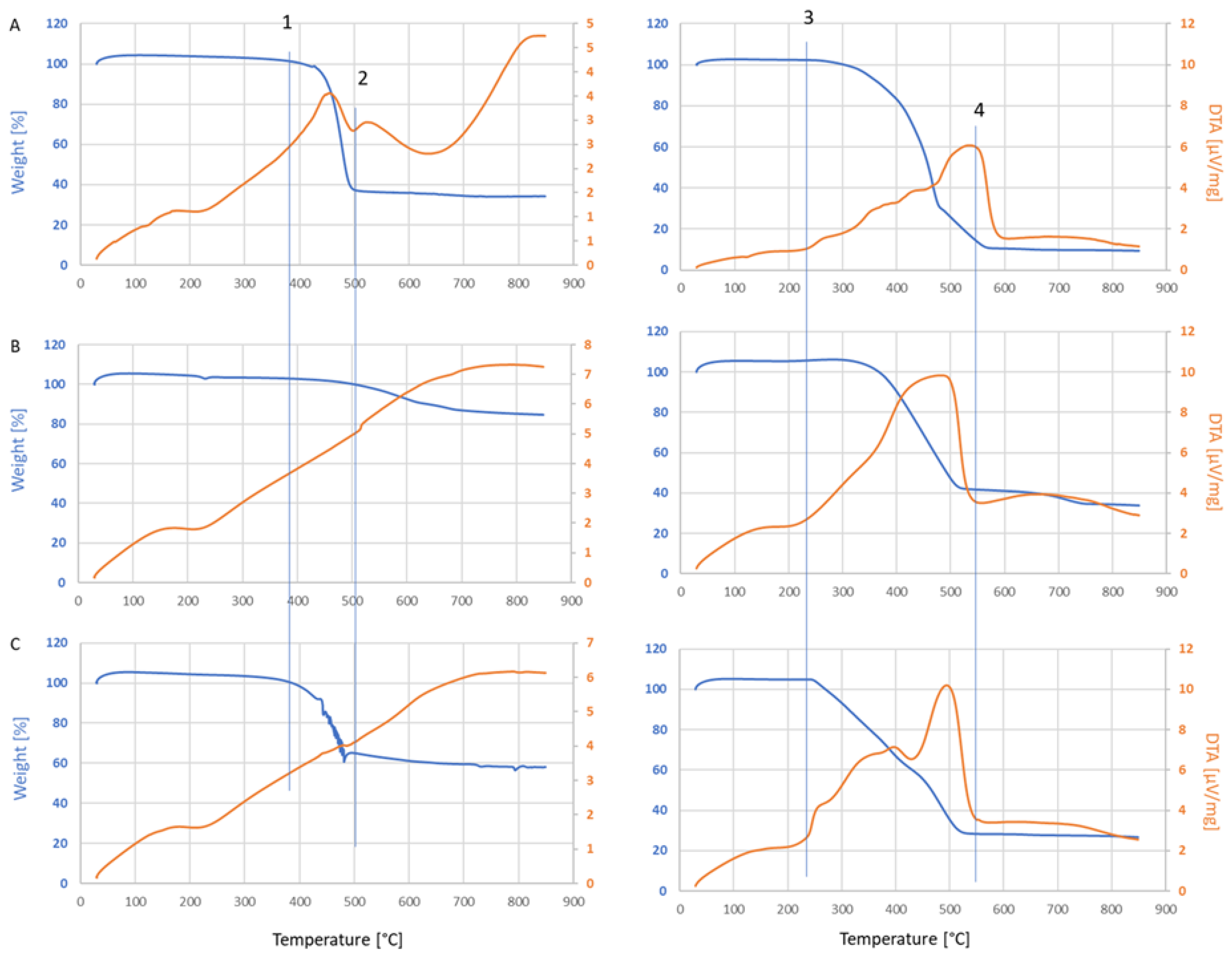
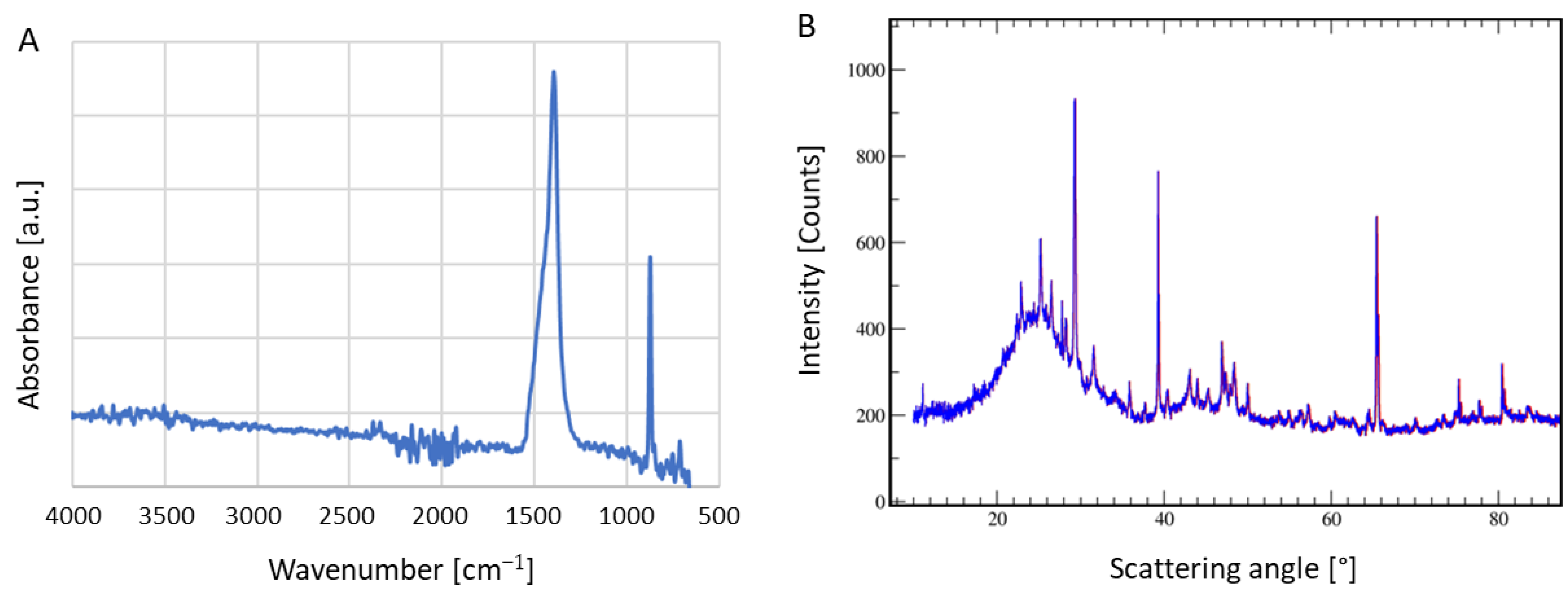
2.4. Char Characterization
3. Results
3.1. Composition Determination
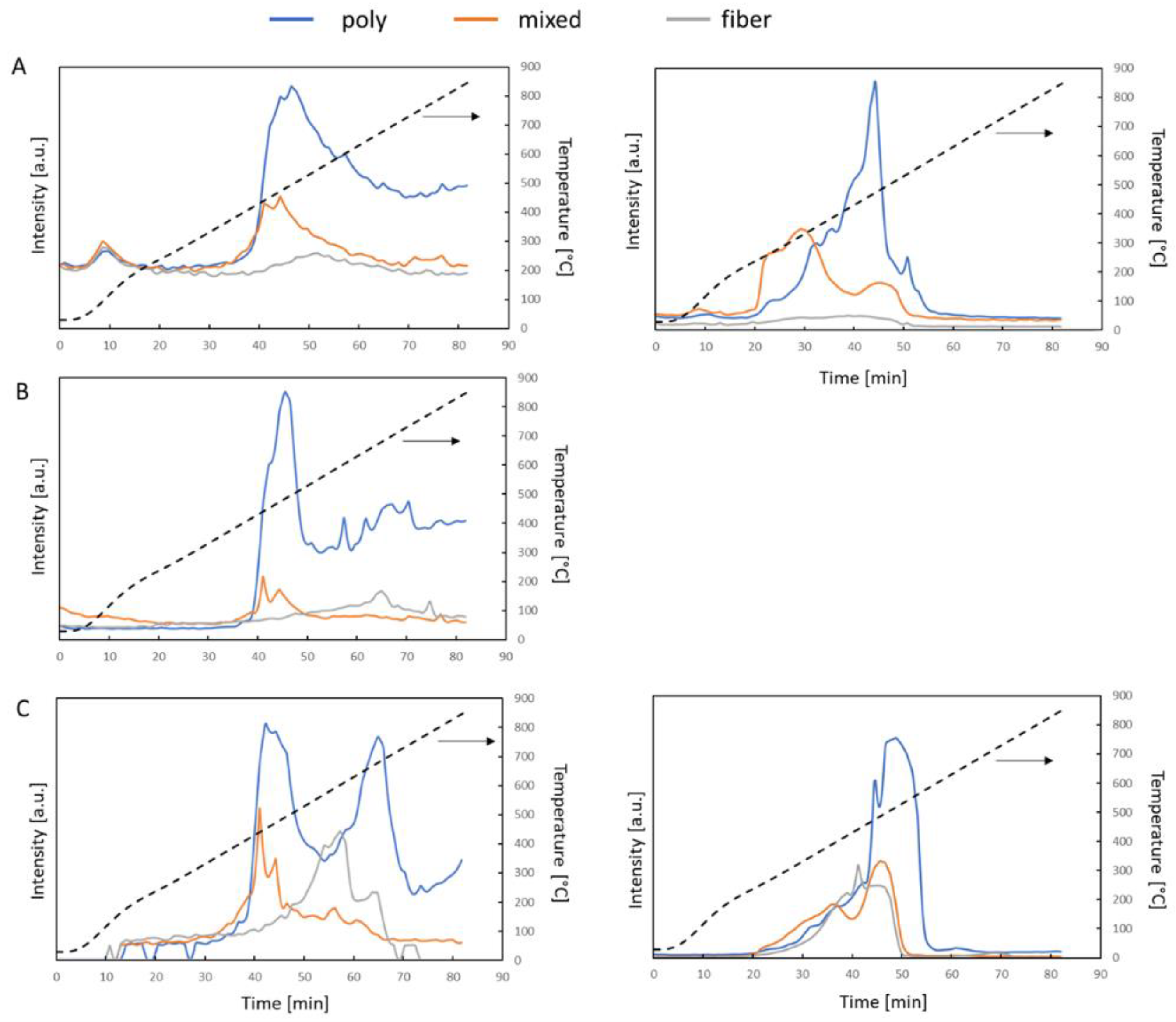
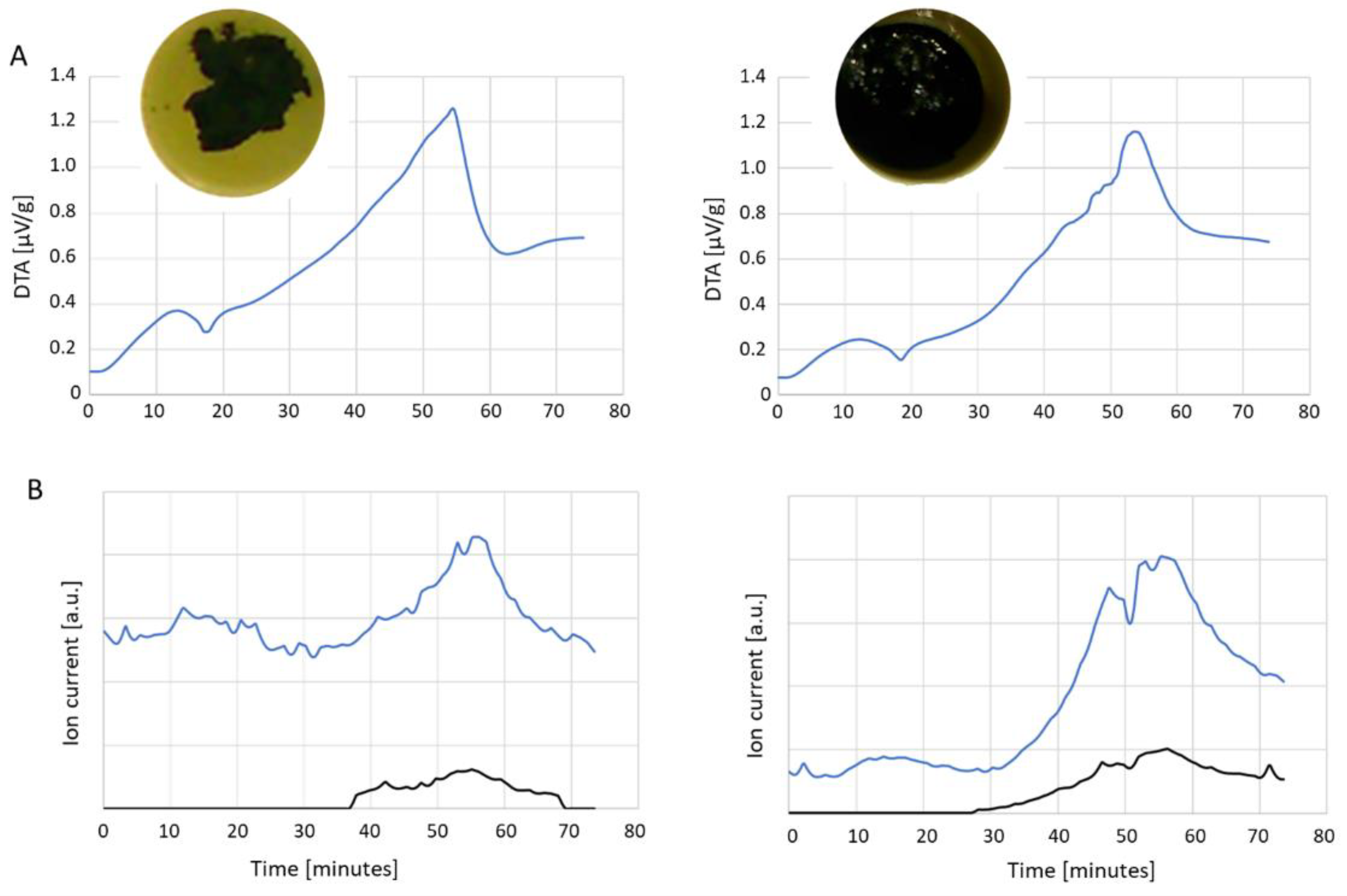
3.2. Char Formation and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Song, M.; Tang, M.; Lv, S.; Wang, X.; Jin, B.; Zhong, Z.; Huang, Y. The pyrolysis of multi-component municipal solid waste in fixed bed reactor for activated carbon production. J. Anal. Appl. Pyrolysis 2014, 109, 278–282. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrit, I. Pyrolysis of plastic wastes. 1. Effect of plastic waste composition on product yield. J. Anal. Appl. Pyrolysis 1999, 51, 39–55. [Google Scholar] [CrossRef]
- Milne, B.J.; Behie, L.A.; Berruti, F. Recycling of waste plastics by ultrapyrolysis using an internally circulating fluidized bed reactor. J. Anal. Appl. Pyrolysis 1999, 51, 157–166. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers. Manag. 2017, 148, 925–934. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Ates, F.; Miskolczi, N.; Borsodi, N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Production yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef]
- Bary, A.I.; Cogger, C.G.; Sullivan, D.M.; Myhre, E.A. Characterization of fresh yard trimmings for agricultural use. Bioresour. Technol. 2005, 96, 1499–1504. [Google Scholar] [CrossRef]
- Boldrin, A.; Andersen, J.K.; Moller, J.; Christensen, T.H.; Favoino, E. Composting and compost utilization: Accounting of greenhouse gases and global warming contributions. Waste Manag. Res. 2009, 27, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Akinbile, C.O.; Suffian, Y.M. Solid waste generation and decomposition using compost bin technique in Pulau Pinang, Malaysia. Waste Manag. Res. 2012, 30, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Huang, X.; Li, Z.; Tan, X.; Zeng, G.; Zhou, L. Potential Benefits of Biochar in Agricultural Soils: A Review. Pedosphere 2017, 27, 645–661. [Google Scholar] [CrossRef]
- Almeida, D.; Marques, M.D.F. Thermal and catalytic pyrolysis of plastic waste. Polímeros 2016, 26, 44–51. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, S.; Yang, X.; Li, G.; Zhou, H.; Li, Q.; Zhang, Y. Thermal behaviour and kinetic study of co-pyrolysis of microalgae with different plastics. Waste Manag. 2021, 126, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of Pyrolysis Processes in the Waste Management Sector. Therm. Sci. Eng. Progress. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- López, A.; Marco, D.I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Pyrolysis of municipal plastic wastes: Influence of raw material composition. Waste Manag. 2010, 30, 620–627. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag. 2021, 127, 101–111. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Yuan, H.; Chen, Y. Catalytic fast pyrolysis of waste mixed cloth for the production of value-added chemicals. Waste Manag. 2021, 127, 141–146. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Jiang, S.; Hou, H. Carbonization: A feasible route for reutilization of plastic wastes. Sci. Tot. Environ. 2020, 710, 136250. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Chiang, P.C.; You, J.H. Use of sewage sludge for manufacturing adsorbents. Can. J. Chem. Eng. 1987, 65, 922–927. [Google Scholar] [CrossRef]
- Lu, G.Q.; Low, J.C.F.; Liu, C.Y.; Lua, A.C. Surface area development of sewage sludge during pyrolysis. Fuel 1995, 74, 344–348. [Google Scholar] [CrossRef]
- Rio, S.; Faur-Brasquet, C.; le Coq, L.; le Cloirec, P. Structure Characterization and Adsorption Properties of Pyrolyzed Sewage Sludge. Environ. Sci. Technol. 2005, 39, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- Rasaq, W.A.; Golonka, M.; Scholz, M.; Białowiec, A. Opportunities and Challenges of High-Pressure Fast Pyrolysis of Biomass: A Review. Energies 2021, 14, 5426. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Plata, M.; Zaborowska, K.; Janus, R.; Lewandowski, M. Py-GC-MS Study on Catalytic Pyrolysis of Biocrude Obtained via HTL of Fruit Pomace. Energies 2021, 14, 7288. [Google Scholar] [CrossRef]
- Grzywacz, P.; Czerski, G.; Gańczarczyk, W. Effect of Pyrolysis Atmosphere on the Gasification of Waste Tire Char. Energies 2022, 15, 34. [Google Scholar] [CrossRef]
- Hu, H.; Gao, L.; Chen, C.; Chen, Q. Low-Cost, Acid/Alkaline-Resistant, and Fluorine-Free Superhydrophobic Fabric Coating from Onionlike Carbon Microspheres Converted from Waste Polyethylene Terephthalate. Environ. Sci. Technol. 2014, 48, 2928–2933. [Google Scholar] [CrossRef]
- Pol, S.V.; Pol, V.G.; Sherman, M.D.; Gedanken, A.S. A solvent free process for the generation of strong, conducting carbon spheres by the thermal degradation of waste polyethylene terephthalate. Green Chem. 2009, 11, 448–451. [Google Scholar] [CrossRef]
- Wei, L.Z.; Yan, N.; Chen, Q.W. Converting Poly(ethylene terephthalate) waste into carbon microspheres in a supercritical CO2 system. Environ. Sci. Technol. 2011, 45, 534–539. [Google Scholar] [CrossRef]
- Grieco, E.M.; Baldi, G. Pyrolysis of polyethylene mixed with paper and wood: Interaction effects on tar, char and gas yields. Waste Manag. 2012, 32, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Boateng, A.A.; Hicks, K.B.; Vogel, K.P. Pyrolysis of switchgrass (Panicum virgatum) harvested at several stages of maturity. J. Anal. Appl. Pyrolysis 2006, 75, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Iwanek, E.; Gliński, M. Application of thermal analysis in determining properties of herbaceous materials. J. Chem. Edu. 2018, 95, 1359–1364. [Google Scholar] [CrossRef]
- Di Blasi, C.; Signorelli, G.; Di Russo, C.; Rea, G. Product Distribution from Pyrolysis of Wood and Agricultural Residues. Ind. Eng. Chem. Res. 1999, 38, 2216–2224. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- McNamara, P.J.; Koch, J.D.; Liu, Z.; Zitomer, D.H. Pyrolysis of Dried Biosolids Can Be Energy Positive. Water Environ. Res. 2016, 88, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; McNamara, P.; Zitomer, D. Autocatalytic pyrolysis of wastewater biosolids for product upgrading. Environ. Sci. Technol. 2017, 51, 9808–9816. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.M.C.; Godina, R.; Oliveira Matias, J.C.; Ribeiro Nunes, L.J. Future Perspectives of Biomass Torrefaction: Review of the Current State-Of-The-Art and Research Development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef] [Green Version]
- Asensio, R.C.; San Andrés Moya, M.; de la Roja, J.M.; Gómez, M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.A.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.H.M.; Obbard, J.P. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Käppler, A.; Fischer, M.; Scholz-Böttcher, B.M.; Oberbeckmann, S.; Labrenz, M.; Fischer, D.; Eichhorn, K.-J.; Voit, B. Comparison of μ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 2018, 410, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Korez, Š.; Gutow, L.; Saborowski, R. Microplastics at the strandlines of Slovenian beaches. Mar. Pollut. Bull. 2019, 145, 334–342. [Google Scholar] [CrossRef]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Mugilarasane, M.; Gurumoorthi, K.; Guganathan, L.; Aboobackera, V.M.; et al. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Critic. Rev. Environ. Sci. Technol. 2020, 50, 1–64. [Google Scholar] [CrossRef]
- Strain, I.N.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A 2015, 3, 1632–1640. [Google Scholar] [CrossRef] [Green Version]
- Signoret, C.; Edo, M.; Caro-Bretelle, A.-S.; Lopez-Cuesta, J.-M.; Ienny, P.; Perrin, D. MIR spectral characterization of plastic to enable discrimination in an industrial recycling context: III. Anticipating impacts of ageing on identification. Waste Manag. 2020, 109, 51–64. [Google Scholar] [CrossRef]
- Choi, D.; Jang, D.; Joh, H.-I.; Reichmanis, E.; Lee, S. High performance graphitic carbon from waste polyethylene: Thermal oxidation as a stabilization pathway revisited. Chem. Mater. 2017, 29, 9518–9527. [Google Scholar] [CrossRef]
- Choi, D.; Yeo, J.S.; Joh, H.I.; Lee, S. Carbon nanosheet from polyethylene thin film as a transparent conducting film: “Upcycling” of waste to organic photovoltaics application. ACS Sustain. Chem. Eng. 2018, 6, 12463–12470. [Google Scholar] [CrossRef]
- Smith, M.W.; Pecha, B.; Helms, G.; Scudiero, L.; Garcia-Perez, M. Chemical and morphological evaluation of chars produced from primary biomass constituents: Cellulose, xylan, and lignin. Biomass Bioenergy 2017, 104, 17–35. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M. A new approach for evaluating biochar quality from Virginia Mallow biomass thermal processing. J. Clean. Prod. 2018, 214, 356–364. [Google Scholar] [CrossRef]
- Morais, J.A.; Gadioli, R.; Paoli, M.-A. Curaua fiber reinforced high-density polyethylene composites: Effect of impact modifier and fiber loading. Polímeros 2016, 26, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Blazsó, M. Recent trends in analytical and applied pyrolysis of polymers. J. Anal. Appl. Pyrolysis 1997, 39, 1–25. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanek, E.M.; Kirk, D.W. Application of Slow Pyrolysis to Convert Waste Plastics from a Compost-Reject Stream into Py-Char. Energies 2022, 15, 3072. https://doi.org/10.3390/en15093072
Iwanek EM, Kirk DW. Application of Slow Pyrolysis to Convert Waste Plastics from a Compost-Reject Stream into Py-Char. Energies. 2022; 15(9):3072. https://doi.org/10.3390/en15093072
Chicago/Turabian StyleIwanek (nee Wilczkowska), Ewa M., and Donald W. Kirk. 2022. "Application of Slow Pyrolysis to Convert Waste Plastics from a Compost-Reject Stream into Py-Char" Energies 15, no. 9: 3072. https://doi.org/10.3390/en15093072





