Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review
Abstract
:1. Introduction
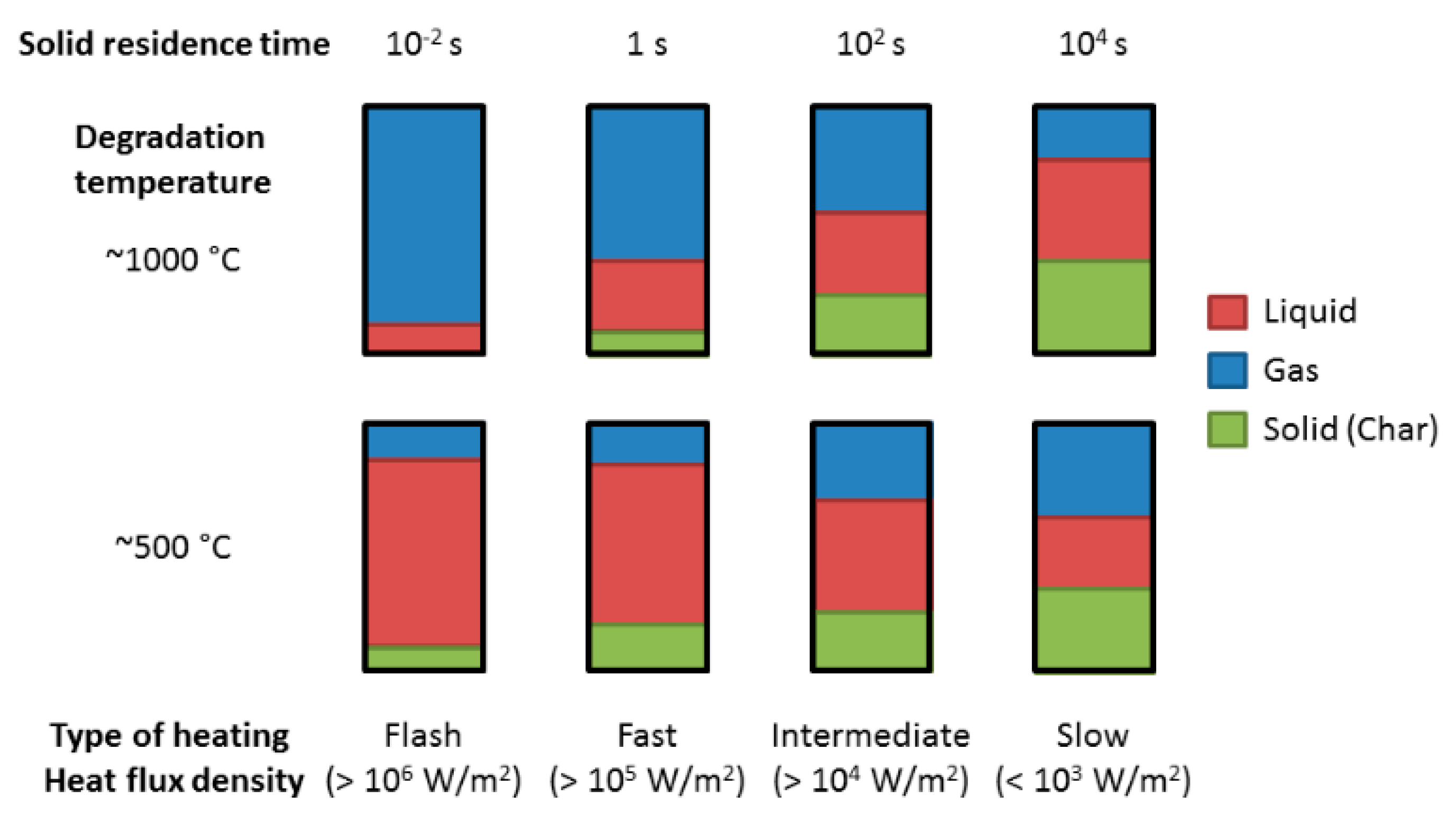
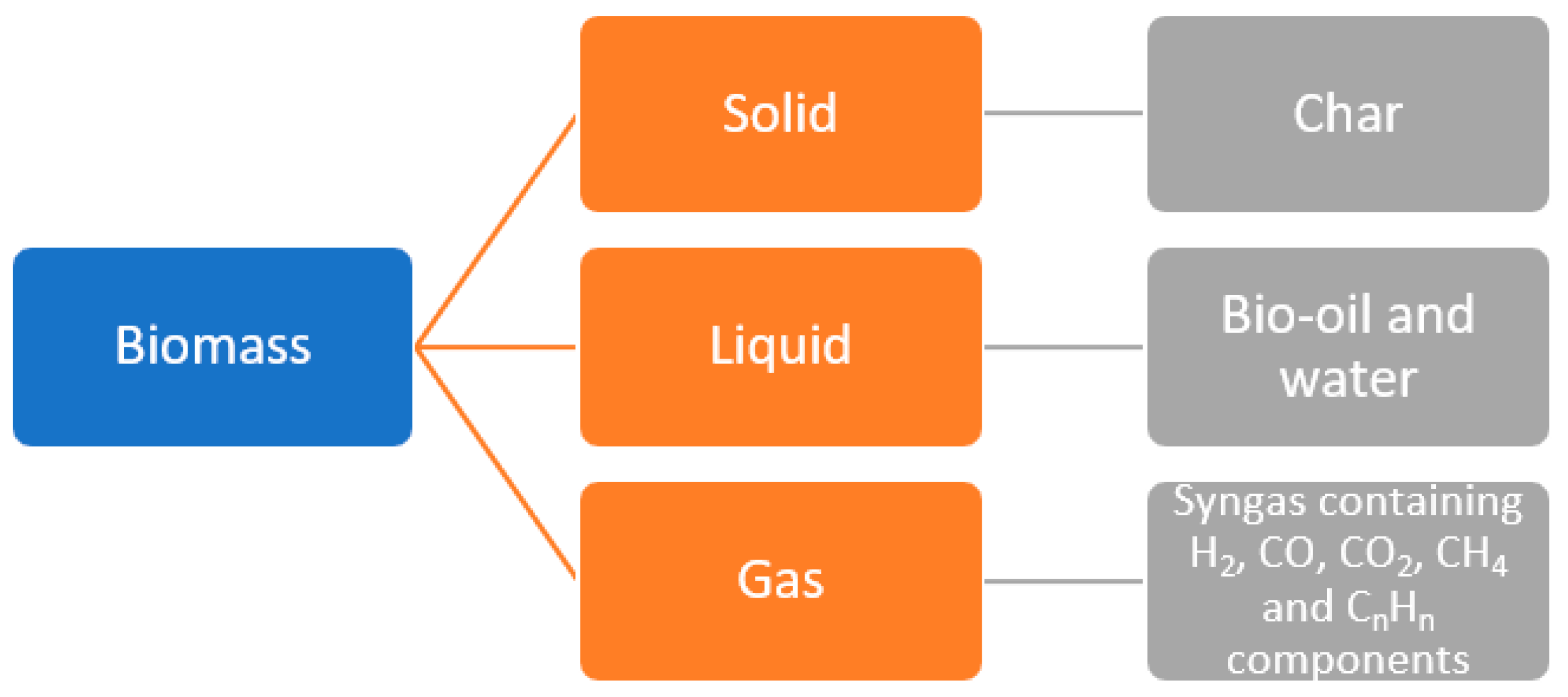
2. Pyrolysis and Catalytic Pyrolysis
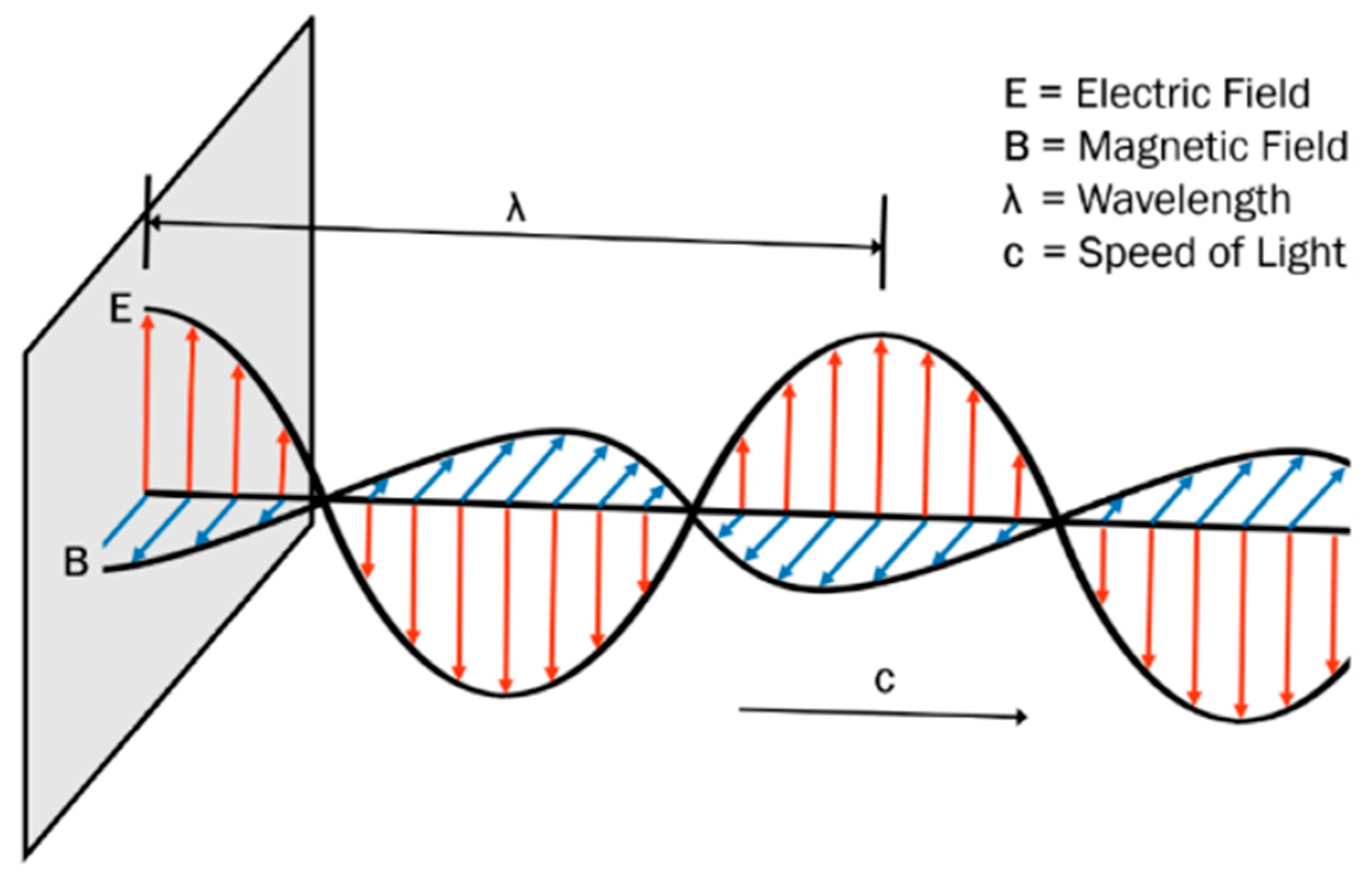
3. Fundamentals on Microwaves
- Insulators or microwave-transparent materials, through which microwaves may pass without any losses (e.g., quartz, Teflon and so on), commonly possess low dielectric loss factors and have very large penetration depths;
- Conductors or reflectors, which microwaves cannot penetrate and are reflected, are generally materials with high conductance and low capacitance (e.g., metals) that have high dielectric loss factors and hence, near-zero penetration depth for microwaves, and;
- Absorbers, where microwave irradiation may be absorbed most effectively (e.g., water, oils and so on), have dielectric loss factors in the middle of the conductivity range.
- Thermal effects (the influence of a high reaction temperature rapidly attained when irradiating polar materials in a microwave field);
- Specific effects (caused by the unique nature of the heating mechanism of microwave irradiation in a microwave field; this cannot be achieved using conventional heating) and;
- Non-thermal, non-specific effects (chemical transformation accelerations that have not been attributed to either thermal or specific microwave effects).
4. Microwave-Assisted Pyrolysis (MAP)
- Multimode applicators: the most commonly used types of applicators for domestic and laboratory purposes, where a large number of resonant modes for operational frequency are hosted.
- Single-mode applicators: most widely used for heterogeneous gas-phase reactions; capacity to attain high temperatures at low powers. These applicators are able to deliver a highly concentrated energy field, leading to faster heating rates.
- Travelling-wave applicators: microwaves travel in only one direction, ensuring no back-reflection of waves and hence, no non-uniform interference in the waveguide (channel through which microwave energy propagates from transmitter to receiver) and no standing wave formation.
4.1. Effect of MAP on Pyrolysis Product Distribution
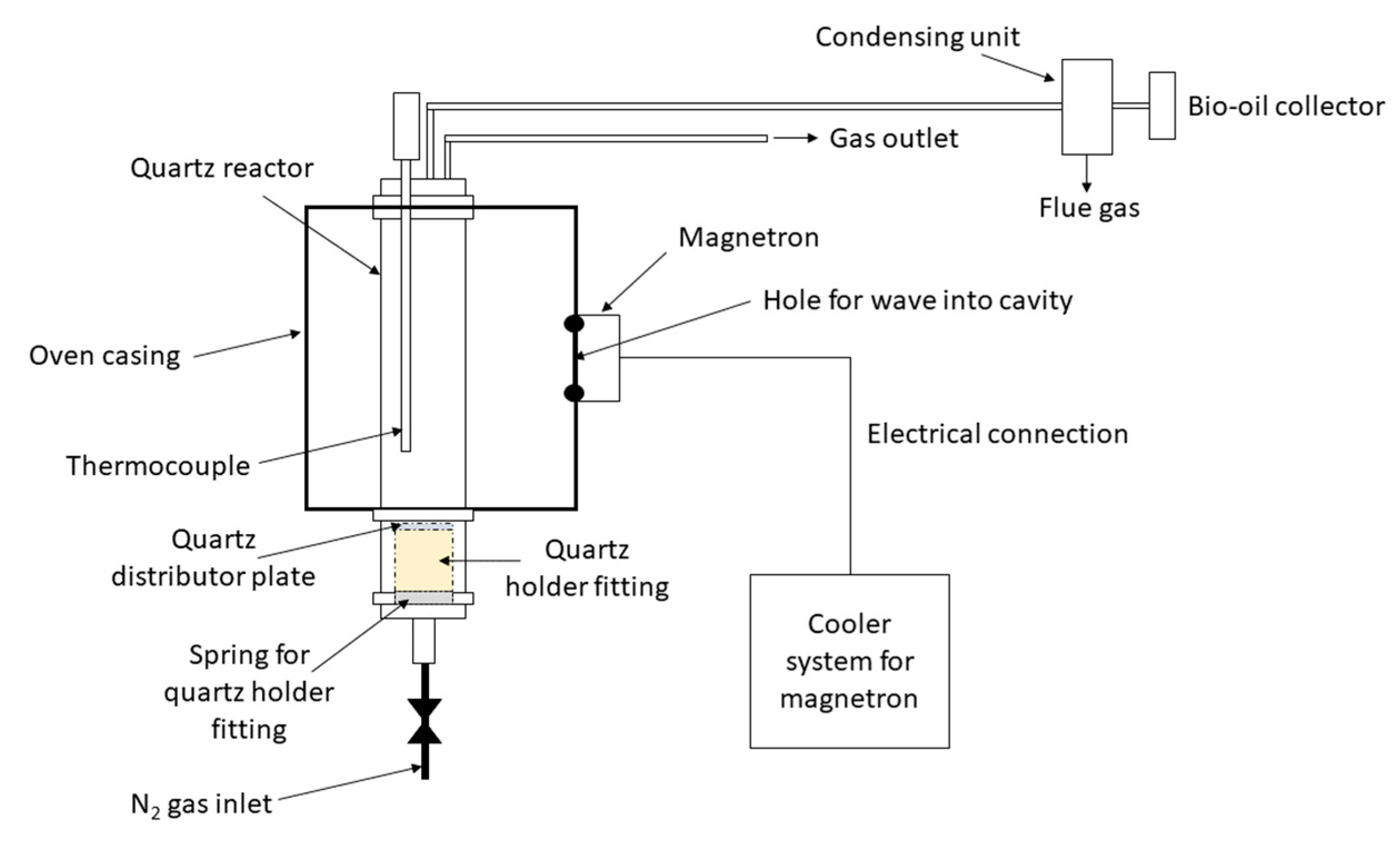
4.2. MAP in Fluidised Bed Reactors
4.3. Catalytic MAP
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Hemansi; Gupta, R.; Yadav, G.; Kumar, G.; Yadav, A.; Saini, J.K.; Kuhad, R.C. Second Generation Bioethanol Production: The State of Art. In Sustainable Approaches for Biofuels Production Technologies: From Current Status to Practical Implementation; Srivastava, N., Srivastava, M., Mishra, P.K., Upadhyay, S.N., Ramteke, P.W., Gupta, V.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–146. [Google Scholar] [CrossRef]
- Maj, G.; Najda, A.; Klimek, K.; Balant, S. Estimation of Energy and Emissions Properties of Waste from Various Species of Mint in the Herbal Products Industry. Energies 2020, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Mettler, M.S.; Vlachos, D.G.; Dauenhauer, P.J. Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ. Sci. 2012, 5, 7797–7809. [Google Scholar] [CrossRef]
- Oasmaa, A.; Czernik, S. Fuel Oil Quality of Biomass Pyrolysis OilsState of the Art for the End Users. Energy Fuels 1999, 13, 914–921. [Google Scholar] [CrossRef]
- Venderbosch, R.H. Fast Pyrolysis. In Thermochemical Processing of Biomass; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 175–206. [Google Scholar] [CrossRef]
- Onwudili, J.; Insura, N.; Williams, P. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Deglise, X. La gazéification thermochimique: Histoire et développement de la recherche. In Colloque ATEE/CIBE: Cogénération Biomasse Dans L’industrie Et Sur Les Réseaux de Chaleur; 2007; p. 39. Available online: https://cibe.fr/wp-content/uploads/2017/01/12_-_Historique_gazeification.pdf (accessed on 28 April 2022).
- Fagbemi, L.; Khezami, L.; Capart, R. Pyrolysis products from different biomasses: Application to the thermal cracking of tar. Appl. Energy 2001, 69, 293–306. [Google Scholar] [CrossRef]
- Rousset, P. From Biomass to Fuel, Power and Chemicals Brazilian Charcoal-Based Pig Iron. 2014. Available online: http://agritrop.cirad.fr/578836/ (accessed on 6 August 2018).
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.; Fonts, I.; Ábrego, J.; Westerhof, R.J.M. Historical Developments of Pyrolysis Reactors: A Review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, J.; Song, Z. Review of the direct thermochemical conversion of lignocellulosic biomass for liquid fuels. Front. Agric. Sci. Eng. 2015, 2, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Ronsse, F. Biochar Production. In Biochar; Bruckman, V.J., Apaydin Varol, E., Uzun, B.B., Liu, J., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 199–226. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in waste oil to sustainable energy, with emphasis on pyrolysis techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Lup, A.N.K.; Khan, S.; Abnisa, F.; Daud, W.M.A.W. A technical review on semi-continuous and continuous pyrolysis process of biomass to bio-oil. J. Anal. Appl. Pyrolysis 2018, 131, 52–75. [Google Scholar] [CrossRef]
- Mohabeer, C.; Reyes, L.; Abdelouahed, L.; Marcotte, S.; Buvat, J.-C.; Tidahy, L.; Abi-Aad, E.; Taouk, B. Production of liquid bio-fuel from catalytic de-oxygenation: Pyrolysis of beech wood and flax shives. J. Fuel Chem. Technol. 2019, 47, 153–166. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on Catalytic Hydrodeoxygenation of Pyrolysis Oil and Its Model Compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.; Lin, Y.-C.; Lee, H.V. A review on catalytic hydrodeoxygenation of lignin to transportation fuels by using nickel-based catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Wang, X.; Arai, M.; Wu, Q.; Zhang, C.; Zhao, F. Hydrodeoxygenation of lignin-derived phenolics—A review on the active sites of supported metal catalysts. Green Chem. 2020, 22, 8140–8168. [Google Scholar] [CrossRef]
- Bui, V.N.; Laurenti, D.; Afanasiev, P.; Geantet, C. Hydrodeoxygenation of guaiacol with CoMo catalysts. Part I: Promoting effect of cobalt on HDO selectivity and activity. Appl. Catal. B Environ. 2011, 101, 239–245. [Google Scholar] [CrossRef]
- Bui, V.N.; Laurenti, D.; Delichère, P.; Geantet, C. Hydrodeoxygenation of guaiacol: Part II: Support effect for CoMoS catalysts on HDO activity and selectivity. Appl. Catal. B Environ. 2011, 101, 246–255. [Google Scholar] [CrossRef]
- Bykova, M.; Ermakov, D.; Kaichev, V.; Bulavchenko, O.; Saraev, A.; Lebedev, M.; Yakovlev, V. Ni-based sol–gel catalysts as promising systems for crude bio-oil upgrading: Guaiacol hydrodeoxygenation study. Appl. Catal. B Environ. 2012, 113-114, 296–307. [Google Scholar] [CrossRef]
- Oh, S.; Choi, H.S.; Choi, I.-G.; Choi, J.W. Evaluation of hydrodeoxygenation reactivity of pyrolysis bio-oil with various Ni-based catalysts for improvement of fuel properties. RSC Adv. 2017, 7, 15116–15126. [Google Scholar] [CrossRef] [Green Version]
- Coumans, A.; Hensen, E. A real support effect on the hydrodeoxygenation of methyl oleate by sulfided NiMo catalysts. Catal. Today 2017, 298, 181–189. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Chen, G.; Zhang, R. Influence of CeO2 Addition to Ni–Cu/HZSM-5 Catalysts on Hydrodeoxygenation of Bio-Oil. Appl. Sci. 2019, 9, 1257. [Google Scholar] [CrossRef] [Green Version]
- Funkenbusch, L.T.; Mullins, M.E.; Salam, M.A.; Creaser, D.; Olsson, L. Catalytic hydrotreatment of pyrolysis oil phenolic compounds over Pt/Al2O3 and Pd/C. Fuel 2019, 243, 441–448. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, J.; Hwang, H.T.; Choi, J.H.; Woo, H.C.; Kim, S.-S. Catalytic Hydrodeoxygenation of Fast Pyrolysis Bio-Oil from Saccharina japonica Alga for Bio-Oil Upgrading. Catalysts 2019, 9, 1043. [Google Scholar] [CrossRef] [Green Version]
- Kavimonica, V.; Parasuraman, S.; Ravikrishnan, V. Kinetic Studies of Catalytic Upgradation of Biomass Model Compounds Using Analytical Py-gc/ms. Chem. Eng. Trans. 2020, 80, 1–6. [Google Scholar] [CrossRef]
- Tran, Q.K.; Han, S.; Ly, H.V.; Kim, S.-S.; Kim, J. Hydrodeoxygenation of a bio-oil model compound derived from woody biomass using spray-pyrolysis-derived spherical γ-Al2O3-SiO2 catalysts. J. Ind. Eng. Chem. 2020, 92, 243–251. [Google Scholar] [CrossRef]
- Williams, P.; Horne, P.A. The influence of catalyst type on the composition of upgraded biomass pyrolysis oils. J. Anal. Appl. Pyrolysis 1995, 31, 39–61. [Google Scholar] [CrossRef]
- Park, H.J.; Dong, J.I.; Jeon, J.K.; Yoo, K.S.; Yim, J.H.; Sohn, J.M.; Park, Y.K. Conversion of the Pyrolytic Vapor of Radiata Pine over Zeolites. J. Ind. Eng. Chem. 2007, 13, 182–189. [Google Scholar]
- Park, H.J.; Park, Y.K.; Kim, J.S.; Jeon, J.K.; Yoo, K.S.; Yim, J.H.; Sohn, J.M. Bio-oil upgrading over Ga modified zeolites in a bubbling fluidized bed reactor. In Studies in Surface Science and Catalysis; Rhee, H.-K., Nam, I.-S., Park, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 159, pp. 553–556. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Jeon, J.-K.; Kim, J.; Ryoo, R.; Jeong, K.-E.; Park, Y.-K. Highly valuable chemicals production from catalytic upgrading of radiata pine sawdust-derived pyrolytic vapors over mesoporous MFI zeolites. Appl. Catal. B Environ. 2010, 95, 365–373. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Lashkul, A.; Eränen, K.; Ziolek, M.; Decyk, P.; Salmi, T.; Holmbom, B.; Hupa, M.; Murzin, D.Y. Catalytic upgrading of woody biomass derived pyrolysis vapours over iron modified zeolites in a dual-fluidized bed reactor. Fuel 2010, 89, 1992–2000. [Google Scholar] [CrossRef]
- Zhang, H.; Carlson, T.R.; Xiao, R.; Huber, G.W. Catalytic fast pyrolysis of wood and alcohol mixtures in a fluidized bed reactor. Green Chem. 2012, 14, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Liu, Y.; Inaba, M.; Takahara, I. Catalytic fast pyrolysis of jatropha wastes. J. Anal. Appl. Pyrolysis 2012, 94, 75–82. [Google Scholar] [CrossRef]
- Choi, S.J.; Park, S.H.; Jeon, J.-K.; Lee, I.G.; Ryu, C.; Suh, D.J.; Park, Y.-K. Catalytic conversion of particle board over microporous catalysts. Renew. Energy 2013, 54, 105–110. [Google Scholar] [CrossRef]
- Guda, V.K.; Toghiani, H. Catalytic upgrading of pinewood fast pyrolysis vapors using an integrated Auger–packed bed reactor system: Effects of acid catalysts on yields and distribution of pyrolysis products. J. For. Prod. Ind. 2015, 4, 33–43. [Google Scholar]
- Jia, L.Y.; Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Bettahar, M.M.; Mauviel, G.; Tarrighi, M.; Pinard, L.; Dufour, A. Catalytic fast pyrolysis of biomass: Superior selectivity of hierarchical zeolites to aromatics. Green Chem. 2017, 19, 5442–5459. [Google Scholar] [CrossRef]
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 8, 100023. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Zlotorzynski, A. The Application of Microwave Radiation to Analytical and Environmental Chemistry. Crit. Rev. Anal. Chem. 1995, 25, 43–76. [Google Scholar] [CrossRef]
- van Loock, W. European Regulations, Safety Issues in RF and Microwave Power. In Advances in Microwave and Radio Frequency Processing; Springer: Berlin/Heidelberg, Germany, 2006; pp. 85–91. [Google Scholar] [CrossRef]
- CEM Corporation. Microwave Heating-Mechanism and Theory. 2020. Available online: https://cem.com/de/microwave-heating-mechanism-and-theory (accessed on 30 March 2022).
- Kappe, C.O.; Stadler, A.; Dallinger, D. Microwaves in Organic and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Xie, Q. Fast Microwave-Assisted Thermochemical Conversion of Biomass for Biofuel Production. 2015. Available online: http://conservancy.umn.edu/handle/11299/177123 (accessed on 14 September 2021).
- Nomanbhay, S.; Salman, B.; Hussain, R.; Ong, M.Y. Microwave pyrolysis of lignocellulosic biomass––A contribution to power Africa. Energy Sustain. Soc. 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2018, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Quitain, A.T.; Sasaki, M.; Goto, M. Microwave-Based Pretreatment for Efficient Biomass-to-Biofuel Conversion. In Pretreatment Techniques for Biofuels and Biorefineries; Fang, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 117–130. [Google Scholar] [CrossRef]
- Lam, S.S.; Chase, H.A. A Review on Waste to Energy Processes Using Microwave Pyrolysis. Energies 2012, 5, 4209–4232. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Ge, S.; Shi, Y.; Xia, C.; Huang, Z.; Manzo, M.; Cai, L.; Ma, H.; Zhang, S.; Jiang, J.; Sonne, C.; et al. Progress in pyrolysis conversion of waste into value-added liquid pyro-oil, with focus on heating source and machine learning analysis. Energy Convers. Manag. 2021, 245, 114638. [Google Scholar] [CrossRef]
- Giorcelli, M.; Das, O.; Sas, G.; Försth, M.; Bartoli, M. A Review of Bio-Oil Production through Microwave-Assisted Pyrolysis. Processes 2021, 9, 561. [Google Scholar] [CrossRef]
- Morgan, H.M.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef]
- State, R.N.; Volceanov, A.; Muley, P.; Boldor, D. A review of catalysts used in microwave assisted pyrolysis and gasification. Bioresour. Technol. 2019, 277, 179–194. [Google Scholar] [CrossRef]
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Vo, D.-V.N.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ. Chem. Lett. 2021, 19, 3609–3629. [Google Scholar] [CrossRef]
- Muley, P.D.; Wang, Y.; Hu, J.; Shekhawat, D. Microwave-assisted heterogeneous catalysis. Catalysis 2021, 33, 1–37. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Ethaib, S.; Omar, R. Effects of microwave absorbers on the products of microwave pyrolysis of oily sludge. J. Eng. Sci. Technol. 2018, 13, 3313–3330. [Google Scholar]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, X.; Chen, C. A study on experimental characteristic of microwave-assisted pyrolysis of microalgae. Bioresour. Technol. 2012, 107, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2011, 102, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Deng, S.; Chen, P.; Liu, Y.; Wan, Y.; Olson, A.; Kittelson, D.; Ruan, R. Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover. Appl. Biochem. Biotechnol. 2007, 137–140, 957–970. [Google Scholar] [CrossRef]
- Domínguez, A.; Menéndez, J.A.; Inguanzo, M.; Bernad, P.L.; Pis, J.J. Gas chromatographic–mass spectrometric study of the oil fractions produced by microwave-assisted pyrolysis of different sewage sludges. J. Chromatogr. A 2003, 1012, 193–206. [Google Scholar] [CrossRef]
- Fernández, Y.; Menéndez, J. Influence of feed characteristics on the microwave-assisted pyrolysis used to produce syngas from biomass wastes. J. Anal. Appl. Pyrolysis 2011, 91, 316–322. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Gao, B.; Meng, R.; Zhou, P.; Chen, G.; Zhan, Y.; Lu, W.; Wang, H. Comparison between hydrogen-rich biogas production from conventional pyrolysis and microwave pyrolysis of sewage sludge: Is microwave pyrolysis always better in the whole temperature range? Int. J. Hydrogen Energy 2021, 46, 23322–23333. [Google Scholar] [CrossRef]
- Salema, A.; Ani, F.N. Microwave induced pyrolysis of oil palm biomass. Bioresour. Technol. 2011, 102, 3388–3395. [Google Scholar] [CrossRef]
- Adam, M.; Beneroso, D.; Katrib, J.; Kingman, S.; Robinson, J.P. Microwave fluidized bed for biomass pyrolysis. Part I: Process design. Biofuels Bioprod. Biorefining 2017, 11, 601–612. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.Y.; Cheng, Y.W.; Xia, C.; Mahari, W.A.W.; Liew, R.K.; Peng, W.; Yuan, T.-Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Peng, Y.; Ke, L.; Yang, Q.; Jiang, L.; Dai, L.; Liu, Y.; Ruan, R.; Xia, D.; et al. Microwave-assisted pyrolysis of waste cooking oil for hydrocarbon bio-oil over metal oxides and HZSM-5 catalysts. Energy Conv. Manag. 2020, 220, 113124. [Google Scholar] [CrossRef]
- Muley, P.; Henkel, C.; Aguilar, G.; Klasson, K.; Boldor, D. Ex situ thermo-catalytic upgrading of biomass pyrolysis vapors using a traveling wave microwave reactor. Appl. Energy 2016, 183, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Wang, Y.; Liu, Y.; Dai, L.; Wu, Q.; Xia, M.; Zhang, S.; Ke, L.; Zou, R.; Ruan, R. Microwave catalytic co-pyrolysis of waste cooking oil and low-density polyethylene to produce monocyclic aromatic hydrocarbons: Effect of different catalysts and pyrolysis parameters. Sci. Total Environ. 2022, 809, 152182. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhong, Z.; Zhang, B. Microwave-Assisted Catalytic Fast Pyrolysis of Biomass for Hydrocarbon Production with Physically Mixed MCM-41 and ZSM-5. Catalysts 2020, 10, 685. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R.; Shukla, A.; Haldar, S. Effective deoxygenation for the production of liquid biofuels via microwave assisted co-pyrolysis of agro residues and waste plastics combined with catalytic upgradation. Bioresour. Technol. 2020, 302, 122775. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Kuan, W.-H.; Chang, C.-C.; Tzou, Y.-M. Catalytic and atmospheric effects on microwave pyrolysis of corn stover. Bioresour. Technol. 2013, 131, 274–280. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Huang, Y.-F.; Chang, C.-C.; Lo, S.-L. Catalytic pyrolysis of sugarcane bagasse by using microwave heating. Bioresour. Technol. 2013, 146, 324–329. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Sun, B.; Yan, Z. Influence of catalyst types on the microwave-induced pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 106, 86–91. [Google Scholar] [CrossRef]
- Ma, R.; Huang, X.; Zhou, Y.; Fang, L.; Sun, S.; Zhang, P.; Zhang, X.; Zhao, X. The effects of catalysts on the conversion of organic matter and bio-fuel production in the microwave pyrolysis of sludge at different temperatures. Bioresour. Technol. 2017, 238, 616–623. [Google Scholar] [CrossRef]
- Fan, L.; Chen, P.; Zhang, Y.; Liu, S.; Liu, Y.; Wang, Y.; Dai, L.; Ruan, R. Fast microwave-assisted catalytic co-pyrolysis of lignin and low-density polyethylene with HZSM-5 and MgO for improved bio-oil yield and quality. Bioresour. Technol. 2017, 225, 199–205. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Yu, J. Production of phenol-rich bio-oil during catalytic fixed-bed and microwave pyrolysis of palm kernel shell. Bioresour. Technol. 2016, 207, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y. High quality syngas production from microwave pyrolysis of rice husk with char-supported metallic catalysts. Bioresour. Technol. 2015, 191, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, Y.; Yin, J.; Li, Z. Bi-objective Mixed Optimal Planning for Distributed Energy Storage System of Active Distribution System. In Proceedings of the 2018 8th International Conference on Power and Energy Systems (ICPES), Colombo, Sri Lanka, 21–22 December 2018; pp. 34–39. [Google Scholar] [CrossRef]
- Guda, V.K.; Steele, P.H.; Penmetsa, V.K.; Li, Q. Chapter 7-Fast Pyrolysis of Biomass: Recent Advances in Fast Pyrolysis Technology. In Recent Advances in Thermo-Chemical Conversion of Biomass; Pandey, A., Bhaskar, T., Stöcker, M., Sukumaran, R.K., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 177–211. [Google Scholar] [CrossRef]



| First-Generation Sources | Virgin resources | Forest resources | Wood such as pine, woody and forest biomass such as willow, poplar, aspen |
| Oil crops | Wheat, barley, corn, canola, rapeseed, palm oil, soybean, sugarcane, flax, oat, straw, pasture grasses | ||
| Second-Generation Sources | Residues | Wood residues | Bark, branches, leftover treetop, leaves from harvest, sawdust, shavings from pulp and sawmills |
| Agricultural residues and waste | Residual fraction from oil crop harvest, waste oil/fat | ||
| Livestock residues | Livestock excrement and carcass | ||
| Municipal solid waste (MSW) | Residential | Cardboard and mixed paper, glass, various metals, electronics, plastics, tyres, organics | |
| Non-residential | Sewage sludge | ||
| Third-Generation Sources | Algae | - | Macroalgae, microalgae |
| Feed | Reaction Conditions | Results | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Catalyst Used | Reactor Used | Temperature (°C), Pressure (bar), H2 Percentage | WHSV * (g feed/g cat. h), Contact Time (s) or F/C * Ratio | Bio-Oil Yield (wt. %) | Products and Conclusions | ||
| Guaiacol | MoS2 | Fixed bed | 300 °C, 40 bar, 100% H2 | 0.3–1 s | - | HDO transformation of guaiacol was significantly enhanced by promoting MoS2-based catalysts with Co. However, the use of Al2O3 as support gave rise to demethylation and production of methyl-substituted products, which render de-oxygenation more difficult. | [21] |
| CoMoS | |||||||
| MoS2/Al2O3 | |||||||
| CoMoS/Al2O3 | |||||||
| Guaiacol | MoS2/γ-Al2O3 | Fixed bed | 300 °C, 40 bar, 100% H2 | 0.3–1 s | - | Out of MoS2-based catalysts, the TiO2-supported one gave the most interesting improvements. As for CoMoS, ZrO2 proved to have the most significant catalytic activities. The CoMoS/ZrO2 system was very selective towards aromatic (C-O) hydrogenolysis as demethoxylation and direct de-oxygenation were both observed. | [22] |
| MoS2/TiO2 | |||||||
| MoS2/ZrO2 | |||||||
| CoMo/γ-Al2O3 | |||||||
| CoMo/TiO2 | |||||||
| CoMo/ZrO2 | |||||||
| Guaiacol | NiCu/ CeO2-ZrO2 | Batch | 280–360 °C, 170 bar, 100% H2 | 337.5:1 | - | NiCu/SiO2-ZrO2-La2O3 was the most efficient catalyst. Deoxygenation degree increased with temperature, while guaiacol conversion decreased due to catalyst coking at higher temperatures. | [23] |
| NiCu/Al2O3 | |||||||
| NiCu/SiO2 | |||||||
| NiCu/SiO2-ZrO2-La2O3 | |||||||
| Ni/SiO2 | |||||||
| Ni-Cu-MgO | |||||||
| Yellow poplar bio-oil | Ni/C | Autoclave | 300 °C, 30 bar, 100% H2 | 25:1 | 63.8 | The mesoporous silica supports (SBA-15 and Al-SBA-15) showed greater catalytic activity than Ni/C. this could be correlated to a higher surface area and pore size, which caused uniform dispersion of NI particles. | [24] |
| Ni/SBA-15 | 76.0 | ||||||
| Ni/Al-SBA-15 | 74.8 | ||||||
| Methyl oleate (as model compound for green diesel) | NiMo/C | Fixed bed | 260 °C, 30 bar, 90% H2 and 10% H2S | 6.5 g feed/g cat. h | - | NiMo/Al2O3 and NiMo/ASA showed the highest global HDO activity, however, they de-activated during the run—owing to Lewis acidity of Al surface active in methyl oleate hydrolysis. NiMo/SiO2 had similar overall activity than the two previous, but did not de-activate. NiMo/C did not de-activate either, and was more active than the others at the end of the run. | [25] |
| NiMo/SiO2 | |||||||
| NiMo/ASA | |||||||
| NiMo/Al2O3 | |||||||
| Rice husk bio-oil | Ni-Cu/HZSM-5 | Autoclave | 270 °C, 20 bar, 100% H2 | 12:1 | 33.9 | Addition of CeO2 to Ni-Cu/HZSM-5 improved the performance of the catalyst. It caused an improvement in the Ni dispersion, redox ability and the Bronsted acidity ratio. It also decreased the particle size of the catalyst and diminished coke deposition on the catalyst surface, which causes catalyst deactivation. In total, 15% CeO2-Ni-Cu/HZSM-5 was the best in terms of catalyst efficiency. | [26] |
| 5% CeO2-Ni-Cu/ HZSM-5 | 37.4 | ||||||
| 15% CeO2-Ni-Cu/ HZSM-5 | 47.6 | ||||||
| 20 % CeO2-Ni-Cu/ HZSM-5 | 35.3 | ||||||
| Anisole, m-cresol and phenol | 5% Pd/C | Parr batch | 250–350 °C, 50 bar, 100% H2 | - | Residence time of 4 h allowed the formation of high yields of deoxygenated compounds. Pt/Al2O3 promoted hydrogenation (ring saturation) and removal of the pendant groups, and a significant pathway shift was observed as temperature increased. Pd/C showed ring saturation followed by methanol abstraction. | [27] | |
| 5% Pt/Al2O3 | |||||||
| Saccharina Japonica bio-oil | HZSM-5 | Autoclave | 350 °C, 3–15 bar, 100% H2 | 10:1 | 80.4 | Pressures of 3 to 15 bar were tested and 15 bar provided the highest bio-oil yield; all catalytic tests were hence performed at 15 bar. HHV* of HDO-upgraded bio-oils improved with use of Co/γ-Al2O3, but decreased with use of metal phosphide catalysts. The latter promoted decarboxylation while metal catalysts elevated demethylation. An increase in kerosene fraction of bio-oil was also observed with the use of catalytic HDO. | [28] |
| Co/γ-Al2O3 | 77.6 | ||||||
| Fe//γ-Al2O3 | 82.1 | ||||||
| CoP//γ-Al2O3 | 82.7 | ||||||
| Fe2P//γ-Al2O3 | 78.8 | ||||||
| CoMoP//γ-Al2O3 | 68.0 | ||||||
| Guaiacol and furfural | Zeolite Y (Si/Al: 5.1:1) | Pyroprobe (Py-GC/MS) | 500 °C, 1.01 bar, 100% H2 | 1:10 | - | Conversion over catalysts with low Si/Al ratios gave rise to the production of aromatics (benzene, toluene, xylene and phenol). Highest benzene yield was 19.5 wt. % over zeolite Y (30:1). Trend of overall BTX yield was: Zeolite Y (30:1), 30.5% > Zeolite Y (5.1:1), 28.1% > Zeolite Y (80:1), 12.0% > Zeolite Y (60:1), 6.4%. It was also shown that zeolite acidity played an important role in the deoxygenation of guaiacol, but not in that of furfural. | [29] |
| Zeolite Y (Si/Al: 30:1) | |||||||
| Zeolite Y (Si/Al: 60:1) | |||||||
| Zeolite Y (Si/Al: 80:1) | |||||||
| Guaiacol | γ-Al2O3-SiO2 | Fixed bed | 275 or 300 °C, 1.01 bar, 25% H2 | 6.50 g guaiacol/g cat. h | - | Catalyst with a 50:50 Al/Si ratio after calcination at 450 °C exhibited the highest guaiacol conversion (81.79%). | [30] |
| Feed | Reaction Conditions | Results | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Catalyst Used | Reactor Used | Temperature (°C) | WHSV (g feed/g cat. h) or F/C Ratio | Bio-Oil Yield (wt. %) | Product Quality, Oxygen Content and Conclusions | ||
| Waste wood shavings | HZSM-5 | Fluidised bed | 500–550 | 1.05–1.14 g biomass/g cat. h | 28.8 | Good aromatics yield (15.9 wt. %) | [31] |
| Na-ZSM-5 | 24.3 | Higher aromatics yield (21.3 wt. %) | |||||
| Y-Zeolite | 28.6 | Average aromatics yield (14.0 wt. %) | |||||
| Activated alumina | 31.2 | Lowest aromatics yield (8.0 wt. %) | |||||
| Radiata pine sawdust | HZSM-5 | Bubbling fluidised bed | 475–625 | 4 g biomass/g cat. h | 43.7 | Formation of mainly aromatic hydrocarbons | [32,33] |
| H-Y | 8 g biomass/g cat. h | 45.7 | Almost no formation of aromatics | ||||
| Ga/HZSM-5 | 4 g biomass/g cat. h | 51.3 | Higher selectivity for aromatics (as compared to HZSM-5) | ||||
| Radiata pine sawdust | HZSM-5 | Fixed bed | 500 | 10:1 | 46.6 | Reduction of non-phenolic oxygenates | [34] |
| MMZHZSM-5 (mesoporous material from HZSM-5) | 50.6 | No change in phenols; reduction in other oxygenates | |||||
| MFI zeolite | 45.9 | High aromatics yield; reduction in non-phenolic oxygenates and in phenols | |||||
| 1% Ga/MFI | |||||||
| 5% Ga/MFI | Similar to HZSM-5; less efficient than 1% Ga/MFI | ||||||
| Pine sawdust | H-β | Dual fluidised bed | 450 | 8.33:1 | 37.8 | β-zeolite was the most efficient for deoxygenation, followed by Y and ferrierite zeolites | [35] |
| Fe-H-β | 37.0 | ||||||
| H-Y | 39.7 | ||||||
| Fe-H-Y | 34.3 | ||||||
| H-Ferrierite | 43.8 | ||||||
| Fe-H-Ferrierite | 44.5 | ||||||
| Pine wood | ZSM-5 | Fluidised bed | 450–600 | 0.35 g biomass/g cat. h | - | Increase in selectivity of aromatics | [36] |
| Jatropha wastes | HZSM-5 | Fixed bed | 550 | 2:1 | 4.1–8.7 | Conversion of 76.7–91.6% found; production of mainly aromatics | [37] |
| Particle board | HZSM-5 | Batch | 500 | 10:1 | 42.5 | Reduction in oxygenates and increase in aromatics and phenolics | [38] |
| Ga/HZSM-5 | 46.3 | Larger reduction in oxygenates and higher increase in aromatics (compared to HZSM-5); lower phenolic content | |||||
| H-β | 44.6 | Similar to HZSM-5, but less efficient | |||||
| Pine wood | β/Al2O3 | Auger | 450 | 12 g biomass/g cat. h | 52.0 | Si/Al and β-zeolite contributed to increase of liquid product while HZSM-5 and H-Y led to formation of aromatics and higher gas percentage. HZSM-5 reduced oxygen content of 46.4 wt. % to 30 wt. %. | [39] |
| Si/Al | 51.0 | ||||||
| H-Y | 43.0 | ||||||
| HZSM-5 | 41.0 | ||||||
| Oak wood cylinder particles | HZSM-5 | Micro fluidised bed | 500 | 0.85:1 | 8.3–10.1 | High selectivity in monoaromatic compounds (4.4 wt. %); CO2:CO = 0.5 | [40] |
| Desilicated HZSM-5 | Higher selectivity in monoaromatic compounds (6.2 wt. %); same CO2:CO | ||||||
| HZSM-5 | Fixed bed | - | High selectivity in monoaromatics (4.1 wt. %), but less efficient than FBR * | ||||
| Desilicated HZSM-5 | Higher selectivity than parent HZSM-5 (5.1 wt. %), but less efficient than FBR * | ||||||
| Beech wood (BW) | HZSM-5 | Semi- continuous | 500 | 4:1 | 50.0 | Fe-HZSM-5 was the most efficient in deoxygenation (33.82 to 17.50% for BW and 34.76 to 17.31% for FS). Zeolites were found to cause decomposition of carboxylic acids to form phenols, H2, H2O, CO2 and CO, mostly. Pt and CoMo-based catalysts did not show high efficiencies; these catalysts might be better suited for HDO applications. | [17] |
| Fe-HZSM-5 | 58.8 | ||||||
| H-Y | 49.0 | ||||||
| Fe-H-Y | 61.0 | ||||||
| Pt/Al2O3 | 60.0 | ||||||
| CoMo/Al2O3 | 54.0 | ||||||
| Flax shives (FS) | HZSM-5 | 50.0 | |||||
| Fe-HZSM-5 | 58.8 | ||||||
| H-Y | 41.0 | ||||||
| Fe-H-Y | 51.0 | ||||||
| Pt/Al2O3 | 52.0 | ||||||
| CoMo/Al2O3 | 47.0 | ||||||
| Feed | Temperature (°C) | Fluidising Gas | Products |
|---|---|---|---|
| Polyethylene (PE), Polypropylene (PP) | 400–550 | Nitrogen, steam and pyrolysis gas | 70–90% waxy products, oil, 2–9% gas |
| Polymethylmethacrylate (PMMA), Polystyrene (PS) | 400–500 | Pyrolysis gas | 70–95% monomers, 5–30% gas, oil |
| Polyethylene terephthalate (PET) | 400–550 | Steam | 45–60% monomers, 40–45% gas, oil |
| PE, PP | 700–800 | Nitrogen, steam | 70–80% gas (olefins), 20–30% oil |
| PE, PP, polyamide (PA) | 700–800 | Pyrolysis gas | 30–50% aromatics, 30–40% oil, gas, 1–10% soot |
| Microwave-Assisted Heating | Conventional Heating |
|---|---|
| Conversion of energy | Transfer of energy |
| Uniform heating at a molecular level from core | Superficial heating through conduction, convection and radiation |
| Frequent hot spot formation | Rarer hot spot formation |
| Rapid | Slow |
| Higher electricity conversion efficiency | Low electricity conversion efficiency |
| Selective | Non-selective |
| Dependent on material properties | Less dependent |
| Precise heating | Less precise |
| Lower thermal inertia and faster response | Higher thermal inertia and slower response |
| Feed | Reaction Conditions | Results | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Catalyst Used | Reactor Used (Power and Frequency) | Temperature (°C) | WHSV (g feed/g cat. h) or F/C Ratio | Bio-Oil Yield (wt. %) | Products and Conclusions | ||
| Waste cooking oil (WCO) and low-density polyethylene (LDPE) | HZSM-5 | Fixed bed (-) | 550 | 2:1 | - | WCO:LDPE used was 1:1. Reaction conditions used produced maximum yield of BTX and minimum yield of polyaromatic hydrocarbons. Efficiency of catalysts on formation increased as follows: SAPO-34 < H-β < H-Y < HZSM-5. | [74] |
| H-β | |||||||
| H-Y | |||||||
| SAPO-34 | |||||||
| Corn straw | MCM-41 mixed with ZSM-5 | Fixed bed (750 W and 2450 MHz) | 450–650 | 1:2 | - | Carbon outputs of aromatics and olefins peaked at 550 °C. Addition of MCM-41 inhibited formation of coke on ZSM-5 surface. | [75] |
| Rice straw | HZSM-5 | Fixed bed (3.2 kW and 2.45 GHz) | 500 | 44.4, 14.7, 11.1 g feed/g cat. h | 21.5 | Catalytic upgradation of pyrolytic vapours of biomass and polymer mixtures produced de-oxygenated bio-oils with properties similar to conventional fuel oil. WHSP of 11 yielded higher selectivity to unsaturated aliphatics and aromatic hydrocarbons. | [76] |
| Bagasse | 22.4 | ||||||
| PP | 74.2 | ||||||
| PS | 92.3 | ||||||
| LDPE | NiO | 2 stage fixed bed (1.8 kW and 2.45 GHz) | 450–600 | 20:1, 15:1, 10:1, 5:1 | 32–57 | Optimum conditions were found to be 500 °C pyrolysis temperature, 450 °C catalytic temperature and LDPE:H-Y of 10:1. Results obtained at these conditions were 56.53 wt. % oil and 93.80% gasoline-range fraction. Addition of catalysts favoured formation of aromatics and inhibited that of aliphatics. | [53] |
| H-Y | |||||||
| Configuration | Catalyst | Feedstock | Microwave Power (W) | Feedstock:Catalyst Ratio | Pyrolytic Temperature (°C) | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| In situ | NiO, CuO, CaO, MgO | Corn stover | 500 | 10:1–33:1 | 450–520 | The authors found that pyrolysis under an N2 atmosphere was more efficient than one under a CO2 atmosphere due to the fact that CO2 possesses better heat absorbability and so, its use reduced heat for pyrolysis. The catalysts were found to increase maximum temperature and mass reduction ratio, but they also decreased the solids’ calorific values. Moreover, use of catalysts diminished PAHs formation, making the bio-oil less toxic. | [77] |
| In situ | NiO, CuO, CaO, MgO | Sugarcane bagasse | 500 | 10:1–33:1 | 490–532 | Addition of catalysts resulted in an increase in mass reduction ratio and reaction rate, but in a decrease in maximum temperature. Use of NiO and CaO enhanced H2 production while use of NiO and CuO lessened it. Addition of CaO and MgO improved gas production while NiO and CuO favoured liquid production. | [78] |
| In situ | CaO, CaCO3, NiO, Ni2O3, γ-Al2O3, TiO2 | Sewage sludge | 700 | 10:1 | - | Addition of catalysts was found to affect temperature evolution of sludge, product distribution and gas composition. The temperature rise rate was found to be highest with CaCO3, followed by, respectively, NiO, TiO2, Ni2O3 ≈ γ-Al2O3 and CaO, which caused virtually no temperature rise. Ni-based catalysts used favoured decomposition of organic matters in sludge and highly increased bio-oil and CO-rich syngas yields. CaO gave rise to a H2-rich syngas while γ-Al2O3 and TiO2 showed no impact on gas percentage or H2:CO ratio. | [79] |
| In situ | CaO, Fe2O3 | Sludge | - | 10:1 | 500–900 | Fe2O3 was found to favour bio-oil production while CaO improved gas formation. The best quality of bio-oil was obtained at 800 °C while using CaO. It was also observed that CaO privileged H2 formation while Fe2O3 enhanced CH4 production. | [80] |
| Ex situ | HZSM-5, MgO | Low-density polyethylene (LDPE) | 750 | 0, 1:2, 1:1 and 2:1 | 450–600 | LDPE was found to be a good H2 donor to improve bio-oil properties; methoxy phenols were converted to phenols and alkylated phenols. HZSM-5 favoured aromatics production while MgO improved alkylation of phenols. Optimum parameters were found to be 500 °C and feedstock to catalyst ratio of 1:1. | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohabeer, C.; Guilhaume, N.; Laurenti, D.; Schuurman, Y. Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review. Energies 2022, 15, 3258. https://doi.org/10.3390/en15093258
Mohabeer C, Guilhaume N, Laurenti D, Schuurman Y. Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review. Energies. 2022; 15(9):3258. https://doi.org/10.3390/en15093258
Chicago/Turabian StyleMohabeer, Chetna, Nolven Guilhaume, Dorothée Laurenti, and Yves Schuurman. 2022. "Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review" Energies 15, no. 9: 3258. https://doi.org/10.3390/en15093258
APA StyleMohabeer, C., Guilhaume, N., Laurenti, D., & Schuurman, Y. (2022). Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review. Energies, 15(9), 3258. https://doi.org/10.3390/en15093258







