Ozone Catalytic Oxidation for Gaseous Dimethyl Sulfide Removal by Using Vacuum-Ultra-Violet Lamp and Impregnated Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
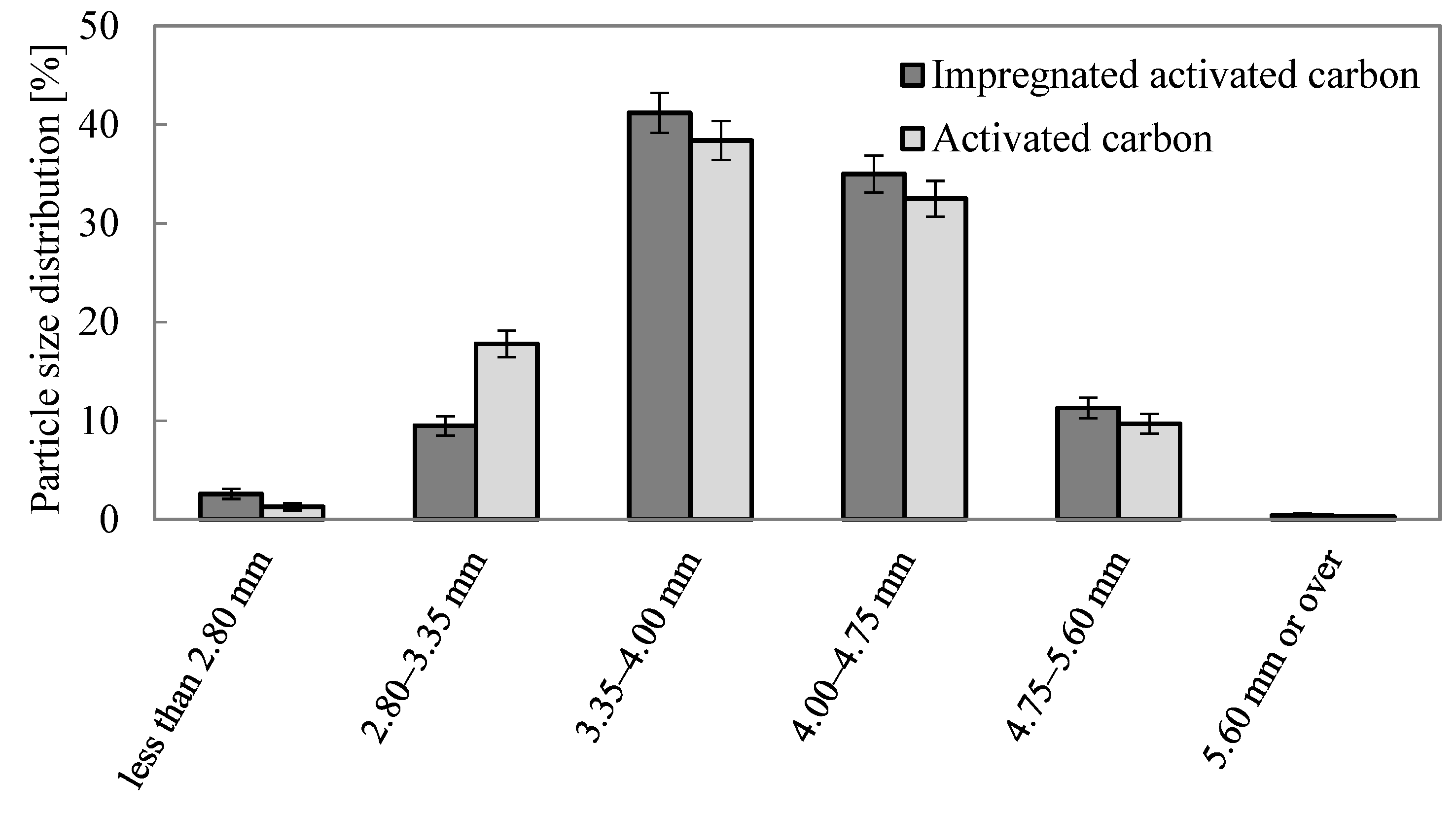
2.1. Characteristics of Granular Activated Carbon Impregnated with Iodic Acid and Inorganic Acid
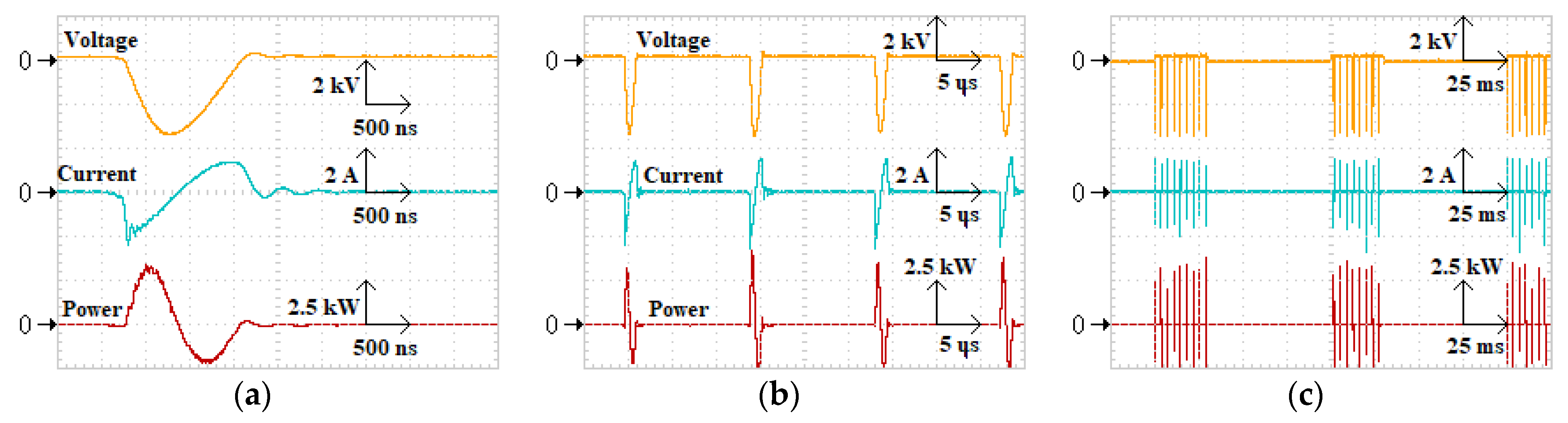
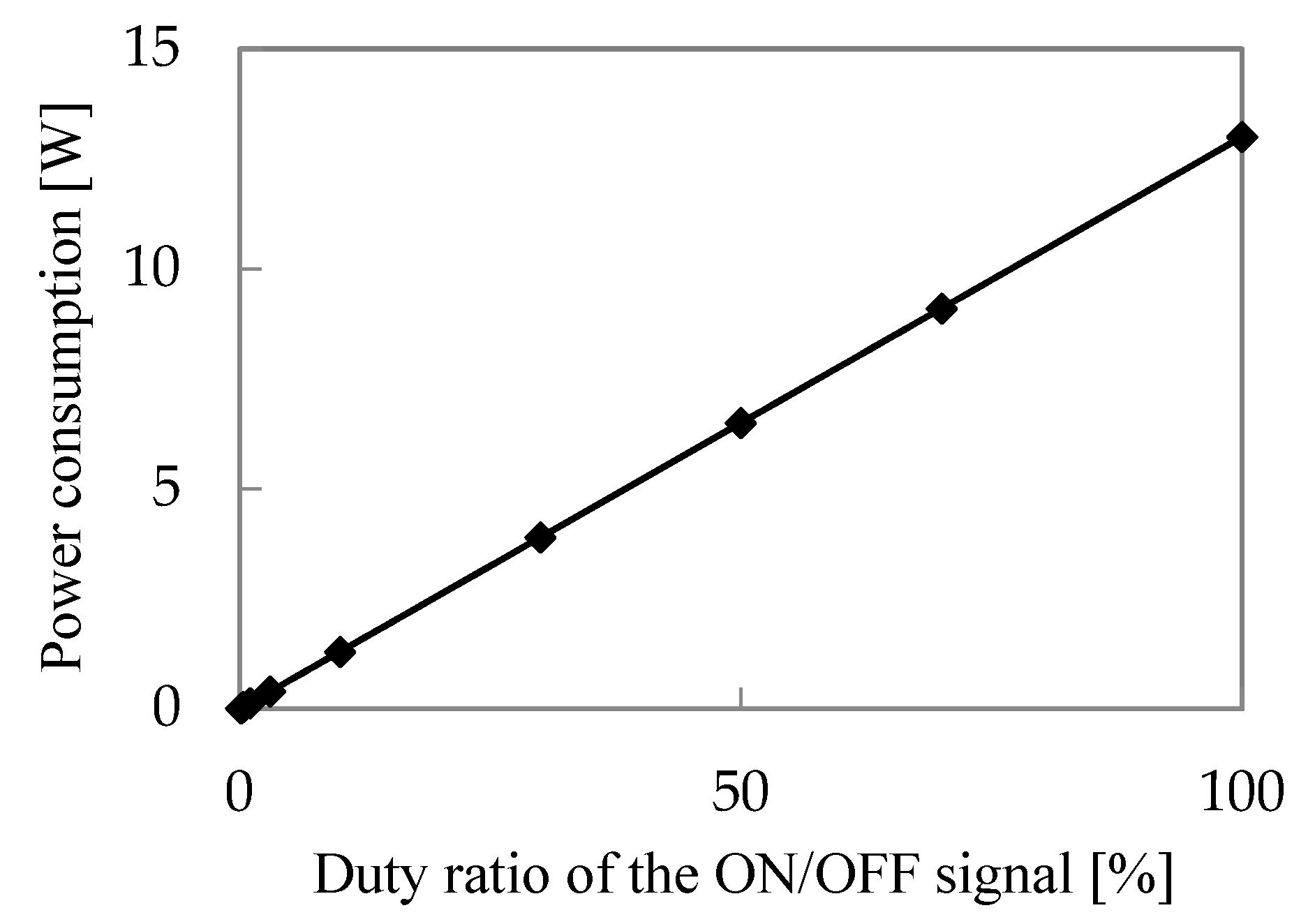
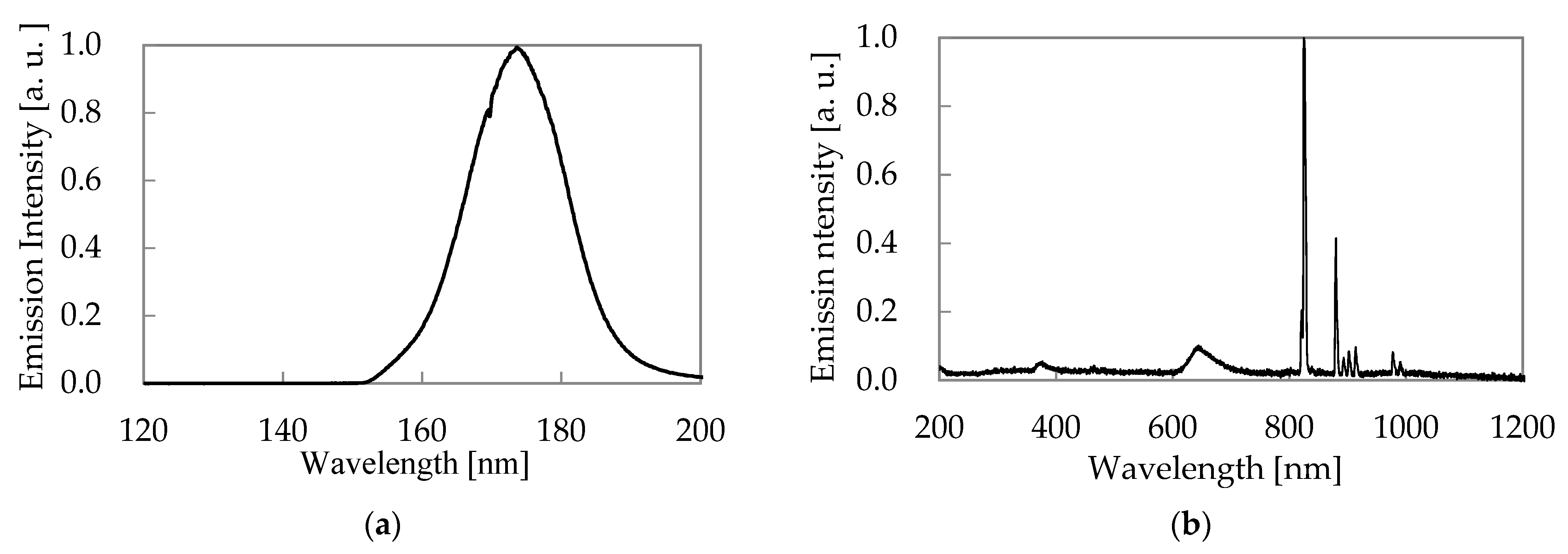
2.2. Characteristics of VUV Xenon Excimer Lamp
2.3. Performance Assessment of Ozone Catalytic Oxidation
2.3.1. Optimizing the Ozone Addition Amount
2.3.2. Dynamic Adsorption Experiment
2.3.3. Activated Carbon Analysis
2.3.4. DMS Desorption Experiment
3. Results and Discussions
3.1. Characteristics of Granular Impregnated Activated Carbon
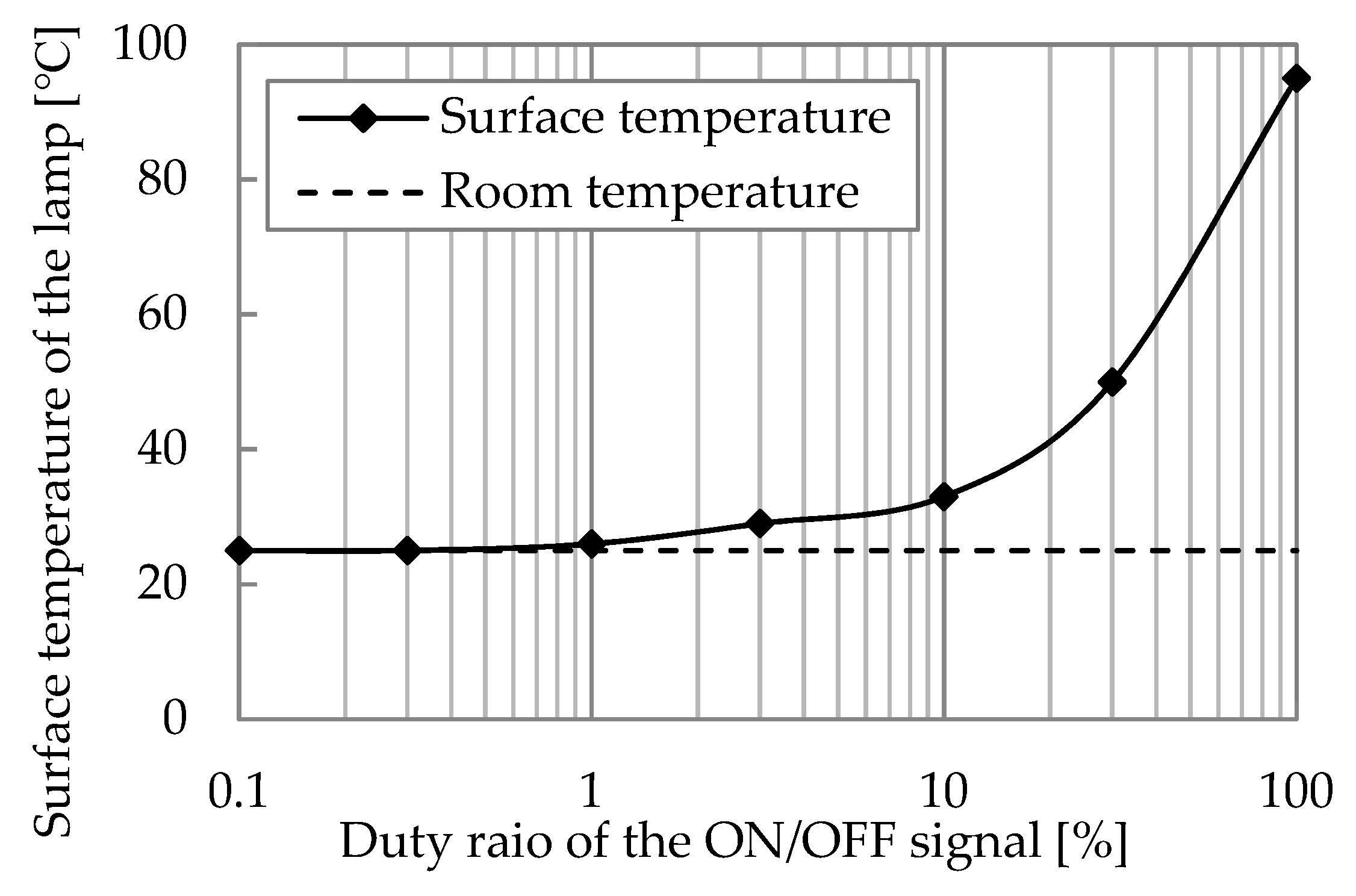
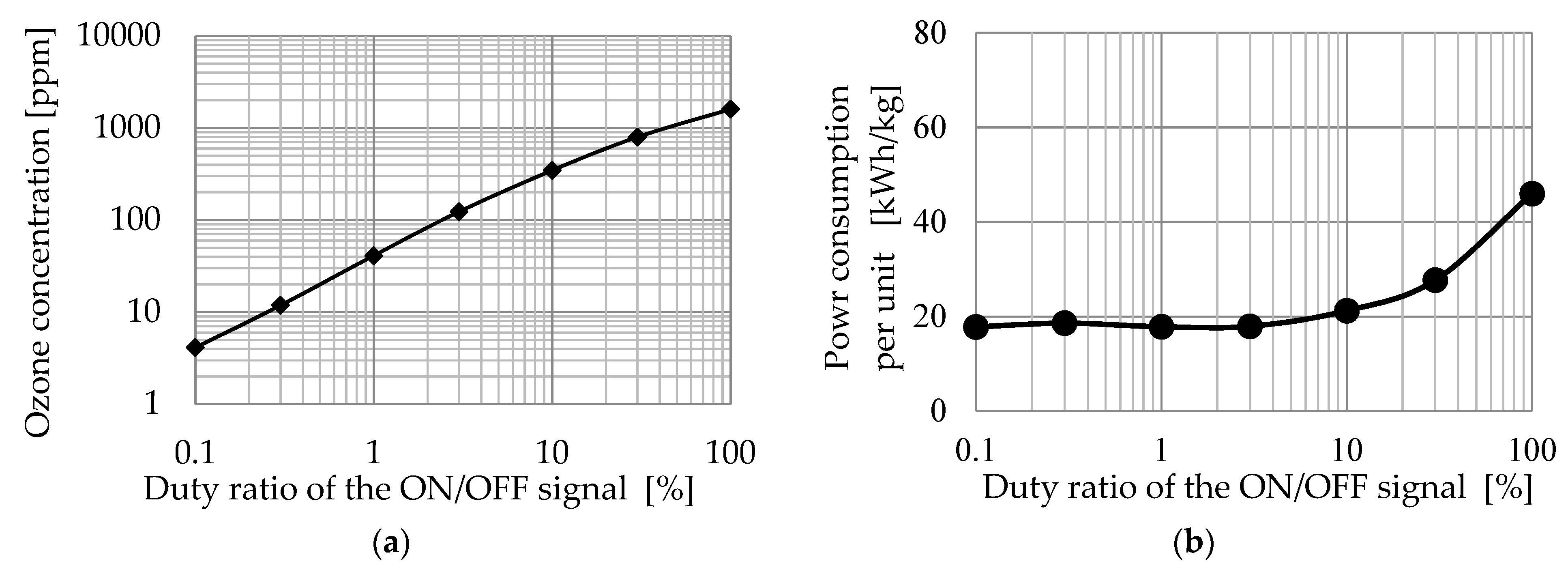
3.2. Characteristics of Excimer Lamp
3.3. Performance Assessment of Ozone Catalytic Oxidation
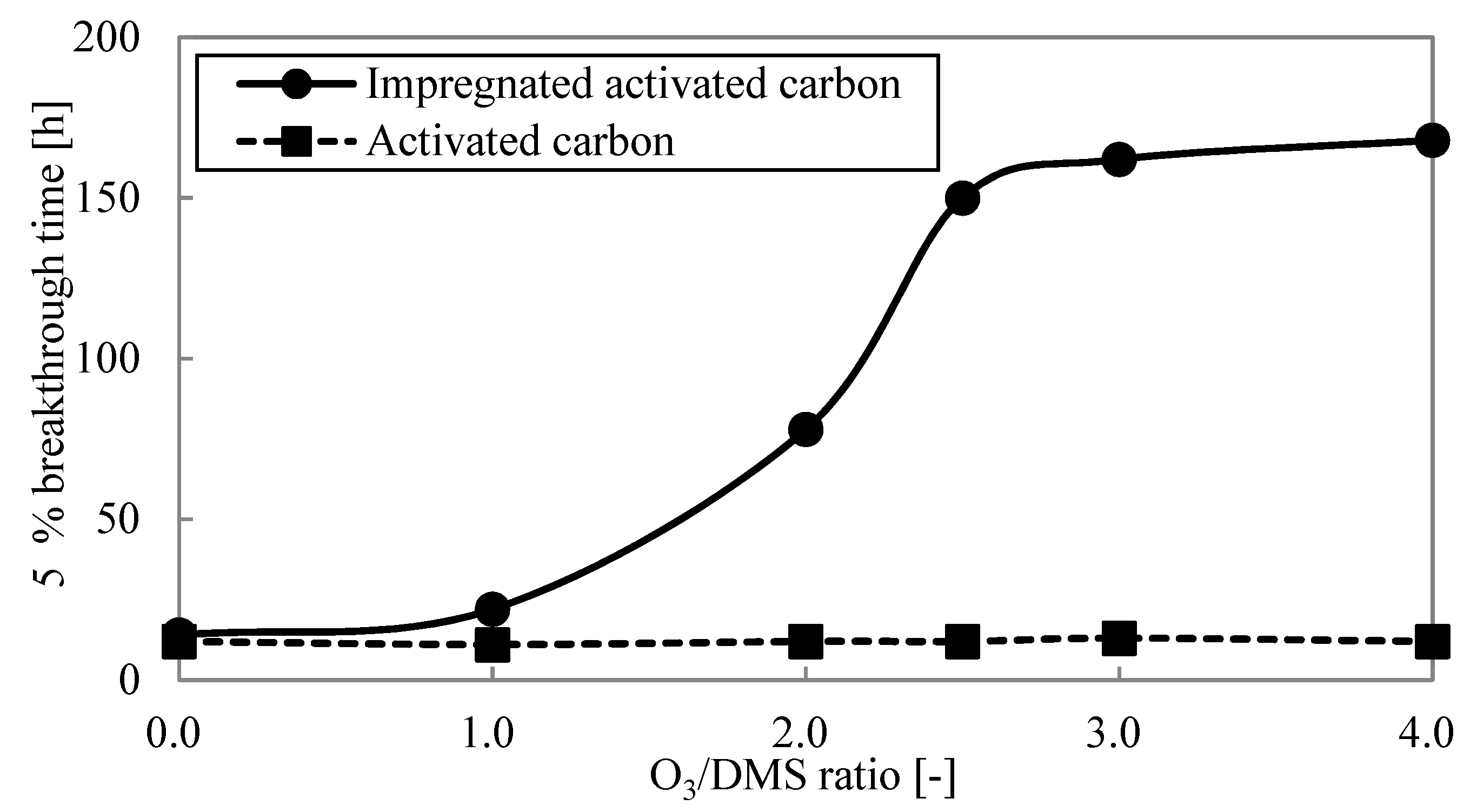
3.3.1. Optimizing the Ozone Addition Amount
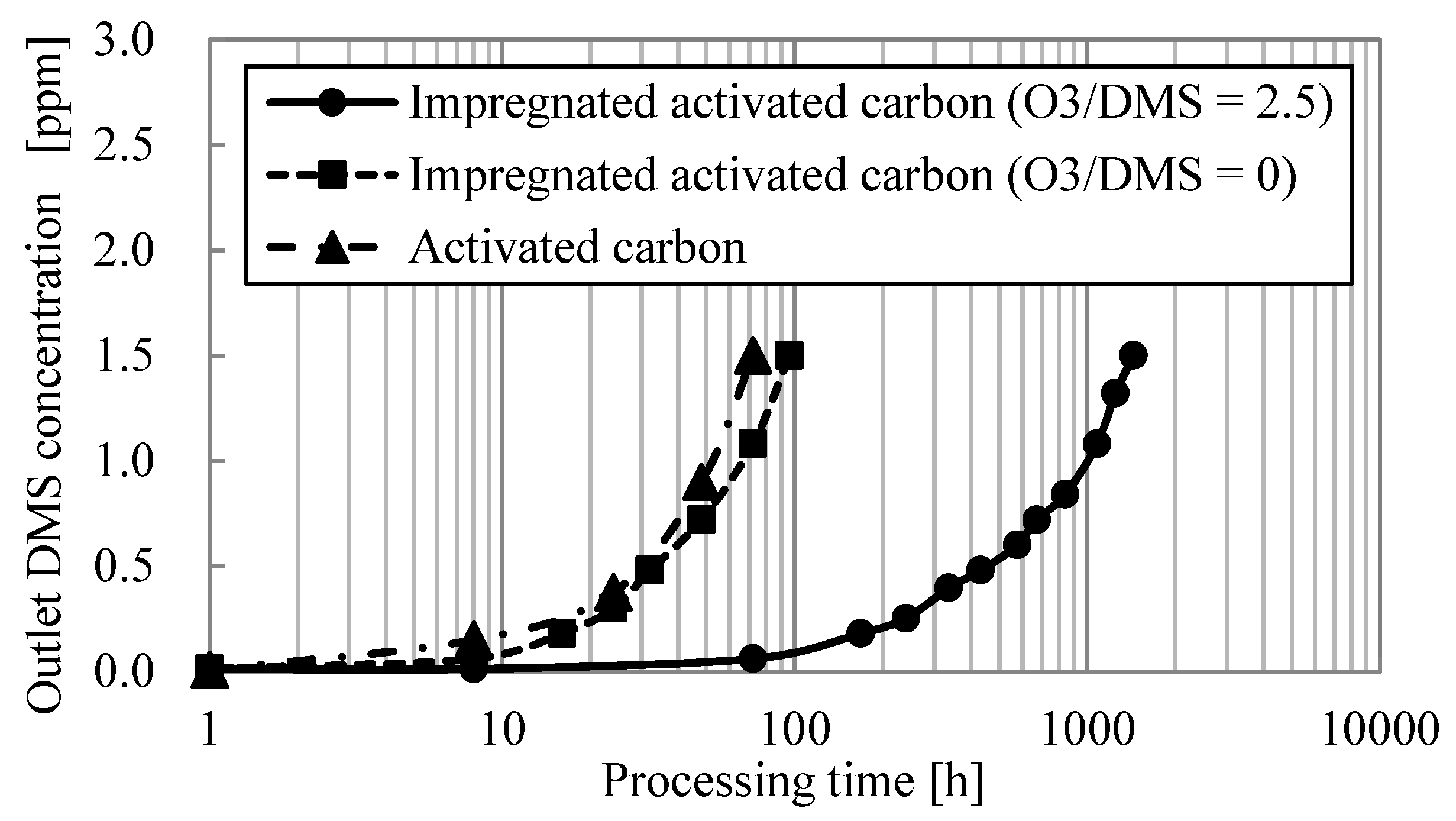
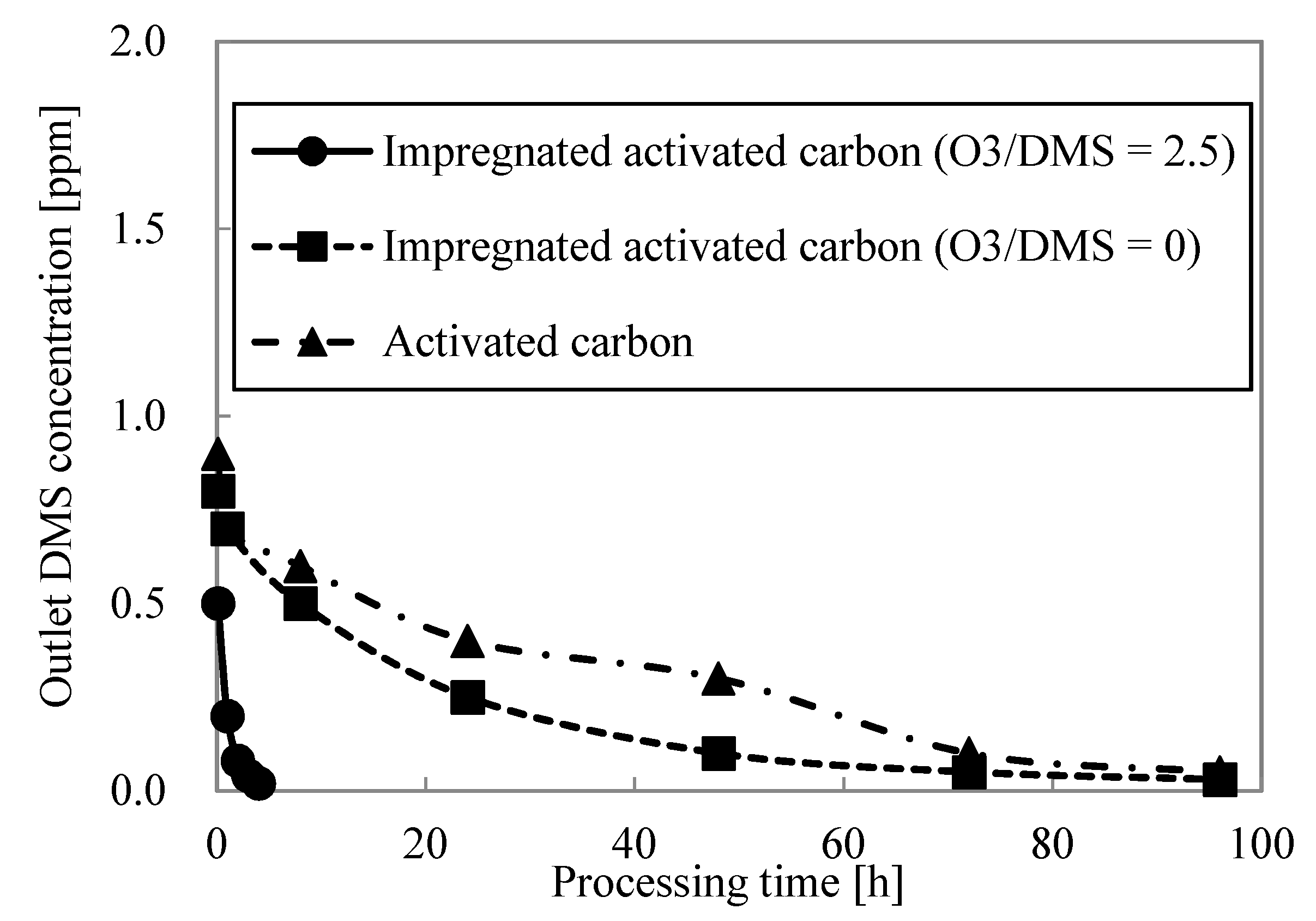
3.3.2. Dynamic Adsorption Experiment
3.3.3. Analysis of Impregnated Activated Carbon
3.3.4. DMS Desorption Experiment
4. Conclusions
- (1)
- The xenon excimer lamp’s energy consumption per unit of ozone generation was 18 kWh/kg. Its ozone generation efficiency was high compared to the atmospheric oxygen discharge. NOx generation ratio over ozone generation was less than 0.32%, confirming that the xenon excimer lamp could generate ozone without NOx generation.
- (2)
- The 5% breakthrough time was measured with varying ozone concentrations in the dynamic adsorption experiment, confirming that impregnated activated carbon could be utilized as a catalyst. The value of ozone concentration over dimethyl sulfide concentration was optimized to 2.5 by measuring the 5% breakthrough time in the dynamic adsorption experiment.
- (3)
- Adsorption capacity and adsorption rate constant were calculated by conducting dynamic dimethyl sulfide adsorption experiments. The adsorption capacity of impregnated activated carbon at 0 and 2.5 O3/DMS was 0.01 kg/kg and 0.15 kg/kg, respectively. Adsorption capacity was increased by ozone addition due to the ozone catalytic oxidation. The adsorption rate constant of impregnated activated carbon at 0 and 2.5 O3/DMS was 3.1 s−1 and 3.0 s−1, respectively. Adsorption rate constant did not change with ozone addition. This shows that dimethyl sulfide oxidation could be formed on the surface of impregnated activated carbon but does not determine processes, the fluid film or fine pore diffusions.
- (4)
- By analyzing the impregnated activated carbon after the dynamic adsorption experiment at 2.5 O3/DMS, methane sulfonic acid was detected. However, when O3/DMS was 0, methane sulfonic acid was generated in very small amounts. The pH of impregnated activated carbon before the adsorption experiment was 2.3. The pH of impregnated activated carbon after the adsorption of O3/DMS at 0 and 2.5 was 2.0 and 2.7, respectively. This pH increment may have occurred because of iodic acid reduction, and a pH decrease may be due to methane sulfonic acid generation and iodic acid oxidation by ozone. These result show the effects on the pH of impregnated activated carbon by H2SO4 could be due to ozone catalytic oxidation.
- (5)
- Dimethyl sulfide desorption occurred by flowing clean air to the impregnated activated carbon after the adsorption experiment. The desorption ratio of impregnated activated carbon at 0 and 2.5 O3/DMS was 0.067 and 0.00015, respectively. Dimethyl sulfide removal by ozone catalytic oxidation occurred because of chemical adsorption not physical adsorption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dada, O.; Mbohwa, C. Energy from waste: A possible way of meeting goal 7 of the sustainable dSevelopment goals. Mater. Today Proc. 2018, 5, 10577–10584. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Hairston, D. Sweeting the smell; silencing the complaints. Chem. Eng. J. 1995, 102, 65–66. [Google Scholar]
- Garber, W.F. Odors and their control in sewers and treatment facilities. Water Sci. Technol. 1980, 12, 657–664. [Google Scholar]
- Freiberg, A.; Scharfe, J.; Murta, V.C.; Seidler, A. The Use of Biomass for Electricity Generation: A Scoping Review of Health Effects on Humans in Residential and Occupational Settings. Int. J. Environ. Res. Public Health 2018, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Biggs, C.; Ryan, C.; Wiseman, J.; Larsen, K. Distributed Water Systems: A networked and localised approach for sustainable water services. In Victorian Eco-Innovation Lab Distributed Systems Briefing Paper 2009, No. 2; Victorian Eco-Innovation Lab (VEIL): Melbourne, Australia, 2009. [Google Scholar]
- Bonnin, C.; Laborie, A.; Paillard, H. Odor nuisances created by sludge treatment: Problems and solutions. Water Sci. Technol. 1990, 22, 65–74. [Google Scholar] [CrossRef]
- Sivret, E.C.; Wang, B.; Parcsi, G.; Stuetz, R.M. Prioritisation of odorants emitted from sewers using odour activity values. Water Res. 2016, 88, 308–321. [Google Scholar] [CrossRef]
- Hanson, R. Scrubbing out odour nuisance. Water Waste Treat. 2002, 45, 28–30. [Google Scholar]
- Robbins, T.L.; Manley, R. Odor preventation and control in process plants. Chem. Eng. J. 2002, 109, 50–55. [Google Scholar]
- Yao, Y.-Y.; Chen, W.-X.; Lu, S.-S. Preparation and Deodorizing Performance of a Novel Air-Purifying Material. J. Appl. Polym. Sci. 2006, 102, 4378–4382. [Google Scholar] [CrossRef]
- Basu, S.; Gu, Z.C.; Shilinsky, K.A. Application of Packed Scrubbers for Air Emissions Control in Municipal Wastewater Treatment Plants. Environ. Prog. 1998, 17, 9–18. [Google Scholar] [CrossRef]
- Shin, C.; Kim, K.; Choi, B. Deodorization Technology at Industrial Facilities Using Impregnated Activated Carbon Fiber. J. Chem. Eng. Jpn. 2001, 34, 401–406. [Google Scholar] [CrossRef]
- Fenske, B.J.; Empie, H.J.; Heedick, G. Reduction of Odorous Emissions from Kraft Pulp Mills Using Green Liquor Dregs. In Proceedings of the TAPPI 2005 Engineering, Pulping & Environmental Conference, Philadelphia, PA, USA, 28–31 August 2000; Volume 2000, pp. 145–161. [Google Scholar]
- National Library of Medicine. PubChem CID 1068: Dimethyl Sulfide; National Library of Medicine: Bethesda, MD, USA, 2004.
- Ministry of the Environment, Government of Japan. Odor Measurement Review; Ministry of the Environment: Tokyo, Japan, 2003; pp. 122–123.
- Hwang, C.-L.; Tai, N.-H. Vapor phase oxidation of dimethyl sulfide with ozone over ion-exchanged zeolites. Appl. Catal. A Gen. 2011, 391, 251–256. [Google Scholar] [CrossRef]
- Soni, K.C.; Shekar, S.C.; Singh, B.; Gopi, T. Catalytic activity of FeZrO2 nanoparticles for dimethyl sulfide oxidation. J. Colloid Interface Sci. 2015, 446, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Endalkachew, S.D.; Devulapelli, V.G. Vapor phase oxidation of dimethyl sulfide with ozone over V2O5/TiO2 catalyst. Appl. Catal. B 2008, 84, 408–419. [Google Scholar]
- Supawat, W.; Chantaraporn, P. Field analysis of PSA O2-ozone production process for water recycling. J. Water Environ. Technol. 2020, 34, 223–231. [Google Scholar]
- Li, X.; Liu, X.; Lin, C.; Qi, C.; Zhang, H.; Ma, J. Enhanced activation of periodate by iodine-doped granular activated carbon for organic contaminant degradation. Chemosphere 2017, 181, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Rimington, D.; Brown, G. Ventilation and the control of odours in sewage treatment industry. Water Serv. 1987, 91, 64–66. [Google Scholar]
- Mohamad, F. Specialty Polymers: Materials and Applications; IK International Publishing House Pvt. Ltd.: Delhi, India, 2013; p. 39. [Google Scholar]
- Williams, S.; Campos, M.F.; Midey, A.J.; Arnold, S.T.; Morris, R.A.; Viggiano, A.A. Negative ion chemistry of ozone in the gas phase. J. Phys. Chem. A 2002, 106, 997–1003. [Google Scholar] [CrossRef]
- Sunder, S.; Vikis, A.C. Raman spectra of iodine oxyacids produced by the gas-phase reaction of iodine with ozone in the presence of water vapour. Can. J. Spectrosc. 1987, 32, 45–48. [Google Scholar]
- Biedermann, G.; Lendeus, R. Preparation of iodic acid by ozonization. Acta Chem. Scand. Ser. A 1984, 38, 825–827. [Google Scholar] [CrossRef]
- Kasahara, T.; Shoji, S.; Mizuno, J. Surface Modification of Polyethylene Terephtalate (PET) by 172-nm Excimer Lamp. Trans. Jpn. Inst. Electron. Packag. 2012, 5, 47–54. [Google Scholar] [CrossRef][Green Version]
- Kling, R.; Roth, M.; Paravia, M. High Efficient Ozone Production with Excimer Lamps. In Proceedings of the Conference record of 20th IOA and 6th IUVA, Paris, France, 22–25 May 2011. [Google Scholar]
- JIS K 1474: 2014; Test Method for Activated Carbon. Japan Industrial Standard Committee: Tokyo, Japan, 2014.
- JWWA A114: 2006; Test Method for Activated Carbon. Japan Water Works Association: Tokyo, Japan, 2006.
- Thermophysical Properties Database System, Fused SiO2 Bulk: Spectral Emissivity; IAEA: Vienna, Austria, 2006.
- Rychter, K.W.; Smolinski, A. A study of dynamic adsorption of propylene and ethylene emitted from the process of coal self-heating. Sci. Rep. 2019, 9, 18277. [Google Scholar] [CrossRef]
- Lodewyckx, P.; Wood, G.O.; Ryu, S.K. The Wheeler-Jonas equation: A versatile tool for the prediction of carbon bed breakthrough time. Carbon 2004, 42, 1351–1355. [Google Scholar] [CrossRef]
- Hori, H.; Tanaka, I.; Akiyama, T. Breakthrough Time on Activated Carbon Fluidized Bed Adsorbers. JAPCA 1988, 38, 269–271. [Google Scholar] [CrossRef]
- Abiko, H.; Furuse, M.; Takano, T. Application of Wheeler–Jonas equation and relative breakthrough time (RBT) in activated carbon beds of respirator gas filters. Air Qual. Atmos. Health 2020, 13, 1057–1063. [Google Scholar] [CrossRef]
- Chen, Q.; Sherwen, T.; Evans, M.; Alexander, B. DMS oxidation and sulfur aerosol formation in the marine troposphere: A focus on reactive halogen and multiphase chemistry. Atmos. Chem. Phys. 2018, 18, 13617–13637. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Li, H.; Keener, T.C. Effects of activated carbon surface properties on the adsorption of volatile organic compounds. J. Air Waste Manag. Assoc. 2012, 62, 1196–1202. [Google Scholar] [CrossRef]
- Bardouki, H.; Mihalopoulos, N.; Zetzsch, C. Oxidation of dimethylsulfoxide (DMSO) by OH radicals in aqueous medium. J. Aerosol. Sci. 2001, 32, S291–S292. [Google Scholar] [CrossRef]
- Scholl, S.; Kajszika, H.; Mersmann, A. Adsorption and desorption kinetics in activated carbon. Gas Sep. Purif. 1993, 7, 207–212. [Google Scholar] [CrossRef]
- Ou, H.; Fang, M.; Chou, M.; Chang, H.; Shiao, T. Long-term evaluation of activated carbon as an adsorbent for biogas desulfurization. J. Air Waste Manag. Assoc. 2020, 70, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Lohwacharin, J.; Maliwan, T.; Osawa, H.; Takizawa, S. Effects of Ferrihydrite-Impregnated Powdered Activated Carbon on Phosphate Removal and Biofouling of Ultrafiltration Membrane. Water 2021, 13, 1178. [Google Scholar] [CrossRef]
- Fijołek, L.; Swietlik, J.; Frankowski, M. The influence of active carbon contaminants on the ozonation mechanism interpretation. Sci. Rep. 2021, 11, 9934. [Google Scholar] [CrossRef] [PubMed]
- Samir, T.; Liu, Y.; Zhao, L. Study on Effect of Neutral Gas Pressure on Plasma Characteristics in Capacitive RF Argon Glow Discharges at Low Pressure by Fluid Modeling. IEEE Trans. Plasma Phys. 2018, 46, 1738–1746. [Google Scholar] [CrossRef]
- Wittenberg, H.H. Gas Tube Design: From Tube Design; Radio Corporation of America: New York, NY, USA, 1962; pp. 792–817. [Google Scholar]
- Sanduloviciu, M.; Leu, C.B.G. Self-organization phenomena in current carrying plasmas related to the non-linearity of the current versus voltage characteristic. Phys. Lett. A 1995, 208, 136–142. [Google Scholar] [CrossRef]
- Salvermoser, M.; Murnick, D.E. Efficient stable; corona discharge 172nm xenon excimer light source. J. Appl. Phys. 2003, 94, 3722–3731. [Google Scholar] [CrossRef]
- Yoshida, Y.; Tanaka, M.; Yukimura, K. Pressure Dependence of Emission Intensity of Rare-gas Excimer Light. T. IEE Jpn. C 1996, 116, 1119–1125. [Google Scholar]
- Schreiber, A.; Kuhn, B.; Arnold, E.; Schilling, F.J.; Witzke, H.D. Radiation Resistance of Quartz Glass for VUV Discharge Lamps. In Proceedings of the 10th International Symposium on the Science and Technology of Light Sources, LS10; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Wu, Y.J.; Wu, C.Y.R.; Chou, S.L.; Lin, M.Y.; Lu, H.C.; Lo, J.I.; Cheng, B.M. Spectra and Photolysis of pure nitrogen and methane dispersed in solid nitrogen with vacuum–ultraviolet light. Astrophys. J. 2012, 746, 175. [Google Scholar] [CrossRef]
- Morimoto, Y.; Sumitomo, T.; Yoshioka, M.; Takemura, T. Recent progress on UV lamps for industries. In Proceedings of the Conference Record of the 2004 IEEE Industry Applications Conference, 39th IAS Annual Meeting, Seattle, WA, USA, 3–7 October 2004; Volume 39, pp. 1008–1015. [Google Scholar]
- Tanaka, M.; Sasaki, S.; Katayama, M. The Near Infrared Emission Band Observed in Electron Irradiated Xe and Xe in Other Rare Gases. Bull. Chem. Soc. Jpn. 1985, 58, 429–432. [Google Scholar] [CrossRef]
- Sunkhatme, S.P. A Textbook on Heat Transfer, 4th ed.; University Press (India) Private Limited: Telangana, India, 2005. [Google Scholar]
- McKenney, D.J.; Laidler, K.J. Elementary processes in the decomposition of ozone. Can. J. Chem. 1962, 40, 539–544. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, B.J.; Im, H.G.; Cha, M.S. Ozone Production With Dielectric Barrier Discharge: Effects of Power Source and Humidity. IEEE Trans. Plasma Sci. 2016, 44, 2288–2296. [Google Scholar] [CrossRef]
- Salvermoser, M.; Murnick, D.E.; Kogelschatz, U. Influence of water vapor on photochemical ozone generation with efficient 172 nm xenon excimer lamps. Ozone Sci. Eng 2008, 30, 228–237. [Google Scholar] [CrossRef]
- Braun, D.; Kuchler, U.; Pietsch, G. Behaviour of NOx in air-fed ozonizers. Pure Appl. Chem. 1988, 60, 741–746. [Google Scholar] [CrossRef]
- Tsuji, M.; Kawahara, M.; Noda, K.; Senda, M.; Sako, H.; Kamo, N.; Kawahara, T.; Sozan, K.; Kamarudin, N. Photochemical removal of NO2 by using 172-nm Xe2 excimer lamp in N2 or air at atmospheric pressure. J. Hazard. Mater. 2009, 162, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, C. Ozone generation. J. Plasma Fusion Res. 1998, 74, 134–139. [Google Scholar]
- Sosnin, E.A. Areas in which vacuum ultraviolet excilamps are used. J. Opt. Technol. 2012, 79, 659–666. [Google Scholar] [CrossRef]
- Marin, P.; Borba, C.E.; Módenes, A.N.; Espinoza-Quiñones, F.R.; De Oliveira, S.P.D.; Kroumov, A.D. Determination of the mass transfer limiting step of dye adsorption onto commercial adsorbent by using mathematical models. Environ. Technol. 2014, 35, 2356–2364. [Google Scholar] [CrossRef]
- Parsons, R.; Salzberg, H.W. Handbook of Electrochemical Constants; Butterworths Scientific Publications: Oxford, UK, 1959; pp. 69–73. [Google Scholar]
- Li, W.; Hun, Z.; Sun, D. Preparation of sludge-based activated carbon for adsorption of dimethyl sulfide and dimethyl disulfide during sludge aerobic composting. Chemosphere 2021, 279, 130924. [Google Scholar] [CrossRef]



| Materials | Boiling Point (°C) |
|---|---|
| Dimethyl sulfide (DMS)—(CH3)2S | 37 |
| Dimethyl sulfoxide (DMSO)—(CH3)2SO | 189 |
| Methane sulfinic acid (MSIA)—CH3SO2H | 256 |
| Methane sulfonic acid (MSA)—CH3SO3H | 167 |
| Sulfur dioxide—SO2 | −10 |
| Sulfuric acid—H2SO4 | 337 |
| Characteristics | Impregnated Activated Carbon | Activated Carbon |
|---|---|---|
| Bulk density (kg/m3) | 540 | 480 |
| Toluene adsorption capacity (kg/kg) | 0.217 | 0.259 |
| pH (-) | 2.4 | 9.7 |
| Adsorbents | 5% Breakthrough Time (h) | 50% Breakthrough Time (h) |
|---|---|---|
| Activated carbon (O3/DMS = 0) | 12 | 72 |
| Impregnated activated carbon (O3/DMS = 0) | 14 | 96 |
| Impregnated activated carbon (O3/DMS = 2.5) | 150 | 1440 |
| Adsorbents | Adsorption Capacity (kg/kg) | Adsorption Rate Constant (s−1) |
|---|---|---|
| Activated carbon (O3/DMS = 0) | 0.0086 | 3.2 |
| Impregnated activated carbon (O3/DMS = 0) | 0.010 | 3.1 |
| Impregnated activated carbon (O3/DMS = 2.5) | 0.15 | 3.0 |
| Adsorbents | MSA (mg/L) | H2SO4 (mg/L) | pH (−) |
|---|---|---|---|
| Activated carbon (before adsorption) | <0.1 | 0.2 | 9.7 |
| Activated carbon (O3/DMS = 0) | 0.1 | 0.2 | 9.7 |
| Impregnated activated carbon (before adsorption) | <0.1 | 340 | 2.3 |
| Impregnated activated carbon (O3/DMS = 0) | 0.3 | 340 | 2.0 |
| Impregnated activated carbon (O3/DMS = 2.5) | 520 | 350 | 2.7 |
| Adsorbents | Adsorption Amount (mg) | Desorption Amount (mg) | Desorption Ratio (−) |
|---|---|---|---|
| Activated carbon(O3/DMS = 0) | 7.6 | 57 | 0.13 |
| Impregnated activated carbon(O3/DMS = 0) | 5.0 | 76 | 0.067 |
| Impregnated activated carbon(O3/DMS = 2.5) | 0.16 | 1100 | 0.00015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, Y.; Yahaya, A.G.; Kristof, J.; Blajan, M.G.; Murakami, E.; Shimizu, K. Ozone Catalytic Oxidation for Gaseous Dimethyl Sulfide Removal by Using Vacuum-Ultra-Violet Lamp and Impregnated Activated Carbon. Energies 2022, 15, 3314. https://doi.org/10.3390/en15093314
Mizuno Y, Yahaya AG, Kristof J, Blajan MG, Murakami E, Shimizu K. Ozone Catalytic Oxidation for Gaseous Dimethyl Sulfide Removal by Using Vacuum-Ultra-Violet Lamp and Impregnated Activated Carbon. Energies. 2022; 15(9):3314. https://doi.org/10.3390/en15093314
Chicago/Turabian StyleMizuno, Yoshinori, Ahmad Guji Yahaya, Jaroslav Kristof, Marius Gabriel Blajan, Eizo Murakami, and Kazuo Shimizu. 2022. "Ozone Catalytic Oxidation for Gaseous Dimethyl Sulfide Removal by Using Vacuum-Ultra-Violet Lamp and Impregnated Activated Carbon" Energies 15, no. 9: 3314. https://doi.org/10.3390/en15093314
APA StyleMizuno, Y., Yahaya, A. G., Kristof, J., Blajan, M. G., Murakami, E., & Shimizu, K. (2022). Ozone Catalytic Oxidation for Gaseous Dimethyl Sulfide Removal by Using Vacuum-Ultra-Violet Lamp and Impregnated Activated Carbon. Energies, 15(9), 3314. https://doi.org/10.3390/en15093314







