Impact of Reservoir Heterogeneity on Diluted Geothermal Brine Reinjection
Abstract
:1. Introduction
2. Materials and Methods
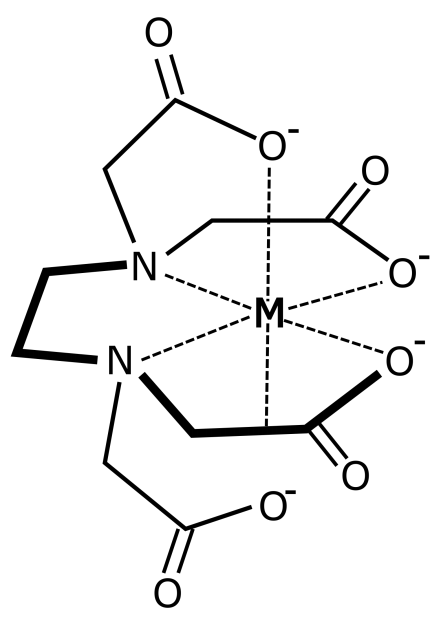
2.1. Brines
2.2. Rock Sample
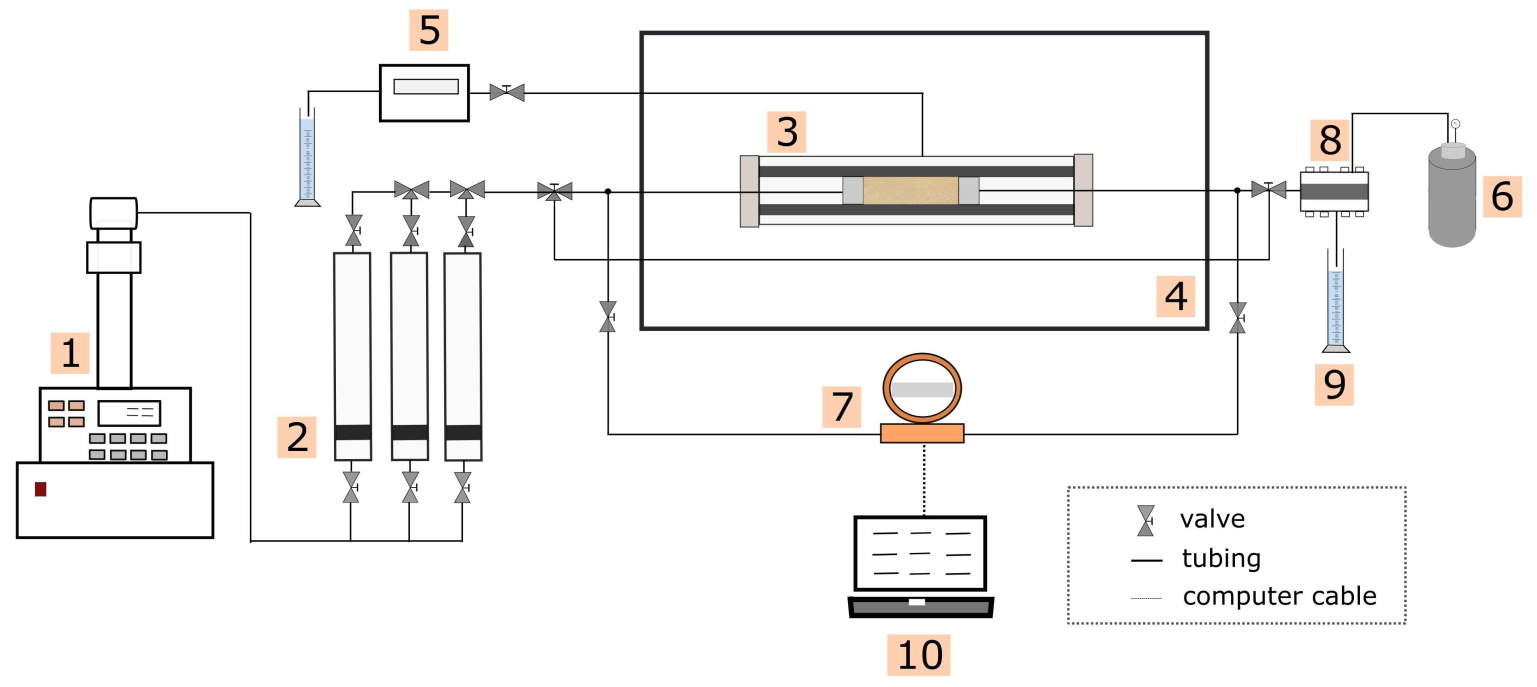
2.3. Core Flooding Apparatus and Procedure
- B-18 and B-19 flooded with 2D*TBEDTA;
- BR-4 and BR-6 flooded with 2D*TBEDTA;
- PR-3 and PR-6 flooded with 2D*TBEDTA.
3. Results and Discussion
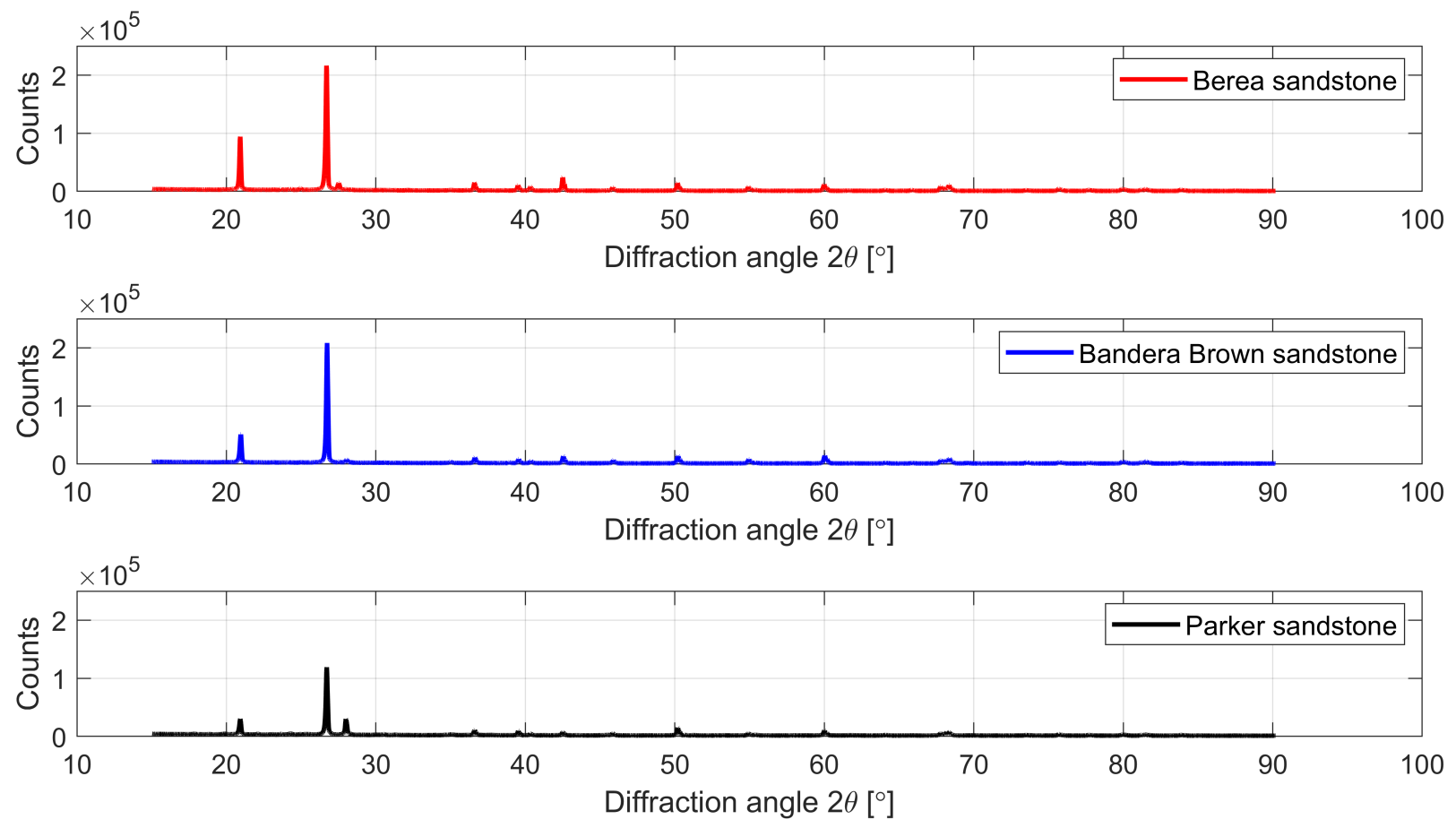
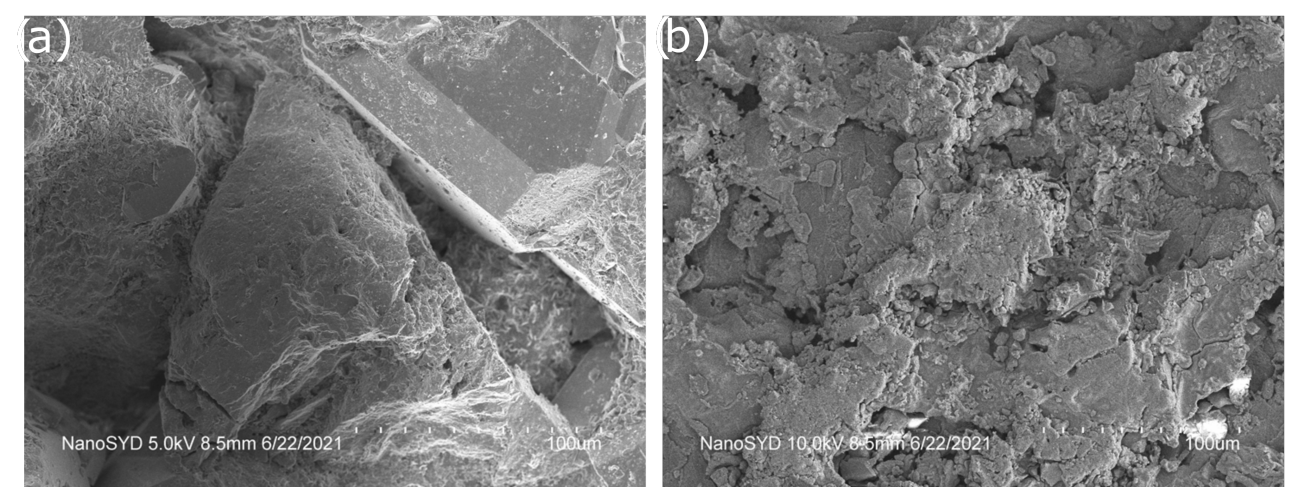
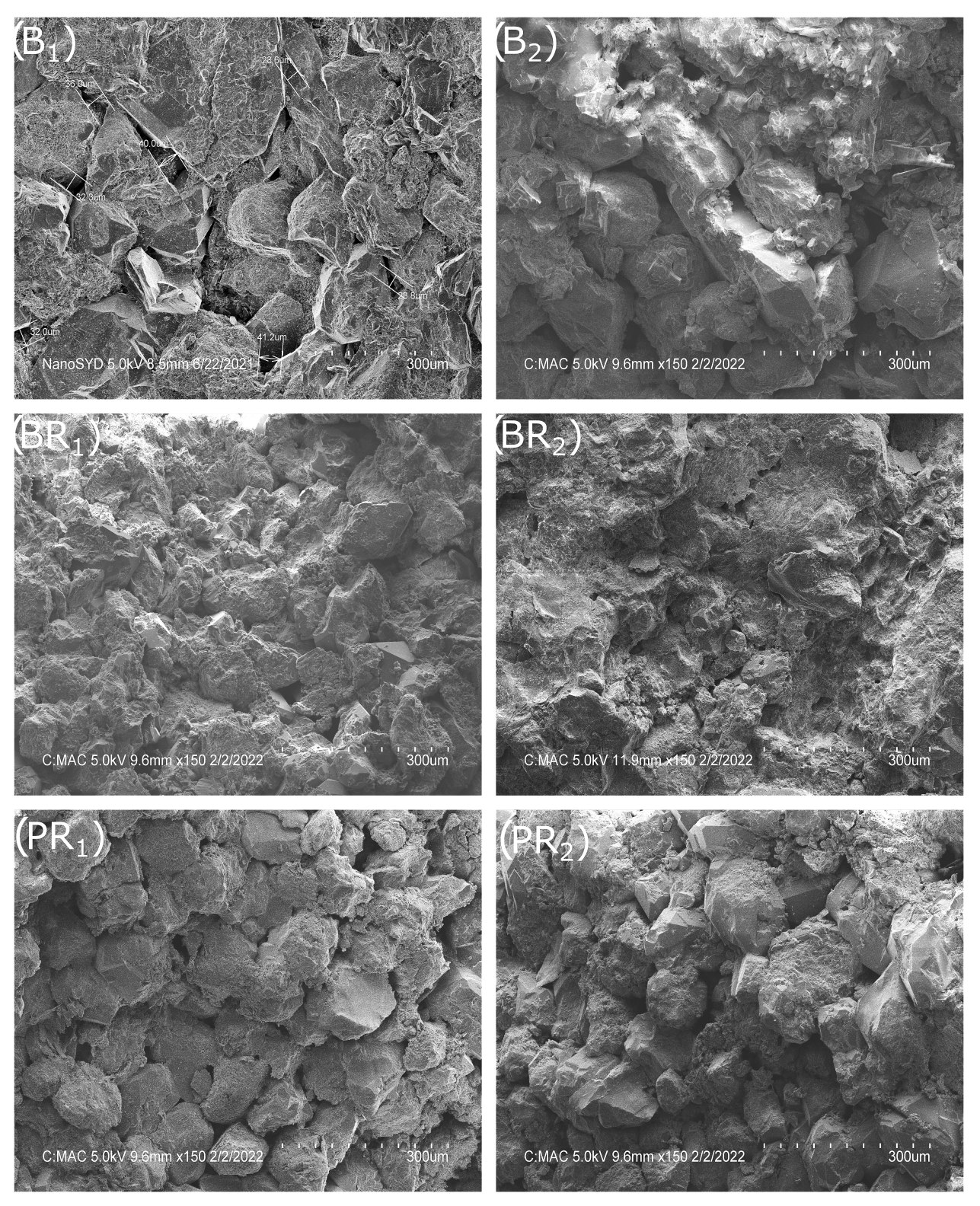
3.1. Chemical and Mineralogical Composition of the Rock Material
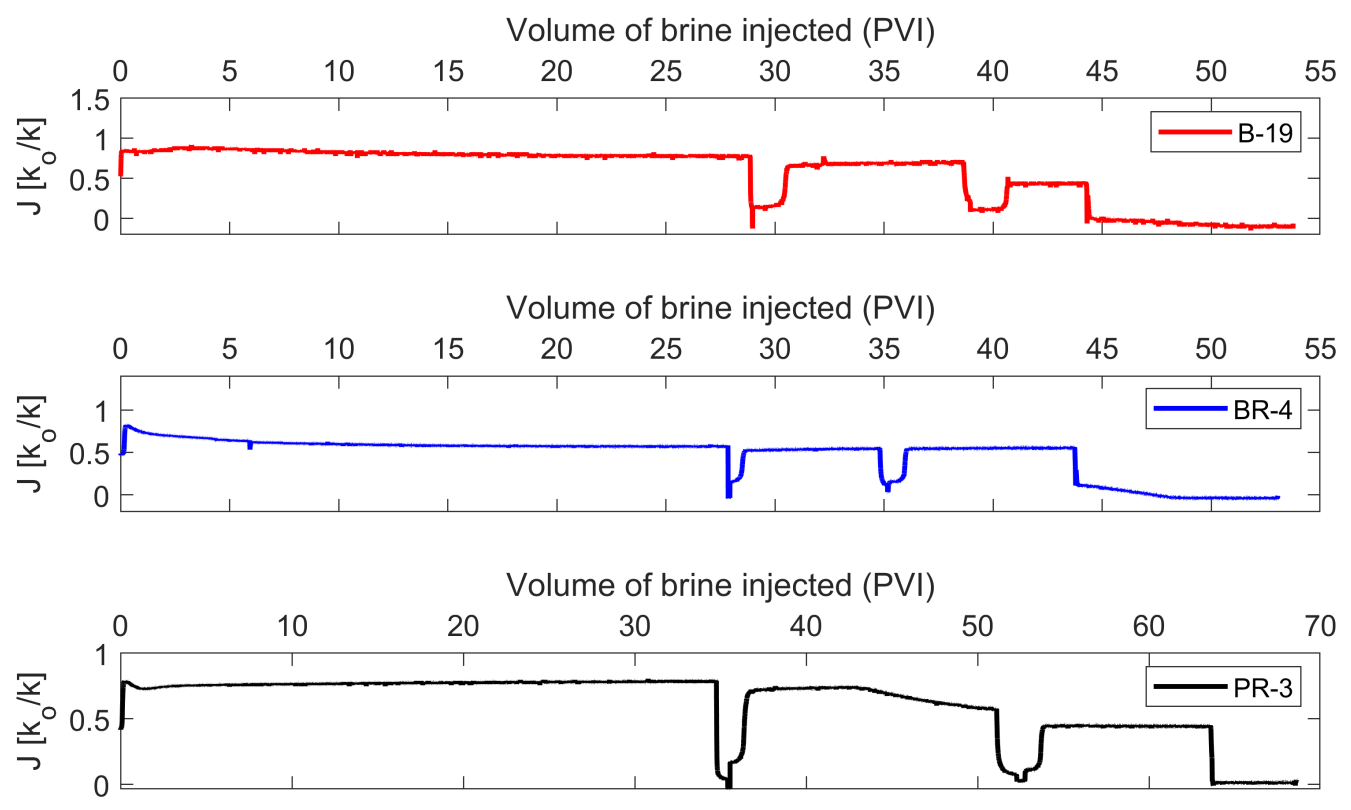
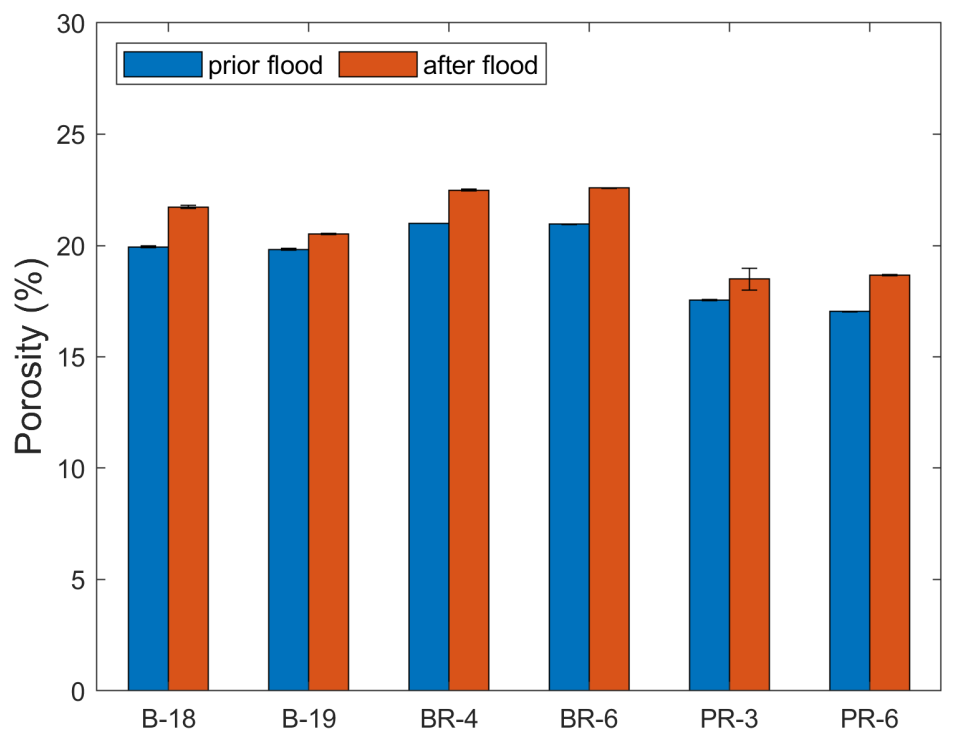
3.2. Brine Permeability Flow Test
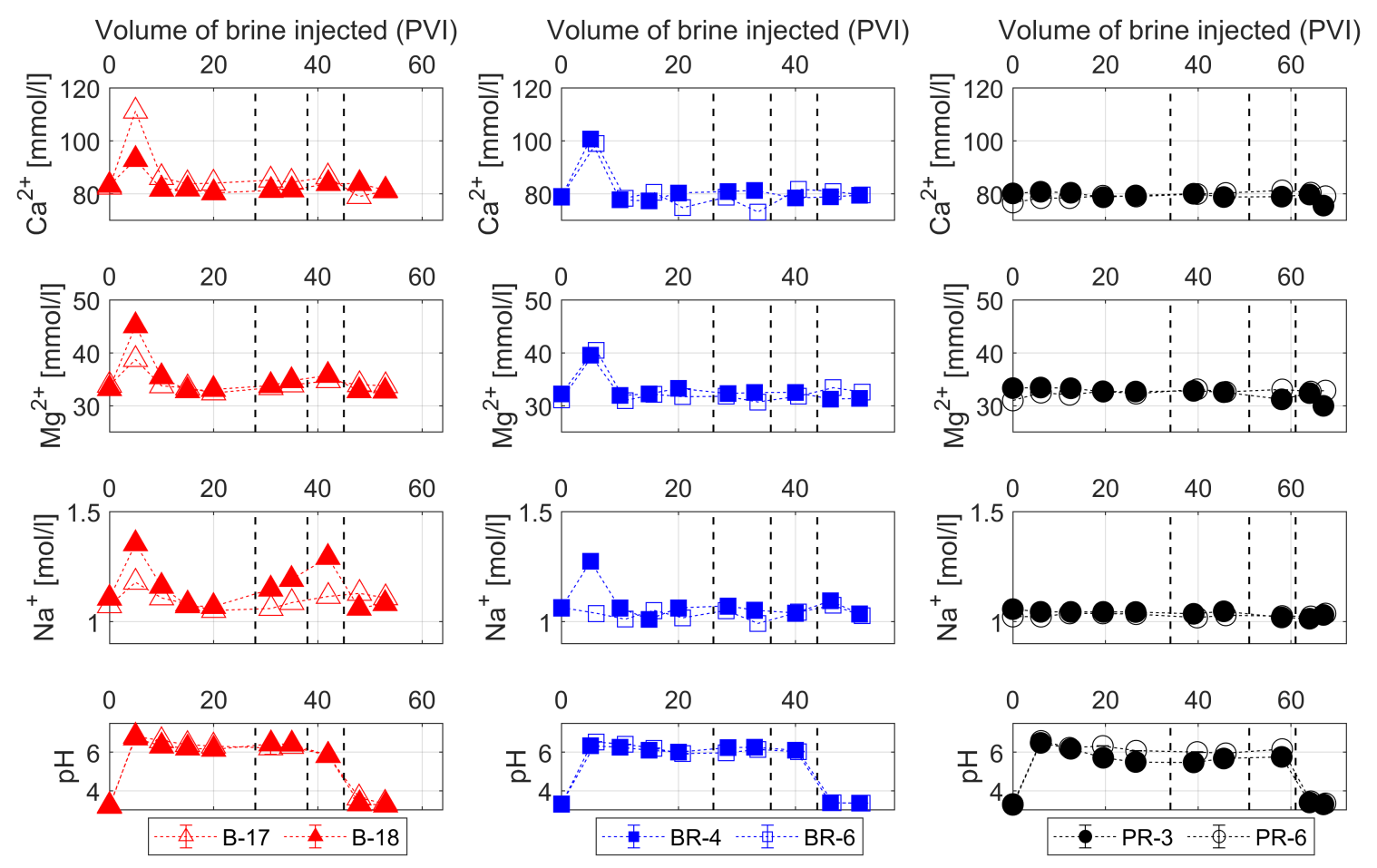
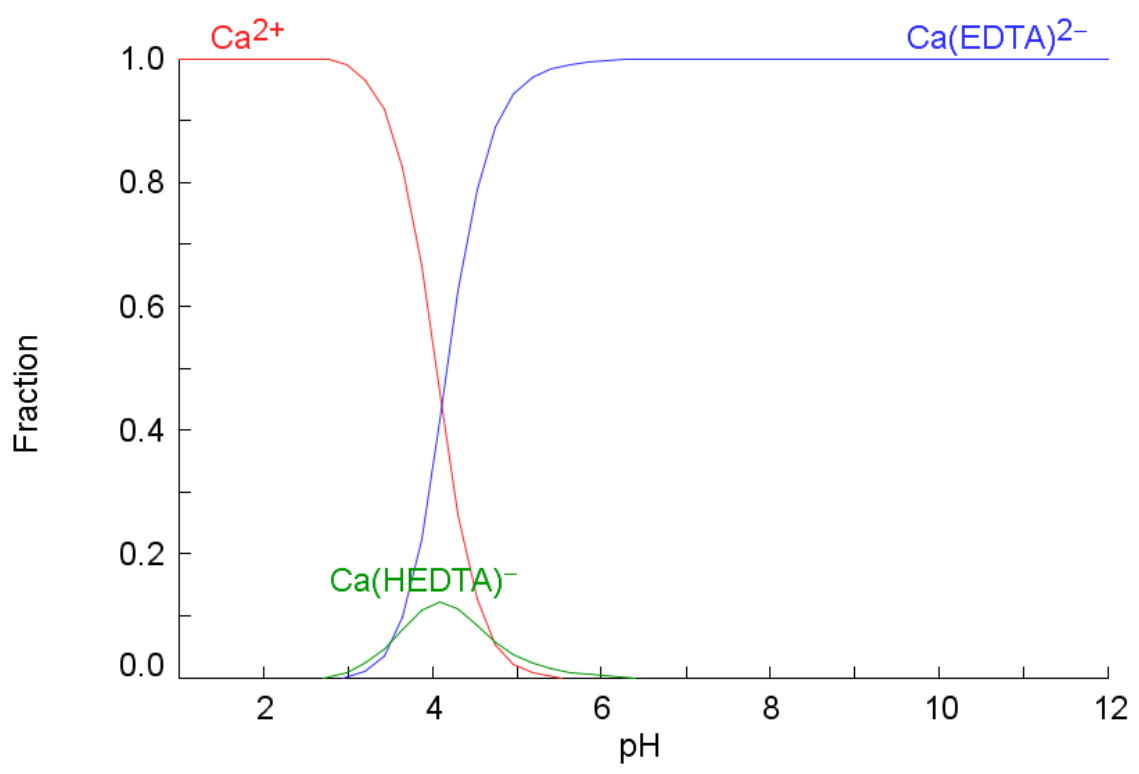
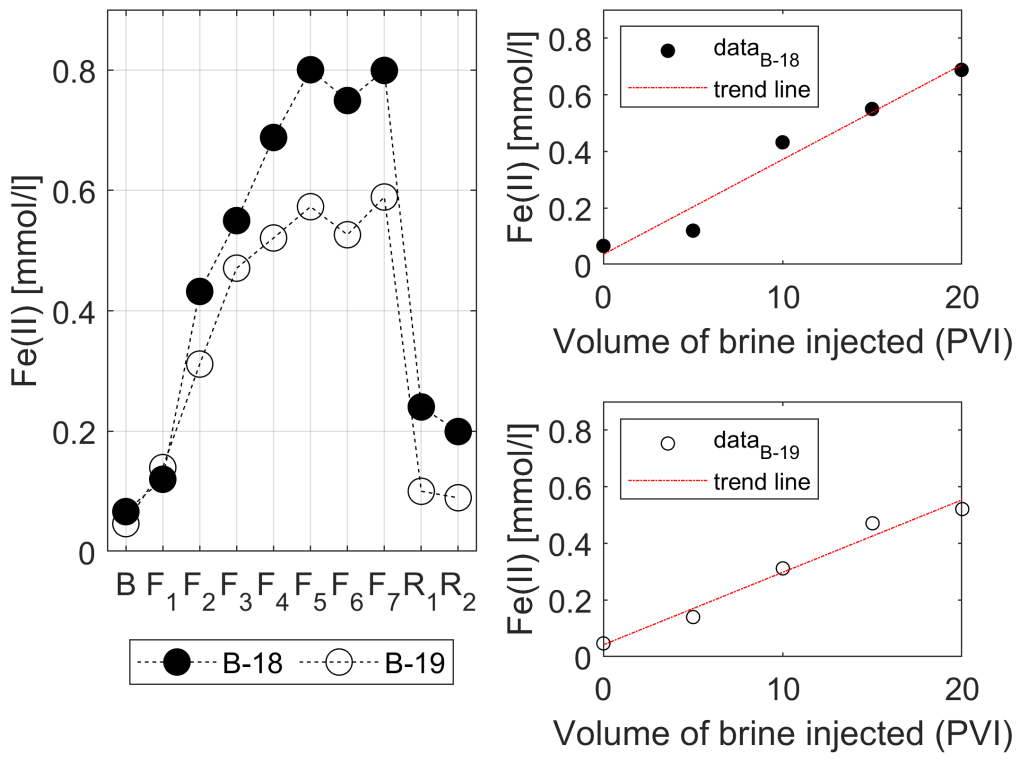
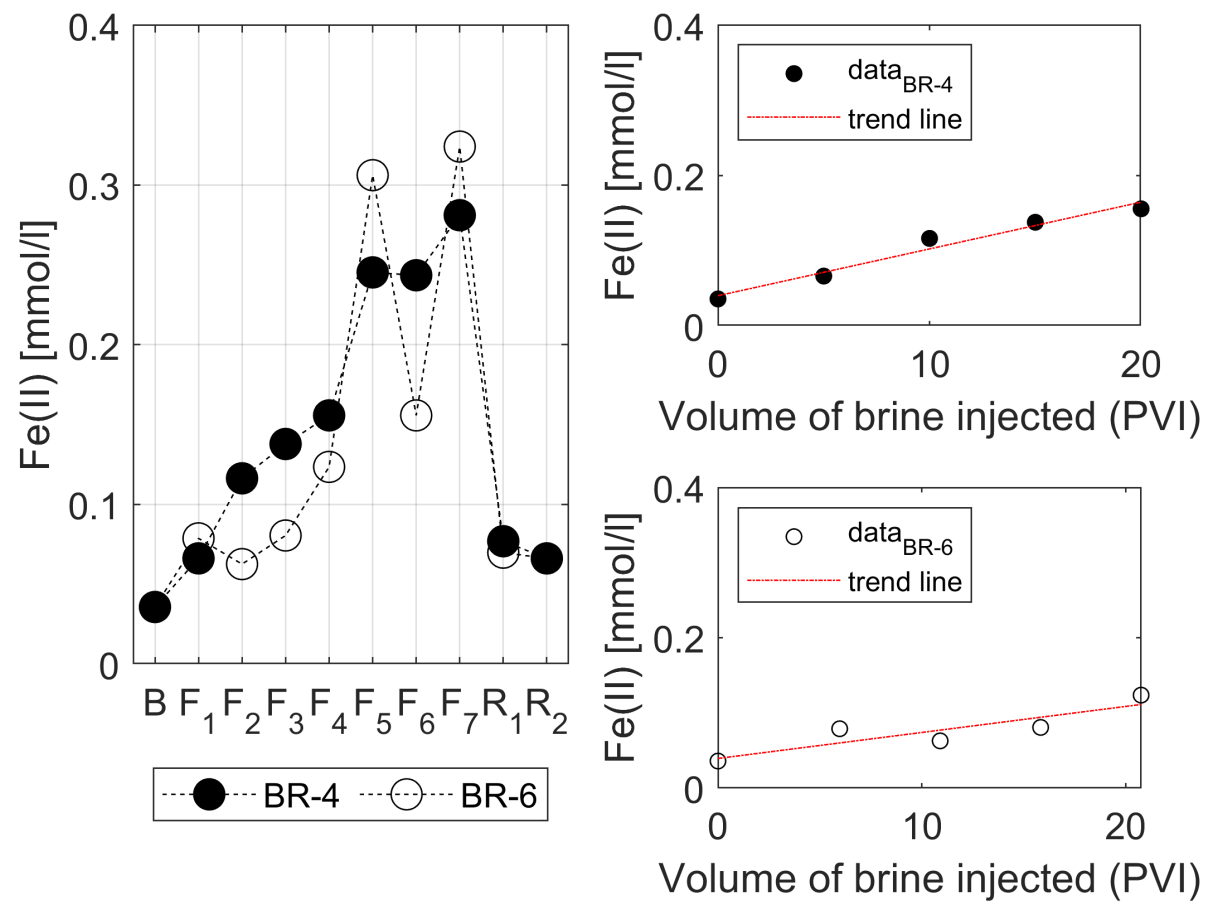
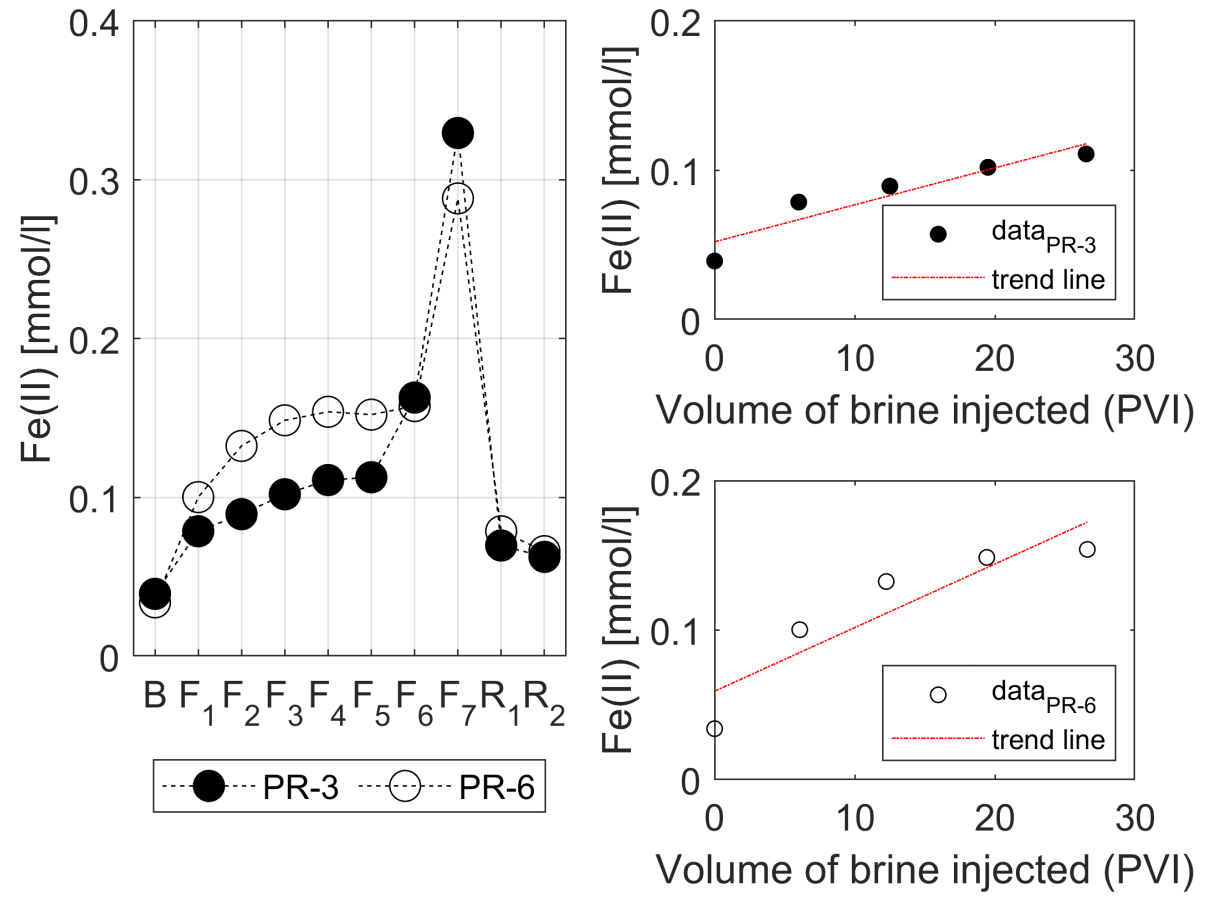
3.3. Water Chemistry
4. Conclusions
- Core flooding experiments indicated that half-diluted Thisted brine with EDTA (2D*TBEDTA) improves the injectivity in all types of sandstone rocks (homogeneous and heterogeneous). This enhancement is even higher if the fluid injection is stopped for a while.
- The addition of EDTA to half-diluted geothermal brine coming from the SaltPower electricity generation unit could avoid not only iron precipitation inside the porous media but also as standalone stimulation fluids for scale removal and matrix acidizing in geothermal reservoirs. Flow test experiments indicated an improvement in the rock properties (porosity) when 2D*TBEDTA was flooded through Berea, Bandera Brown, and Parker sandstone core plugs. The injection of 2D*TBEDTA could be used to increase the permeability in reinjection wells that have been clogged by precipitated salts instead of building a new one.
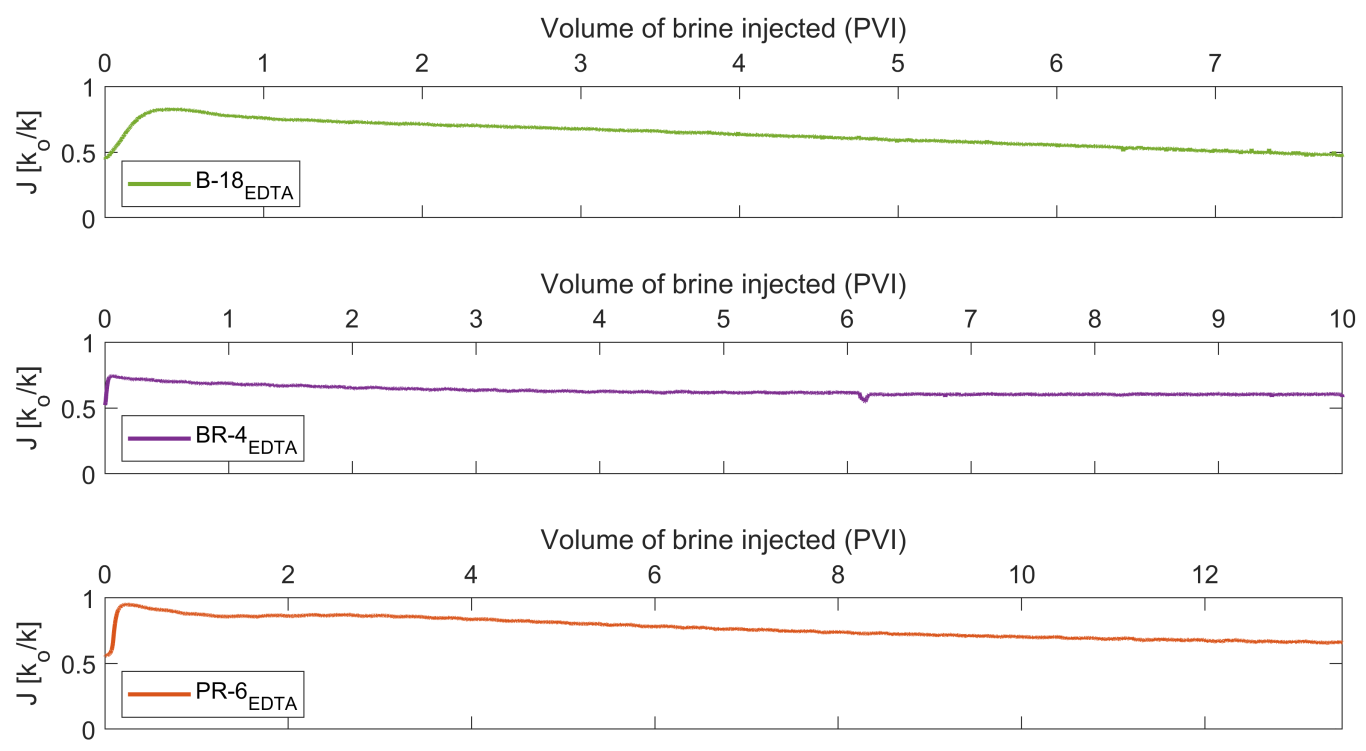
- The injection of 2D*TBEDTA causes the dissolution of carbonate cement in both homogeneous and heterogeneous rocks throughout the complexation of EDTA with divalent cations (Ca2+ and Mg2+) and their transport away from the mineral surface into the bulk solution. This process reaches a maximum at about 5PVI and then it decreases due to the formation of wider flow paths.
- The additional flooding experiments in which half-diluted geothermal brine without EDTA (2D*TB) was injected through the homogeneous and heterogeneous rocks show that the usage of EDTA can be decreased after some time due to the resulting increase of permeability and porosity in the heat reservoir. The reinjection of a half-diluted geothermal brine with EDTA could be a good choice to overcome the scaling problems in the reinjection wells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madsen, H.; Bruun Hansen, T.; Nakao, T.; Goda, S.; Søgaard, E. Combined geothermal heat and pressure retarded osmosis as a new green power system. Energy Convers. Manag. 2020, 226, 113504. [Google Scholar] [CrossRef]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.M.; Nijmeijer, K. Salinity gradients for sustainable energy: Primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef] [PubMed]
- Straub, A.P.; Deshmukh, A.; Elimelech, M. Pressure-retarded osmosis for power generation from salinity gradients: Is it viable? Energy Environ. Sci. 2016, 9, 31–48. [Google Scholar] [CrossRef]
- Madsen, H.T.; Søndergaard Nissen, S.; Søgaard, E.G. Theoretical framework for energy analysis of hypersaline pressure retarded osmosis. Chem. Eng. Sci. 2016, 139, 211–220. [Google Scholar] [CrossRef]
- Skilhagen, S.; Dugstad, J.; Aaberg, R. Osmotic power—Power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 2008, 220, 476–482. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power generation with pressure retarded osmosis: An experimental and theoretical investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Performance limiting effects in power generation from salinity gradients by pressure retarded osmosis. Environ. Sci. Technol. 2011, 45, 10273–10282. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A. Pressure retarded osmosis: Advancement in the process applications for power generation and desalination. Desalination 2015, 356, 31–46. [Google Scholar] [CrossRef]
- Madsen, H.; Nissen, S.; Muff, J.; Søgaard, E. Pressure retarded osmosis from hypersaline solutions: Investigating commercial FO membranes at high pressures. Desalination 2017, 420, 183–190. [Google Scholar] [CrossRef]
- Cobos, J.; Søgaard, E. Study of geothermal brine reinjection by microcalorimetry and core flooding experiments. Geothermics 2020, 87. [Google Scholar] [CrossRef]
- Cobos, J.; Søgaard, E. Systematic study of geothermal brine reinjection for SaltPower generation purposes: Citric acid as a potential iron control agent. Geothermics 2021, 95, 102116. [Google Scholar] [CrossRef]
- Crooijmans, R.; Willems, C.; Nick, H.; Bruhn, D. The influence of facies heterogeneity on the doublet performance in low-enthalpy geothermal sedimentary reservoirs. Geothermics 2016, 64, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Hovadik, J.M.; Larue, D.K. Static characterizations of reservoirs: Refining the concepts of connectivity and continuity. Pet. Geosci. 2007, 13, 195–211. [Google Scholar] [CrossRef]
- Nicolaides, C.; Jha, B.; Cueto-Felgueroso, L.; Juanes, R. Impact of viscous fingering and permeability heterogeneity on fluid mixing in porous media. Water Resour. Res. 2015, 51, 2634–2647. [Google Scholar] [CrossRef]
- Alqahtani, F.M.; Dahouk, M.M.; Whitson, C.H.; Chuparova, E. Impact of Fluid Heterogeneity on Tight Unconventional Well GOR Performance. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Virtual, 20–22 July 2020. [Google Scholar] [CrossRef]
- Prete, P.; Fiorentino, A.; Rizzo, L.; Proto, A.; Cucciniello, R. Review of aminopolycarboxylic acids–based metal complexes application to water and wastewater treatment by (photo-)Fenton process at neutral pH. Curr. Opin. Green Sustain. Chem. 2021, 28, 100451. [Google Scholar] [CrossRef]
- Stumm, W. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; John Wiley & Sons: Hoboken, NJ, USA, 1992. [Google Scholar]
- Rosenbrand, E.; Kjøller, C.; Fabricius, J.; Kets, F.; Fabricius, I. Different effects of temperature and salinity on permeability reduction by fines migration in Berea sandstone. Geothermics 2015, 53, 225–235. [Google Scholar] [CrossRef]
- Kamal, M.S.; Mahmoud, M.; Hanfi, M.; Elkatatny, S.; Hussein, I. Clay minerals damage quantification in sandstone rocks using core flooding and NMR. J. Pet. Explor. Prod. Technol. 2019, 9, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Gamal, H.; Elkatatny, S.; Adebayo, A. Influence of mud filtrate on the pore system of different sandstone rocks. J. Pet. Sci. Eng. 2021, 202, 108595. [Google Scholar] [CrossRef]
- Hubbert, M. Darcy’s law and the field equations of the flow of underground fluids. Int. Assoc. Sci. Hydrol. Bull. 1957, 2, 23–59. [Google Scholar] [CrossRef] [Green Version]
- University of Calgary. Crewes Properties Calculator. 2022. Available online: https://www.crewes.org/ (accessed on 13 January 2022).
- Klinkenberg, L.J. The permeability of porous media to liquids and gases. Drill. Prod. Pract. 1941, 108, 200–213. [Google Scholar] [CrossRef]
- Dawson, G.; Pearce, J.; Biddle, D.; Golding, S. Experimental mineral dissolution in Berea Sandstone reacted with CO2 or SO2-CO2 in NaCl brine under CO2 sequestration conditions. Chem. Geol. 2015, 399, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Cerasi, P.; Kjøller, C.; Sigalas, L.; Bhuiyan, D.; Frykman, P. Mechanical Effect of CO2 Flooding of a Sandstone Specimen. Energy Procedia 2016, 86, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Shehata, A.; Nasr-El-Din, H. The role of sandstone mineralogy and rock quality in the performance of low-salinity waterflooding. SPE Reserv. Eval. Eng. 2016, 20. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Vaz Alexandre, S.L.; Fernando, D.S.; Yulong, Y.; Zhenjiang, Y.; Pavel, B. Slow migration of mobilised fines during flow in reservoir rocks: Laboratory study. J. Pet. Sci. Eng. 2014, 122, 534–541. [Google Scholar] [CrossRef]
- Selley, R. Sedimentary Rocks | Mineralogy and Classification. In Encyclopedia of Geology; Selley, R.C., Cocks, L., Plimer, I.R., Eds.; Elsevier: Oxford, UK, 2005; pp. 25–37. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Influence of transport and reaction on wormhole formation in porous media. AIChE J. 1998, 44, 1933–1949. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.; Nasr-El-Din, H. Modeling flow of chelating agents during stimulation of carbonate reservoirs. Arab. J. Sci. Eng. 2014, 39, 9239–9248. [Google Scholar] [CrossRef]
- Alkhaldi, M.; Nasr-El-Din, H.; Sarma, H. Application of citric acid in acid stimulation treatments. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 16–18 June 2009; p. 16. [Google Scholar]
- Portier, S.; André, L. Review on Chemical Stimulation Techniques in Oil Industry and Applications to Geothermal Systems. 2007. Available online: http://engine.brgm.fr/Deliverables/Period2/ENGINE_D28_WP4_ChemicalStimulation_DHMA_052007.pdf (accessed on 27 April 2022).
- Moghadasi, J.; Müller-Steinhagen, H.; Jamialahmadi, M.; Sharif, A. Chemical stimulation for EGS: The use of chelating agents at high pH for the simultaneous dissolution of calcium carbonate, silicas, and silicates. In Proceedings of the World Geothermal Congress 2010, Bali, Indonesia, 25–30 April 2010; p. 11. [Google Scholar]
| Ionic Composition | Intrinsic Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Mg2+ | Na+ | Sr2+ | Ca2+ | Cl− | Fe(II) | pH | ||||
| 2D*TBEDTA | 11.7 | 33.2 | 1106.8 | 1.8 | 82.5 | 1437.3 | 0.05 | 1.51 | 1.06 | 3.19 | 0.87 |
| Core id. | l (cm) | d (cm) | PV (mL) | % | kgas (MD) | kklink (MD) |
|---|---|---|---|---|---|---|
| B-18 | 7.64 | 3.80 | 15.57 | 17.90 | 26.23 | 24.19 |
| B-19 | 7.64 | 3.80 | 15.71 | 18.09 | 24.49 | 22.51 |
| BR-4 | 7.64 | 3.80 | 17.42 | 20.22 | 21.00 | 7.36 |
| BR-6 | 7.64 | 3.80 | 17.45 | 20.22 | 20.95 | 7.68 |
| PR-3 | 7.64 | 3.80 | 14.03 | 16.12 | 17.54 | 7.67 |
| PR-6 | 7.64 | 3.80 | 13.76 | 15.80 | 17.04 | 5.19 |
| Berea | Bandera Brown | Parker | |||
|---|---|---|---|---|---|
| Mineral | Concentration (wt%) | Mineral | Concentration (wt%) | Mineral | Concentration (wt%) |
| Albite | 14.1 | Albite | 3.2 | Albite | 7.2 |
| Kaolinite | 25.8 | Kaolinite | 3.7 | Kaolinite | 8.0 |
| Quartz | 60.1 | Illite | 3.9 | Illite | 2.7 |
| - | - | Quartz | 80.2 | Quartz | 73.3 |
| - | - | Muscovite | 8.9 | Muscovite | 8.7 |
| Sample | k0 (mD) | kB1 (mD) | kB2 (mD) | kB3 (mD) |
|---|---|---|---|---|
| B-18 | 28.2 | 47.6 | 46.1 | - |
| B-19 | 35.0 | 42.8 | 50.7 | 80.7 |
| BR-4 | 15.4 | 25.8 | 27.9 | 27.4 |
| BR-6 | 15.6 | 24.1 | 26.9 | 27.3 |
| PR-3 | 11.7 | 15.1 | 19.6 | 26.3 |
| PR-6 | 9.7 | 20.9 | 22.7 | 22.8 |
| Ion | Equation | R2 |
|---|---|---|
| B-18 | 0.96 | |
| B-19 | 0.97 | |
| BR-4 | 0.96 | |
| BR-6 | 0.78 | |
| PR-3 | 0.87 | |
| PR-6 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobos, J.E.; Søgaard, E.G. Impact of Reservoir Heterogeneity on Diluted Geothermal Brine Reinjection. Energies 2022, 15, 3321. https://doi.org/10.3390/en15093321
Cobos JE, Søgaard EG. Impact of Reservoir Heterogeneity on Diluted Geothermal Brine Reinjection. Energies. 2022; 15(9):3321. https://doi.org/10.3390/en15093321
Chicago/Turabian StyleCobos, Jacquelin E., and Erik G. Søgaard. 2022. "Impact of Reservoir Heterogeneity on Diluted Geothermal Brine Reinjection" Energies 15, no. 9: 3321. https://doi.org/10.3390/en15093321
APA StyleCobos, J. E., & Søgaard, E. G. (2022). Impact of Reservoir Heterogeneity on Diluted Geothermal Brine Reinjection. Energies, 15(9), 3321. https://doi.org/10.3390/en15093321






