Thermochemical Modeling of Metal Composition and Its Impact on the Molten Corium–Concrete Interaction: New Insights with Sensitivity Analysis
Abstract
:1. Introduction
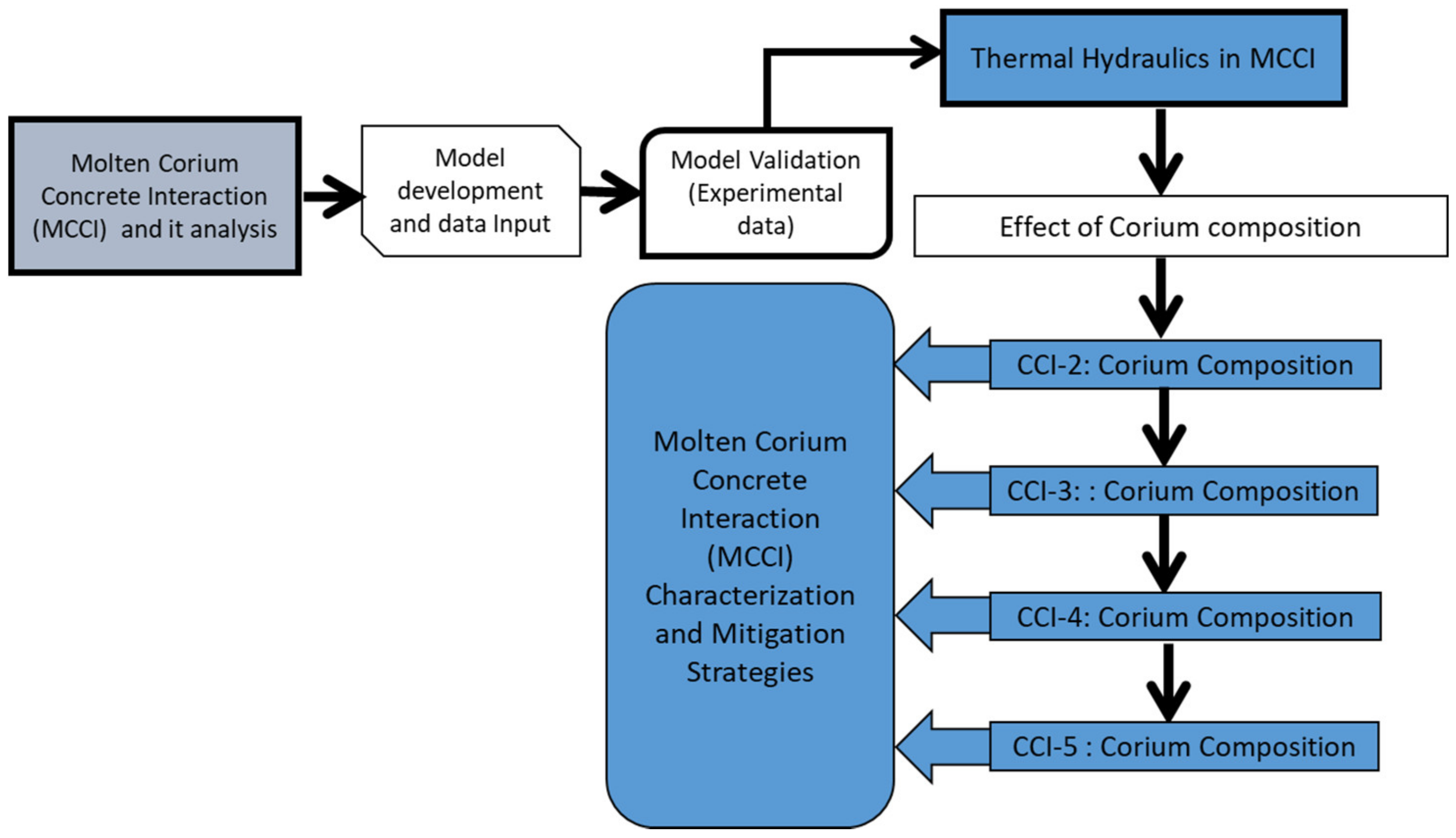
2. Modeling Corium Composition, Viscosity, Heat Transfer, and Ablation
- a.
- Dry-out concrete model;
- b.
- Dry-out transient concrete model;
- c.
- Ablation quasi-steady concrete model.
3. Results
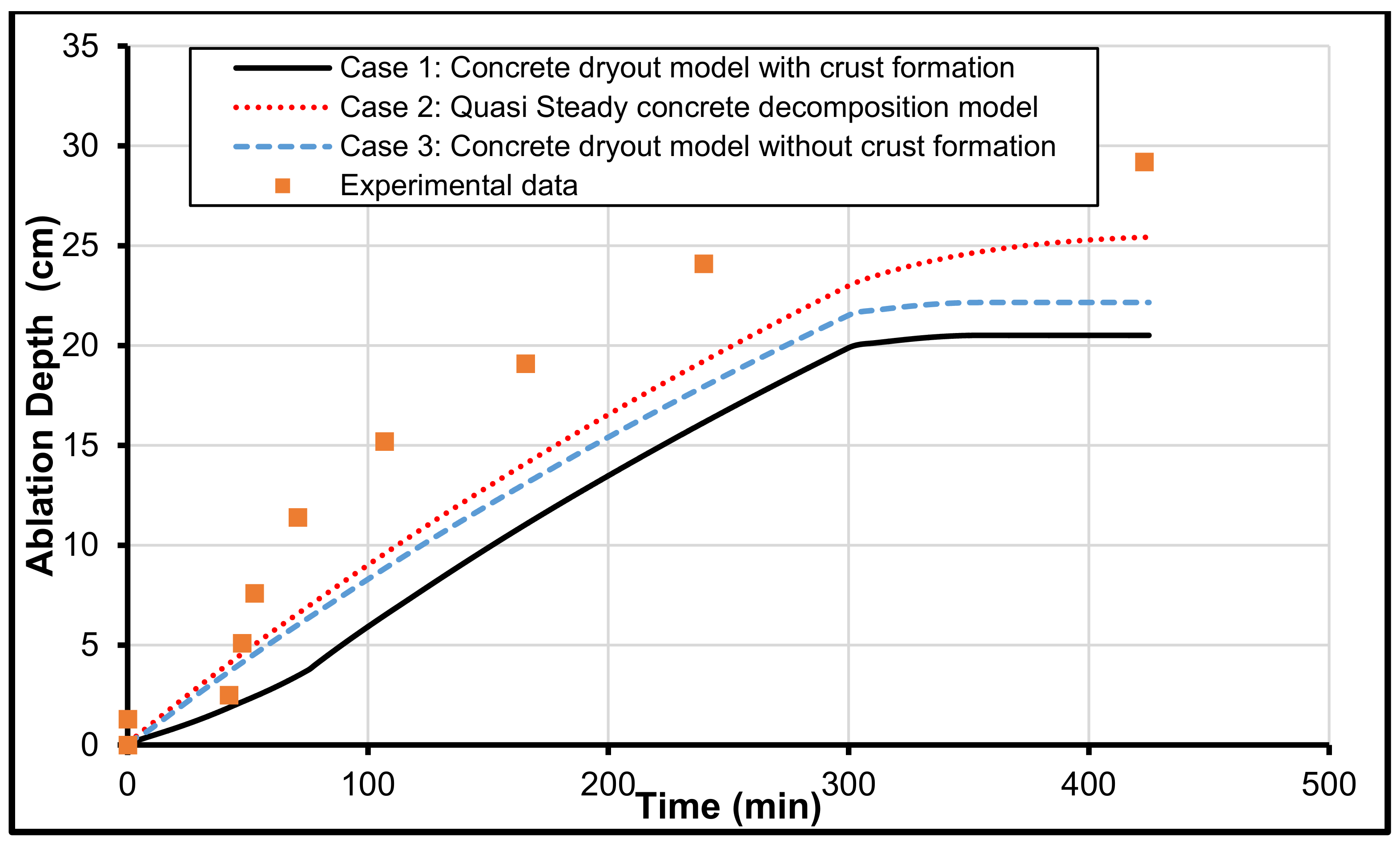
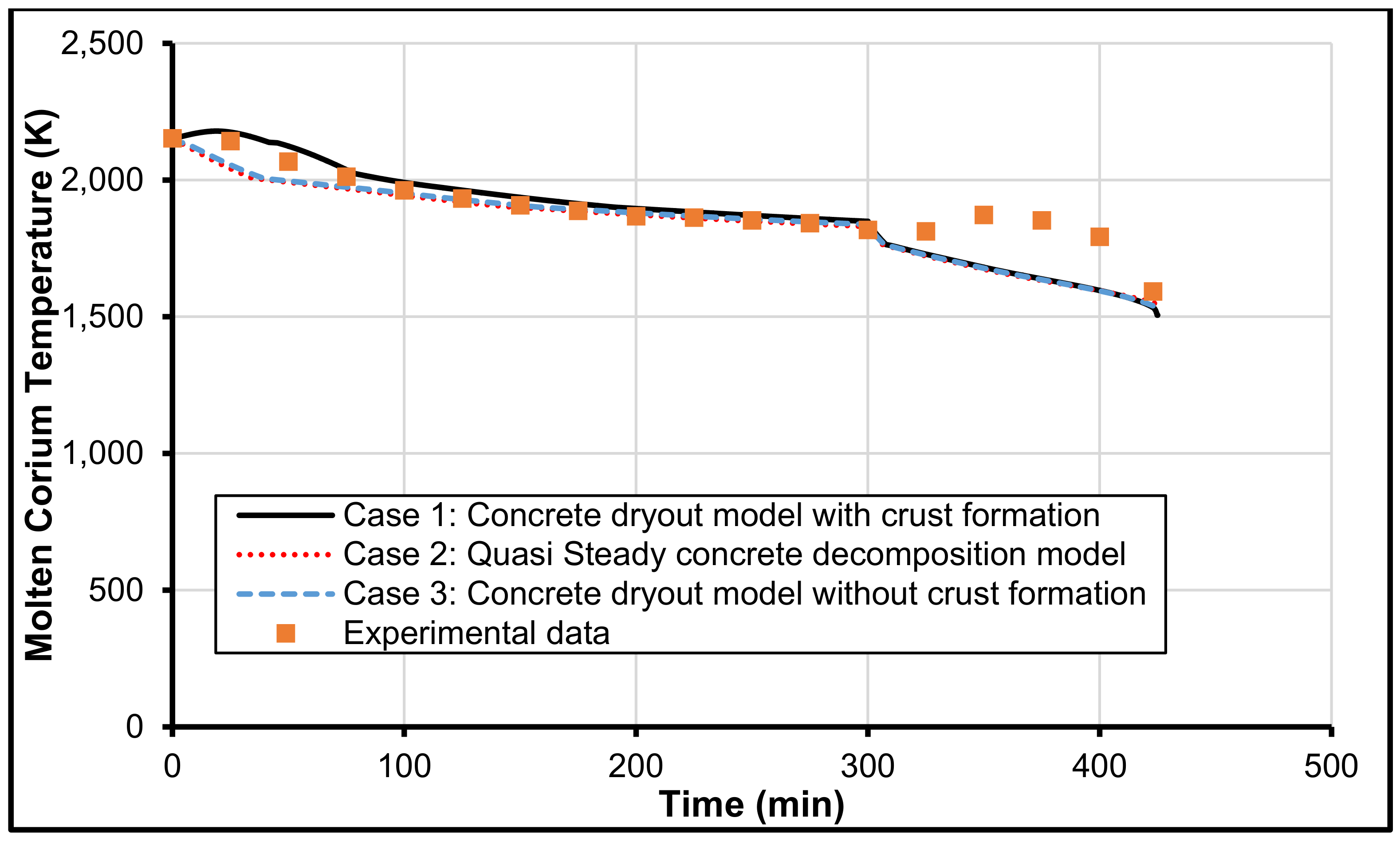
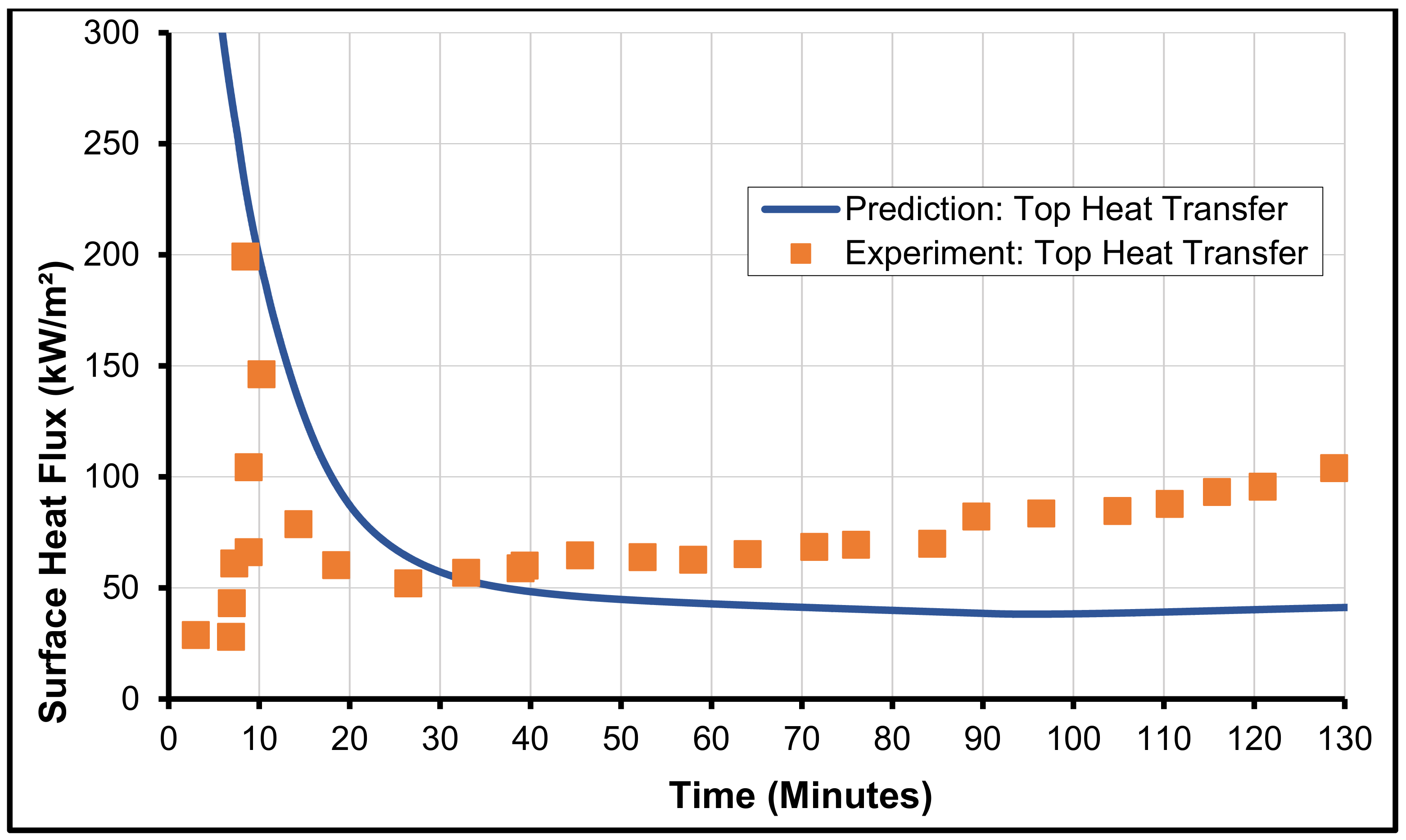
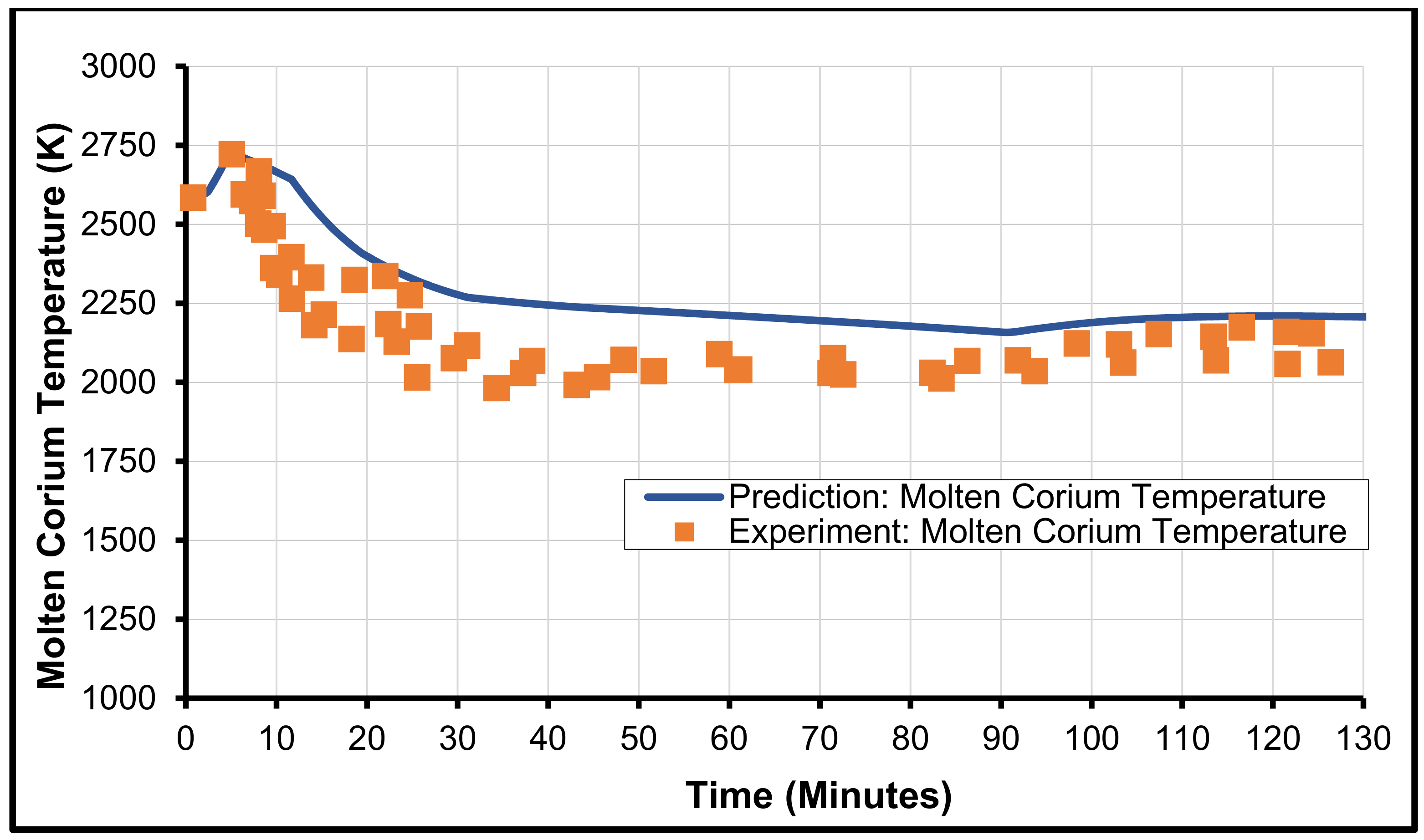
3.1. MCCI Model Justification/Validation
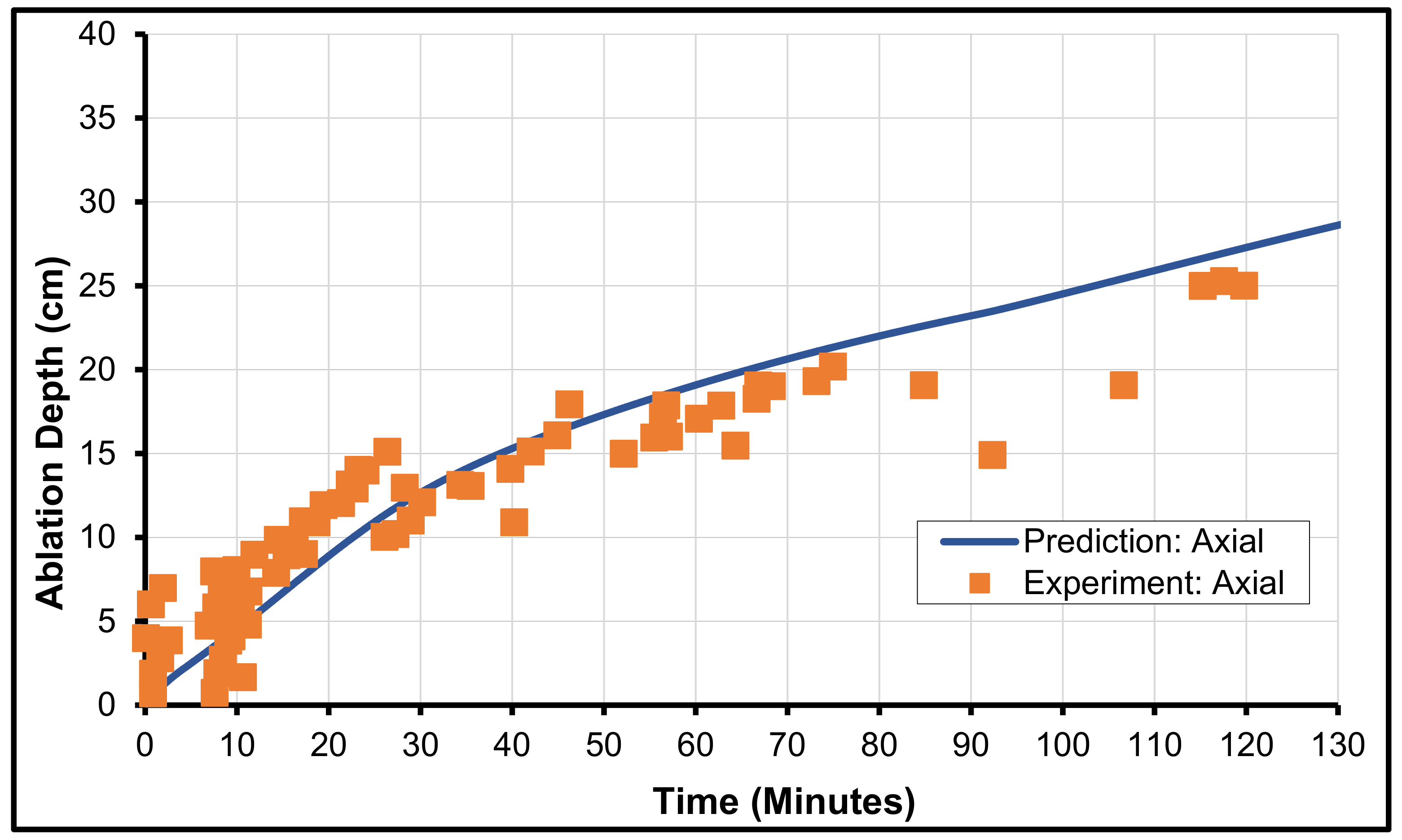
3.2. Validation with MCCI Data (Experimental)
3.3. CCI-2 from OECD/MCCI
3.4. SURC-2 from SNL
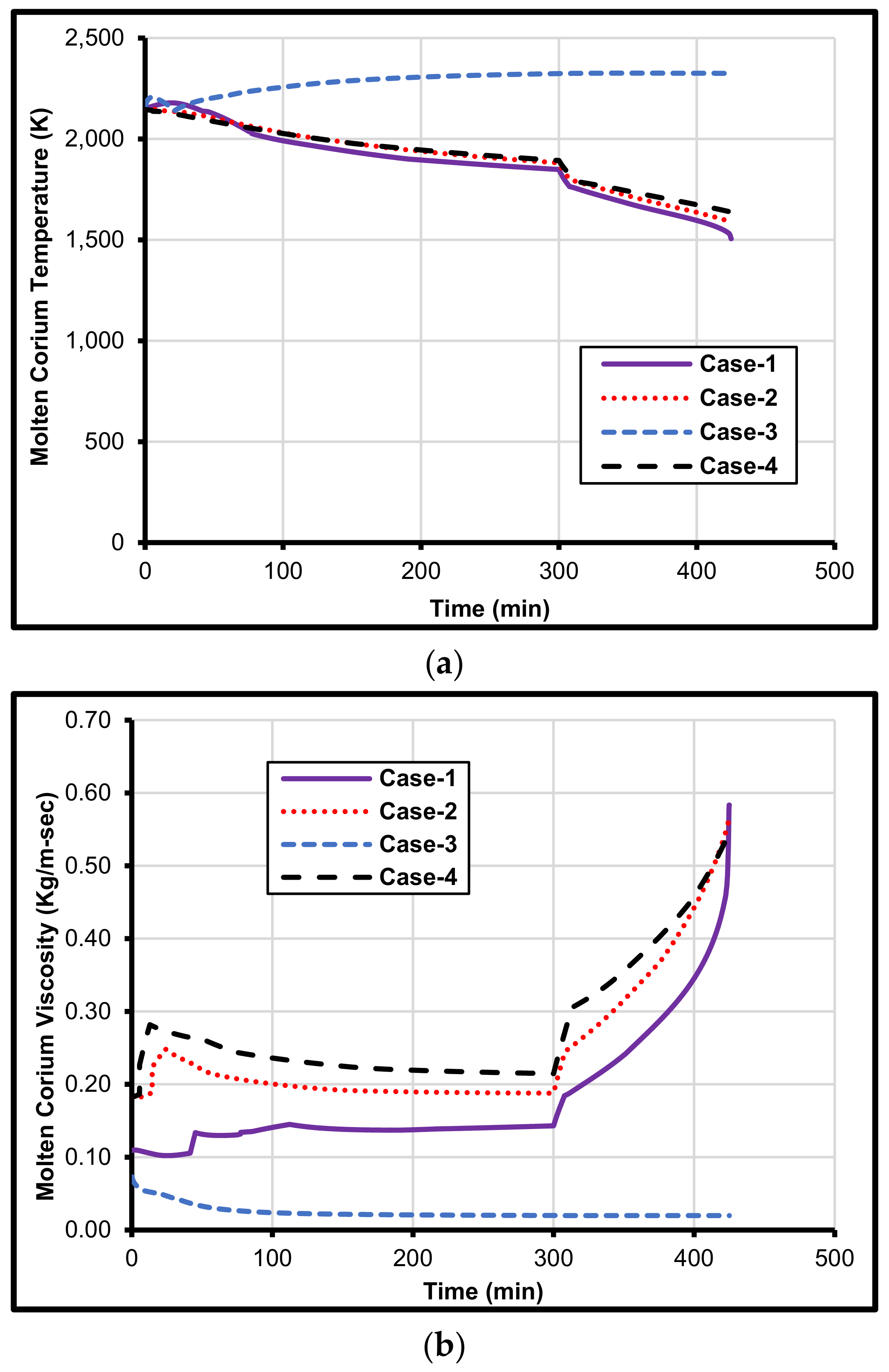
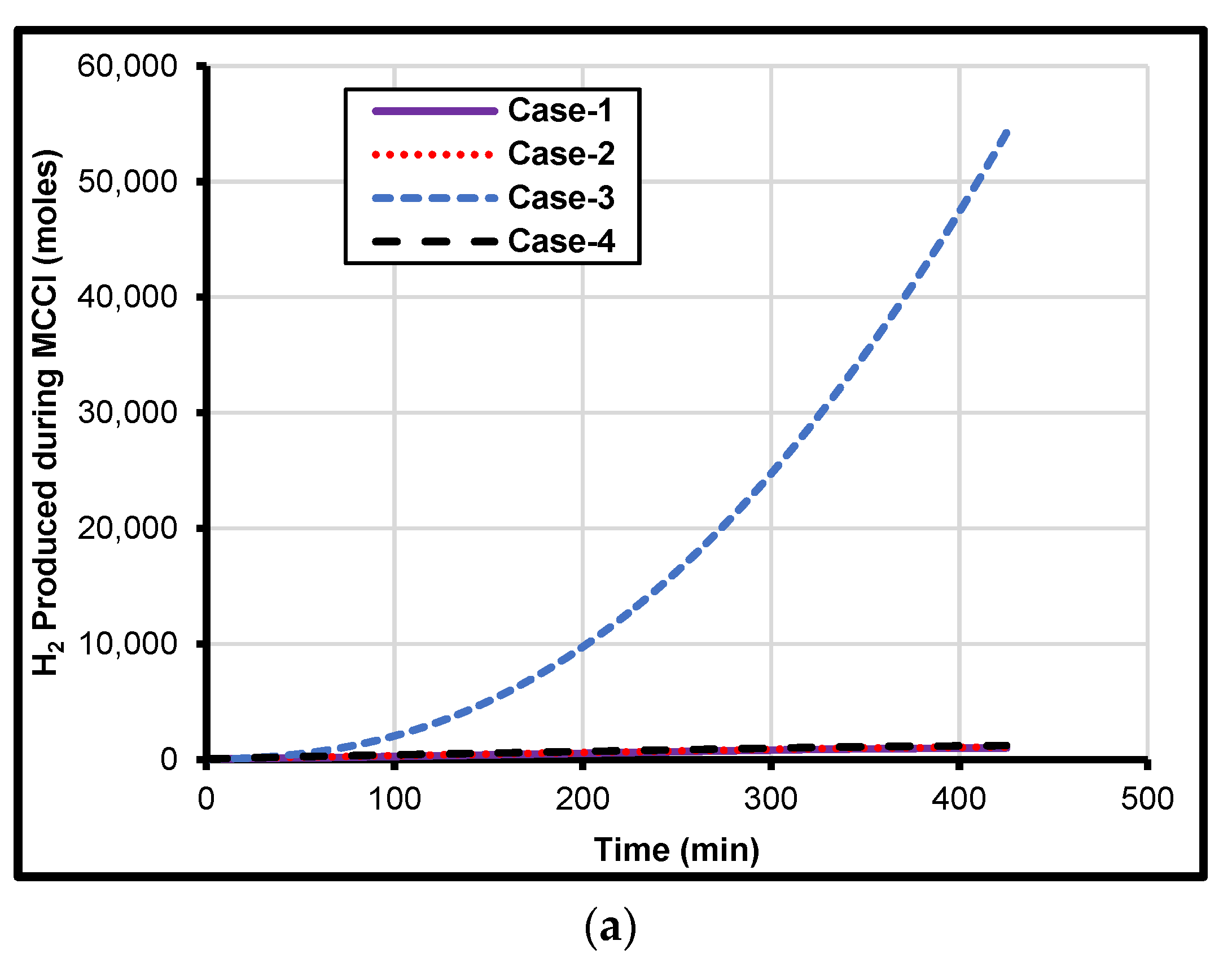
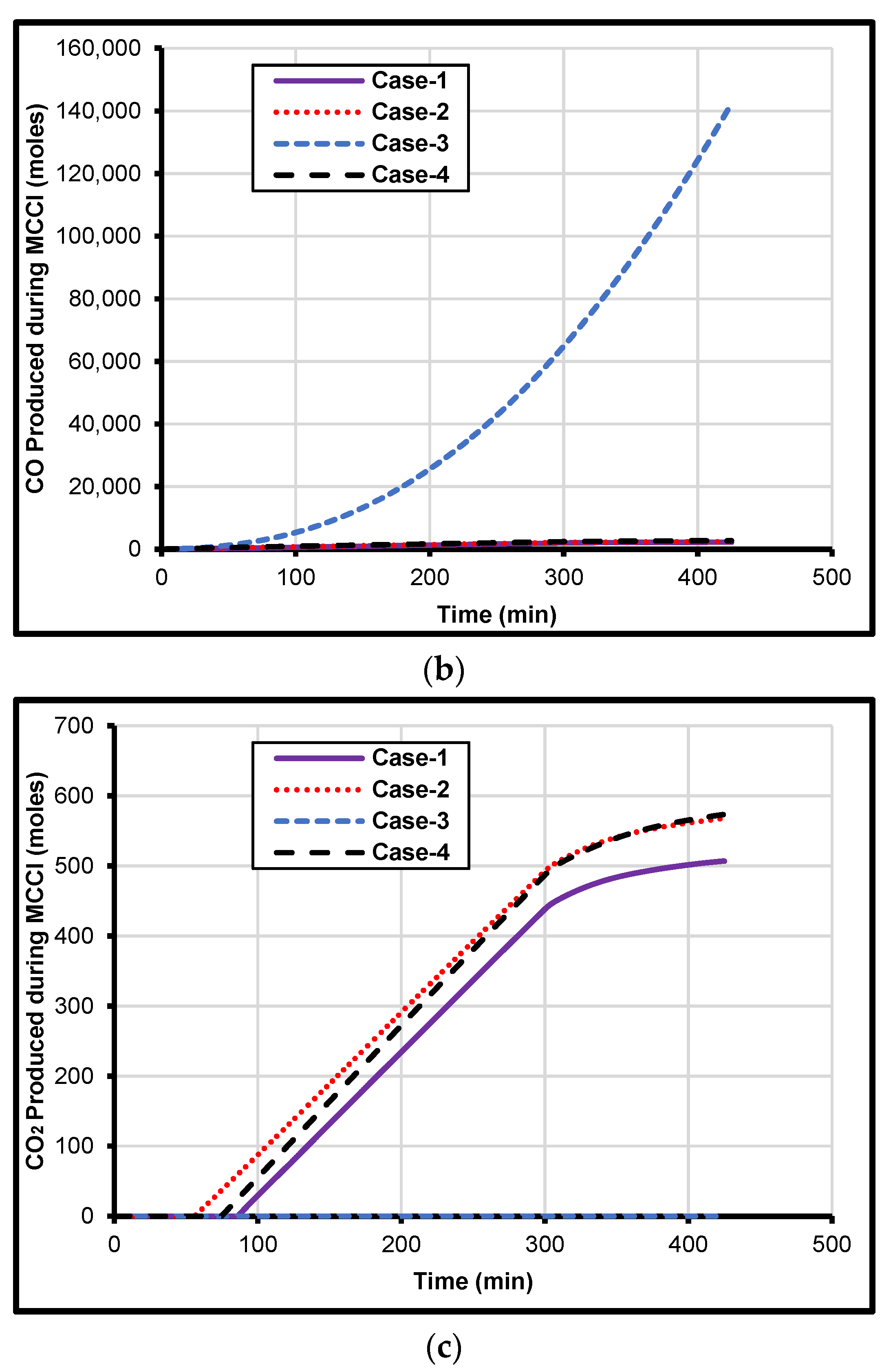
4. Prediction of Metal Composition on MCCI
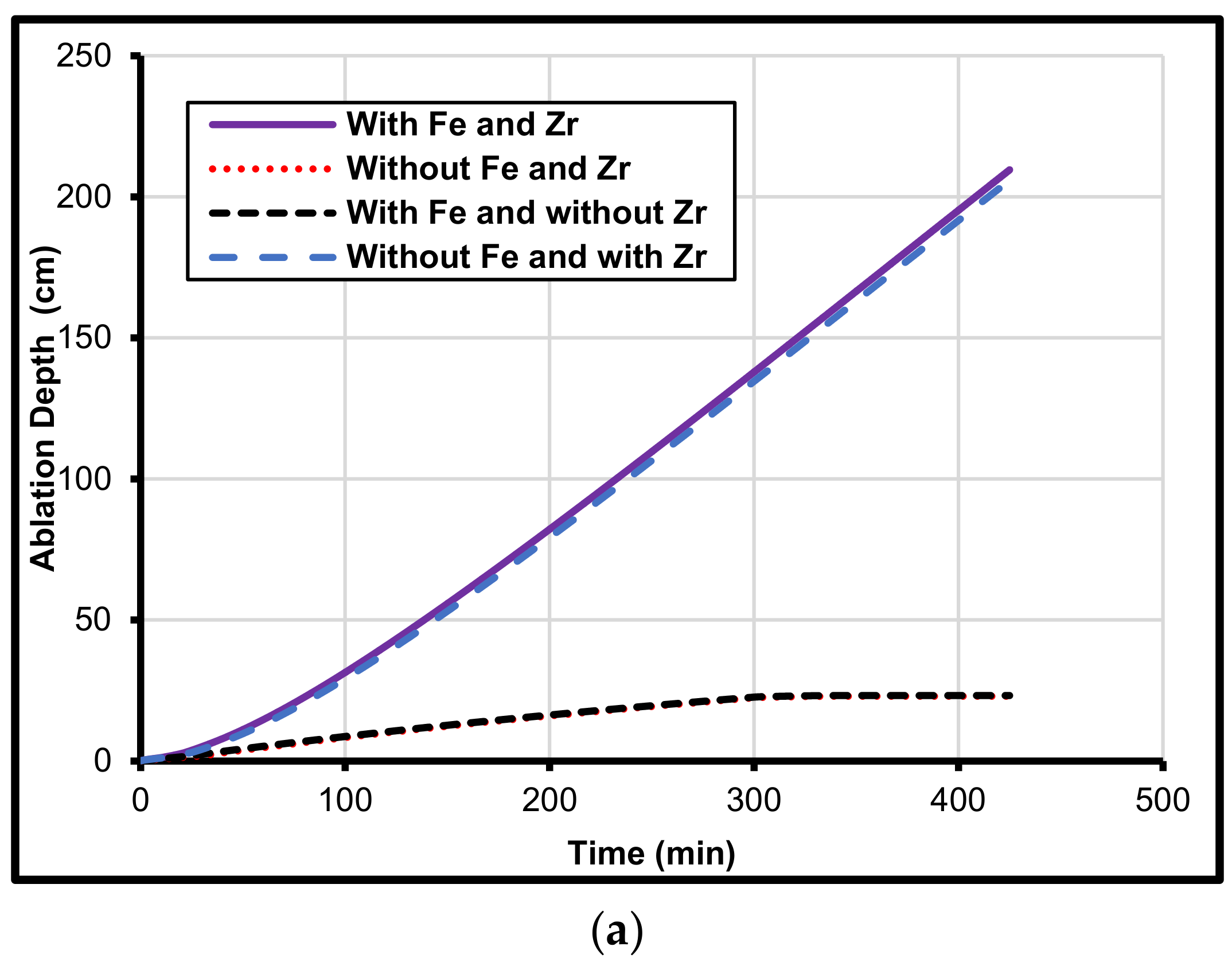
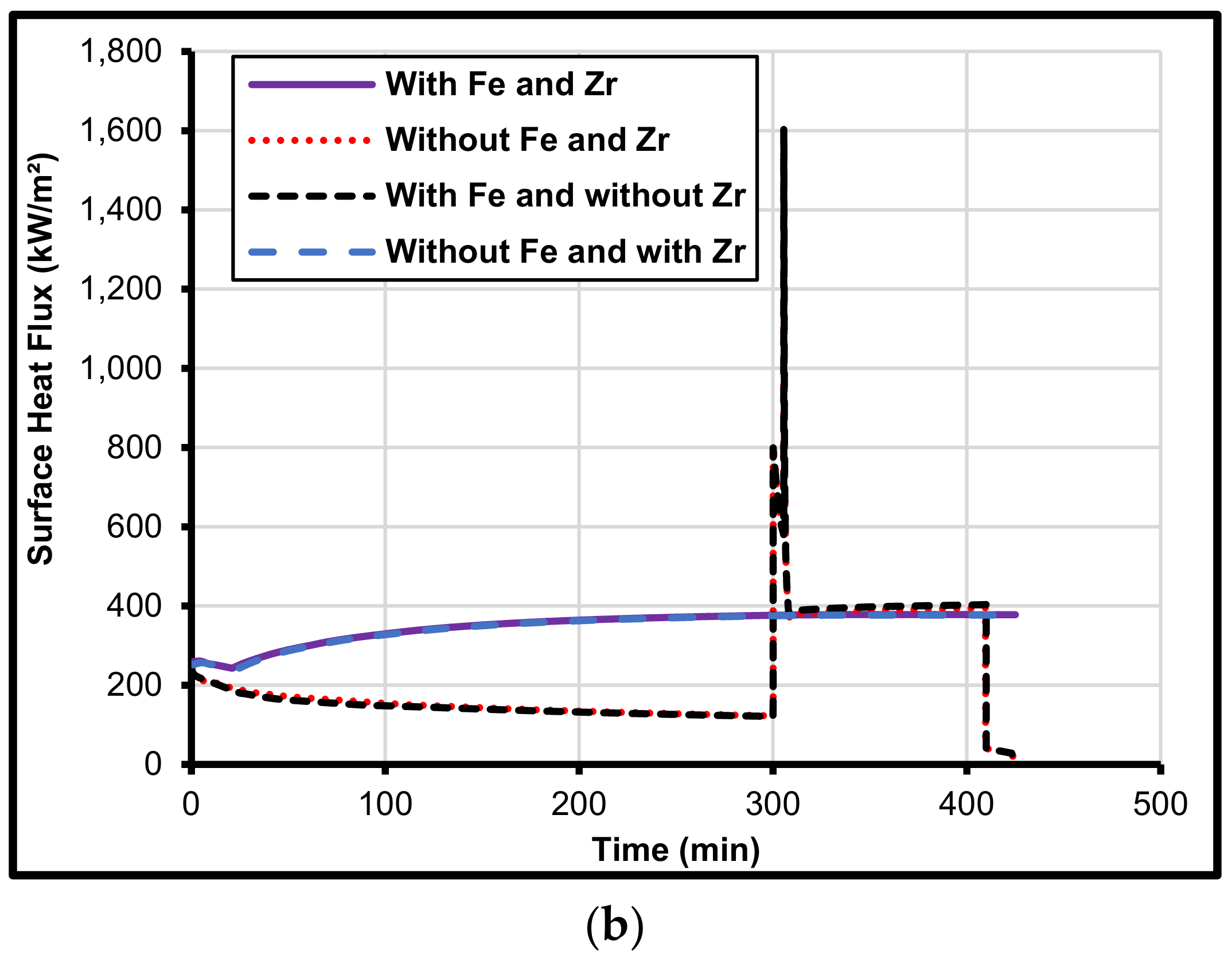
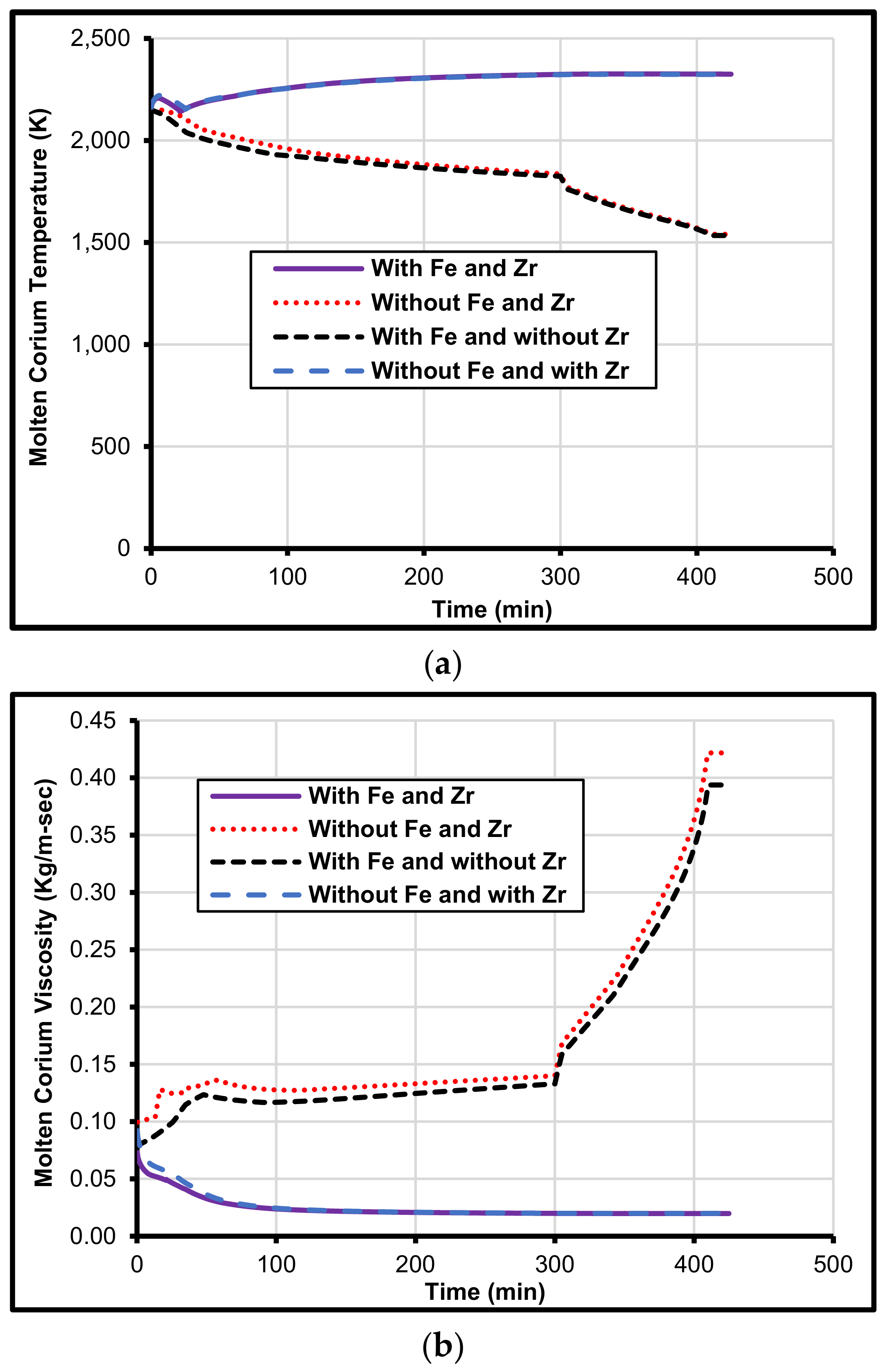
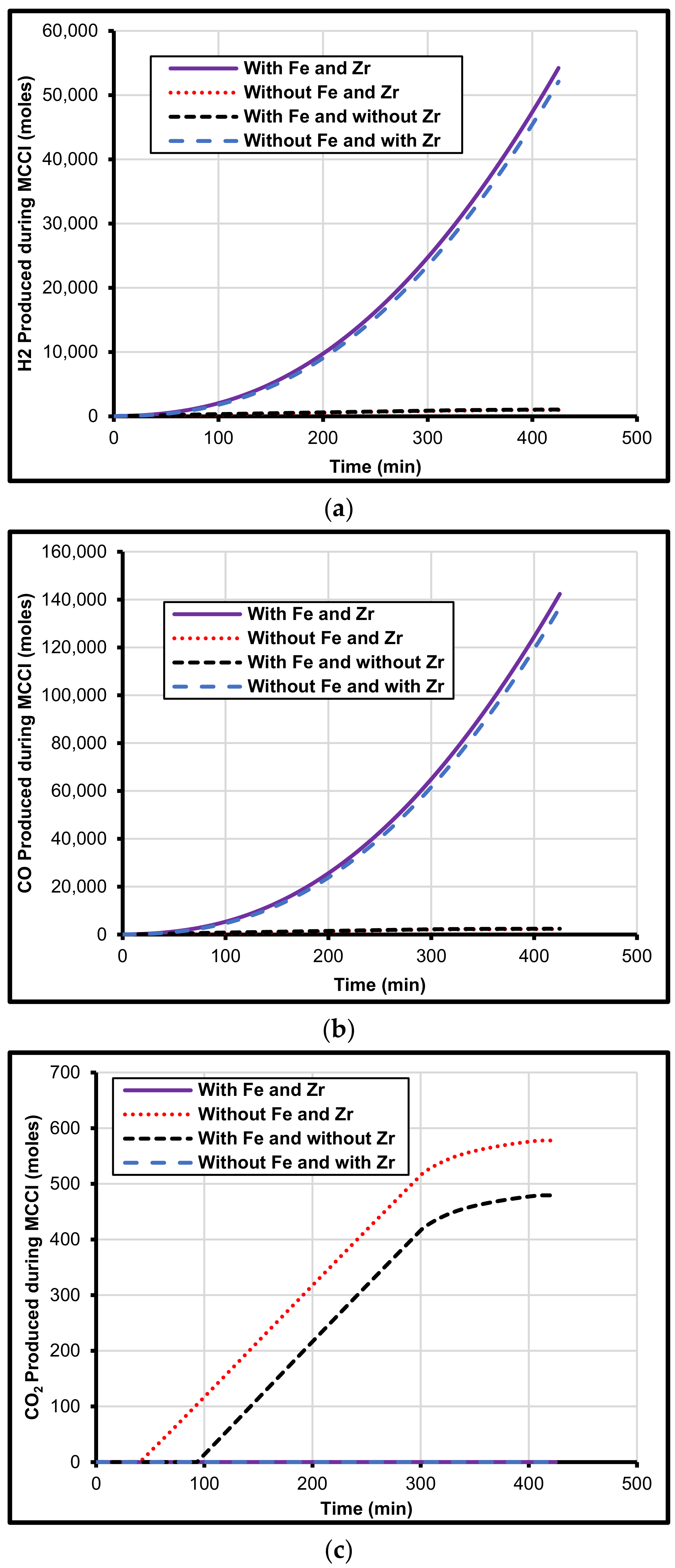
5. Iron and Zirconium Effect on MCCI
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amidu, M.A.; Olatubosun, S.A.; Ayodeji, A.; Addad, Y. Severe accident in high-power light water reactors: Mitigating strategies, assessment methods, and research opportunities. Prog. Nucl. Energy 2021, 143, 104062. [Google Scholar] [CrossRef]
- Farmer, M.T. The CORQUENCH Code for Modeling of Ex-Vessel Corium Coolability under Top FLooding Conditions; The United States; Argonne National Laboratory: Argonne, IL, USA, 2018. [Google Scholar] [CrossRef]
- Bonnet, J.-M.; Cranga, M.; Vola, D.; Marchetto, C.; Kissane, M.; Robledo, F.; Farmer, M.T.; Spengler, C.; Basu, S.; Atkhen, K.; et al. State-of-the-Art Report on Molten Corium Concrete Interaction and Ex-Vessel Molten Core Coolability; (NEA--7392). Nuclear Energy Agency of the OECD (NEA): Paris, France, 2017. [Google Scholar]
- Wu, Z.; Laurence, D.; Iacovides, H.; Afgan, I. Direct simulation of conjugate heat transfer of jet in channel crossflow. Int. J. Heat Mass Transf. 2017, 110, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Abed, N.; Afgan, I. A CFD study of flow quantities and heat transfer by changing a vertical to diameter ratio and horizontal to diameter ratio in inline tube banks using URANS turbulence models. Int. Commun. Heat Mass Transf. 2017, 89, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Laurence, D.; Afgan, I. Direct numerical simulation of a low momentum round jet in channel crossflow. Nucl. Eng. Des. 2017, 313, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Kahil, Y.; Benhamadouche, S.; Berrouk, A.S.; Afgan, I. Simulation of subcritical-Reynolds-number flow around four cylinders in square arrangement configuration using LES. Eur. J. Mech.-B/Fluids 2019, 74, 111–122. [Google Scholar] [CrossRef]
- Wu, Z.; Laurence, D.; Utyuzhnikov, S.; Afgan, I. Proper orthogonal decomposition and dynamic mode decomposition of the jet in channel crossflow. Nucl. Eng. Des. 2019, 344, 54–68. [Google Scholar] [CrossRef]
- Nguyen, P.T.L.; Uribe, J.C.; Afgan, I.; Laurence, D.R. A Dual-Grid Hybrid RANS/LES Model for Under-Resolved Near-Wall Regions and its Application to Heated and Separating Flows. Flow Turbul. Combust. 2020, 104, 835–859. [Google Scholar] [CrossRef] [Green Version]
- Benhamadouche, S.; Afgan, I.; Manceau, R. Numerical Simulations of Flow and Heat Transfer in a Wall-Bounded Pin Matrix. Flow Turbu. Comb. 2020, 104, 19–44. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, U.; Apsley, D.; Stallard, T.; Stansby, P.; Afgan, I. Turbulent length scales and budgets of Reynolds stress-transport for open-channel flows; friction Reynolds numbers Reτ = 150, 400 and 1020. J. Hydraul. Res. 2021, 59, 36–50. [Google Scholar] [CrossRef]
- Ali, A.E.A.; Afgan, I.; Laurence, D.; Revell, A. A dual-mesh hybrid RANS-LES simulation of the buoyant flow in a differentially heated square cavity with an improved resolution criterion. Comput. Fluids 2021, 224, 104949. [Google Scholar] [CrossRef]
- Guleren, K.M.; Afgan, I.; Turan, A. Predictions of turbulent flow for the impeller of a NASA low-speed centrifugal compressor. ASME J. Turbomach. 2010, 132, 021005. [Google Scholar] [CrossRef]
- Han, X.; Sagaut, P.; Lucor, D.; Afgan, I. Stochastic response of the laminar flow past a flat plate under uncertain inflow conditions. Int. J. Comput. Fluid Dyn. 2012, 26, 101–117. [Google Scholar] [CrossRef]
- Abed, N. Assessment and Evaluation of the Thermal Performance of Various Working Fluids in Parabolic Trough Collectors of Solar Thermal Power Plants under Non-Uniform Heat Flux Distribution Conditions. Energies 2020, 13, 3776. [Google Scholar] [CrossRef]
- Abed, N.; Afgan, I.; Cioncolini, A.; Iacovides, H.; Nasser, A.; Mekhail, T. Thermal performance evaluation of various nanofluids with non-uniform heating for parabolic trough collectors. Case Stud. Therm. Eng. 2020, 22, 100769. [Google Scholar] [CrossRef]
- D’Auria, F.; Hassan, Y. Challenges and concerns for development of nuclear thermal-hydraulics. Nucl. Eng. Des. 2021, 375, 111074. [Google Scholar] [CrossRef]
- Zhdanov, V.; Vasilyev, Y.; Kolodeshnikov, A.; Cherepnin, Y.; Sakaki, I.; Nagasaka, H. COTELS Project (4): Structural Investigation of Solidified Debris in MCCI. In Proceedings of the OECD Workshop on Ex-Vessel Debris Coolability, Karlsruhe, Germany, 15–18 November 1999. Organized in collaboration with Forschungszentrum Karlsruhe (FZK) GmbH. [Google Scholar]
- Blose, R.E.; Gronager, J.E.; Suo-Anttila, A.; Brockmann, J.E. SWISS: Sustained Heated Metallic Melt/Concrete Interactions with Overlying Water Pools; Sandia National Laboratories: Albuquerque, NM, USA, 1987. [Google Scholar]
- Nagasaka, H.; Sakaki, I.; Kato, M.; Vasilyev, Y.; Kolodeshnikov, A.; Zhdanov, V. COTELS Project (3): Ex-vessel Debris Cooling Tests. In Proceedings of the OECD Workshop on Ex-Vessel Debris Coolability, Karlsruhe, Germany, 15–18 November 1999. Organized in Collaboration with Forschungszentrum Karlsruhe (FZK) GmbH. [Google Scholar]
- Blose, R.E.; Powers, D.A.; Copus, E.R.; Brockmann, J.E.; Simpson, R.B.; Lucero, D.A. Core-Concrete Interactions with Overlying Water Pools; The WETCOR-1 Test; Nuclear Regulatory Commission: Washington, DC, USA, 1993. [Google Scholar]
- Kato, M.; Nagasaka, H.; Vasilyev, Y.; Kolodeshnikov, A.; Zhdanov, V. COTELS Project (2): Fuel Coolant Interaction Tests under Ex-Vessel Conditions. In Proceedings of the OECD Workshop on Ex-Vessel Debris Coolability, Karlsruhe, Germany, 15–18 November 1999. Organized in Collaboration with Forschungszentrum Karlsruhe (FZK) GmbH. [Google Scholar]
- Maruyama, Y.; Tahara, M.; Nagasaka, H.; Kolodeshnikov, A.; Zhdanov, V.; Vassiliev, Y. Kyeongju Recent Results of Mcci Studies in Cotels Project. In Proceedings of the NTHAS3: Third Korea-Japan Symposium on Nuclear Thermal Hydraulics and Safety, Kyeongju, Korea, 13–16 October 2002. [Google Scholar]
- Alsmeyer, H.; Cron, T.; Foit, J.J.; Messemer, G.; Schmidt-Stiefel, S.; Haefner, W. Forschungszentrum Karlsruhe GmbH Technik und Umwelt (Germany). In Inst. Fuer Kern- und Energietechnik, Programm Nukleare Sicherheitsforschung; Kriscio, H [Becker Technologies, Eschborn (Germany), Experiment ECOKATS-2: On Melt Spreading and Subsequent Top Flooding; Test and Data Report; Commission of the European Communities: Brussels, Belgium, 2005. [Google Scholar]
- Lomperski, S.; Farmer, M.T.; Basu, S. Experimental investigation of corium quenching at elevated pressure. Nucl. Eng. Des. 2006, 236, 2271–2280. [Google Scholar] [CrossRef]
- Lomperski, S.; Farmer, M.T. Corium crust strength measurements. Nucl. Eng. Des. 2009, 239, 2551–2561. [Google Scholar] [CrossRef]
- Miassoedov, A.; Alsmeyer, H.; Cron, T.; Foit, J. The COMET-L3 experiment on long-term melt–concrete interaction and cooling by surface flooding. Nucl. Eng. Des. 2010, 240, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Journeau, C.; Piluso, P.; Correggio, P.; Ferry, L.; Fritz, G.; Haquet, J.; Monerris, J.; Ruggieri, J.; Sanchez-Brusset, M.; Parga, C. Contributions of the VULCANO experimental programme to the Understanding of MCCI phenomena. Nucl. Eng. Technol. 2012, 44, 261–272. [Google Scholar]
- Foit, J.J.; Fischer, M.; Journeau, C.; Langrock, G. Experiments on MCCI with oxide and steel. Ann. Nucl. Energy 2014, 74, 100–109. [Google Scholar] [CrossRef]
- Gencheva, R.; Stefanova, A.; Groudev, P.; Cranga, M.; Tyrpekl, V.; Duspiva, J.; Kujal, B.; Barc, H.; Chatterjee, B. Investigation of Some Phenomena and Parametrical Studies on VVER1000 MCCI. In Proceedings of the 6th European Review Meeting on Severe Accident Research (ERMSAR-2013), Avignon, France, 2–4 October 2013. [Google Scholar]
- Bixler, N.; Gauntt, R.; Jones, J.; Leonard, M. State-of-the-Art-Reactor Consequence Analyses Project: Volume 1, Peach Bottom Integrated Analysis; U.S. Nuclear Regulatory Commission: Washington, DC, USA, 2013. [Google Scholar]
- Spengler, C.; Foit, J.; Fargette, A.; Agethen, K.; Cranga, M. Transposition of 2D Molten Corium–Concrete Interactions (MCCI) from experiment to reactor. Ann. Nucl. Energy 2014, 74, 89–99. [Google Scholar] [CrossRef]
- Bradley, D.R.; Gardner, D.R.; Brockmann, J.E.; Griffith, R.O. CORCON-MOD3: An Integrated Computer Model for Analysis of Molten Core-Concrete Interactions; User’s Manual; Nuclear Regulatory Commission: Washington, DC, USA, 1993. [Google Scholar]
- Cranga, M.; Fabianelli, R.; Jacq, F.; Barrachin, M.; Duval, F. The MEDICIS code, a versatile tool for MCCI modeling. In Proceedings of the ICAPP05, Seoul, Korea, 15–19 May 2005. [Google Scholar]
- Bolshov, L.; Strizhov, V. SOCRAT: The System of Codes for Realistic Analysis of Severe Accidents; American Nuclear Society—ANS: La Grange Park, IL, USA, 2006. [Google Scholar]
- Polidoro, F.; Parozzi, F.; Manzini, G. Preliminary Assessment of the Thermal Interaction among Corium and In-Vessel Core Catcher in a Large Sodium Fast Reactor. In Proceedings of the 15th International Topical Meeting on Nuclear Reactor Thermal-Hydraulics, NURETH-15 NURETH15-138, Pisa, Italy, 12–17 May 2013. [Google Scholar]
- Amidu, M.A.; Addad, Y. The influence of the water ingression and melt eruption model on the MELCOR code prediction of molten corium-concrete interaction in the APR-1400 reactor cavity. Nucl. Eng. Technol. 2022, 54, 1508–1515. [Google Scholar] [CrossRef]
- Farmer, M.T.; Spencer, B.W.; Binder, J.L.; Hill, D.J. Status and Future Direction of the Melt Attack and Coolability Experiments (MACE) Program at Argonne National Laboratory; US Department of Energy: Washington, DC, USA, 2001. [Google Scholar]
- Farmer, M.T.; Spencer, B.W.; Kilsdonk, D.J.; Aeschlimann, R.W.; Engineering, R. Results of MACE Core Coolability Experiments M0 and M1b; Argonne National Laboratory: Argonne, IL, USA, 2000. [Google Scholar]
- Farmer, M.T.; Lomperski, S.; Kilsdonks, D.J.; Aeschlimann, R.W. OEC MCCI-2 Project; Final Report; Argonne National Laboratory: Argonne, IL, USA, 2010. [Google Scholar]
- Khurshid, I.; Afgan, I.; Alade, A.M.; Yacine, A. Influence of corium temperature, concrete composition and water injection time on concrete ablation during MCCI: New insights. Prog. Nucl. Energy 2022, 144, 104102. [Google Scholar] [CrossRef]
- Kunitz, M. An empirical formula for the relation between viscosity of solution and volume of solute. J. Gen. Physiol. 1926, 9, 715–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, M.; Zuber, N. Drag coefficient and relative velocity in bubbly, droplet, or particulate flows. AIChE J. 1979, 25, 843–855. [Google Scholar] [CrossRef]
- Bradley, D.R. Modeling of heat transfer between core debris and concrete. In Proceedings of the ANS Proceedings of the 1988 National Heat Transfer Conference, Houston, TX, USA, 24–28 July 1988. [Google Scholar]
- Kutateladze, S.S.; Malenkov, I.G. Boiling and Bubbling Heat Transfer under the Conditions of Free and Forced Convection. In Proceedings of the 6th International Heat Transfer Conference Digital Library, Toronto, ON, Canada, 7–11 August 1978; Volume 1, pp. 281–286. [Google Scholar]
- Jones, S.W.; Epstein, M.; Bankoff, S.G.; Pedersen, D.R. Dryout Heat Fluxes in Particulate Beds Heated Through the Base. J. Heat Transf. 1984, 106, 176–183. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Choi, J.; Cho, Y.J. Concrete ablation analysis for molten corium-concrete interaction mitigation strategy. Ann. Nucl. Energy 2019, 132, 615–627. [Google Scholar] [CrossRef]
- Ricou, F.P.; Spalding, D.B. Measurements of entrainment by axisymmetrical turbulent jets. J. Fluid Mech. 1961, 11, 21–32. [Google Scholar] [CrossRef]
- Farmer, M.T. Phenomenological Modeling of the Melt Eruption Cooling Mechanism During Molten Corium Concrete Interaction (MCCI); American Nuclear Society—ANS: La Grange Park, IL, USA, 2006. [Google Scholar]
- Spencer, B.W.; Wang, K.; Blomquist, C.A.; McUmber, L.M.; Schneider, J.P. Fragmentation and Quench Behavior of Corium Melt Streams in Water; Argonne National Laboratory: Argonne, IL, USA, 1994. [Google Scholar]
- Magallon, D. Characteristics of corium debris bed generated in large-scale fuel-coolant interaction experiments. Nucl. Eng. Des. 2006, 236, 1998–2009. [Google Scholar] [CrossRef]
- Khurshid, I.; Afgan, I.; Alade, A.M.; Yacine, A. A new insight into molten corium concrete interaction with concrete ablation analysis for mitigation scheme. V002T07A028. In Proceedings of the International Conference on Nuclear Engineering, Proceedings, ICONE, Virtual Online, 4–6 August 2021. [Google Scholar]
- Khurshid, I.; Alade, A.M.; Yacine, A.; Afgan, I. Thermo-physiochemical analysis of molten corium concrete interaction with concrete: A new insight into ablation analysis. NURETH-35447. In Proceedings of the 19th International Topical Meeting on Nuclear Reactor Thermal Hydraulics (NURETH-19), Virtual Online, 6–11 March 2022. [Google Scholar]



| Oxidation Based Chemical Reactions | Component ith | |||
|---|---|---|---|---|
| Zr + 2H2O → ZrO2 + 2H2 Zr + 2CO2 → ZrO2 + 2CO | Zr | |||
| ZrO2 | ||||
| Gas Phase: Si + 2H2O → SiO2 + 2H2 Si + 2CO2 → SiO2 + 2CO Condensed Phase: Zr + SiO2 → ZrO2 + Si(l) (T 2784) Zr + 2SiO2 → ZrO2 + 2SiO(g) (T 2784) | Si | |||
| SiO2 | ||||
| 2Cr + 3H2O → Cr2O3 + 3H2 2Cr + 3CO2 → Cr2O3 + 3CO | Cr | 0 | ||
| Cr2O3 | 0 | |||
| Fe + H2O → FeO + H2 Fe + CO2 → FeO + CO | Fe | 0 | ||
| FeO | 0 |
| Component | CCI-2 | |
|---|---|---|
| Concrete composition (Wt %) | CO2 | 30.42 |
| CaO | 26.42 | |
| SiO2 | 22.01 | |
| MgO | 11.71 | |
| H2O | 4.46 | |
| Al2O3 | 2.54 | |
| Fe2O3 | 1.42 | |
| K2O | 0.56 | |
| Na2O | 0.32 | |
| TiO2 | 0.14 | |
| Concrete Properties | Solidus temperature (K) | 1393 |
| Liquidus temperature (K) | 1568 | |
| Ablation temperature (K) | 1500 | |
| Fraction H2O liberated | 1.00 | |
| Fraction CO2 liberated | 1.00 |
| Component | CCI-2 | |
|---|---|---|
| Composition of melt/corium (Kg) | U2O | 242.48 |
| ZrO2 | 99.60 | |
| Cr | 25.64 | |
| SiO2 | 13.56 | |
| CaO | 12.52 | |
| MgO | 4.56 | |
| Al2O3 | 1.64 | |
| Zr | 0 | |
| Fe | 0 | |
| Total | 400.0 | |
| Melt Temperature (K) | Initial melt temperature | 2150 |
| Outer structure temperature | 750 |
| Component | SURC-2 | |
|---|---|---|
| Composition of melt/corium (kg) | UO2 | 140.9 |
| ZrO2 | 46.1 | |
| Zr | 16.9 | |
| Total | 203.9 | |
| Melt Temperature (K) | Initial melt temperature | 2600 |
| Outer structure temperature | 900 |
| Component | SURC-2 | |
|---|---|---|
| Composition of Concrete (Wt %) | SiO2 | 57.90 |
| CaO | 13.80 | |
| Al2O3 | 7.20 | |
| H2O | 5.00 | |
| Fe2O3 | 4.40 | |
| MgO | 4.00 | |
| K2O | 3.80 | |
| CO2 | 1.50 | |
| Na2O | 1.40 | |
| TiO2 | 0.80 | |
| Properties of Concrete | Solidus temperature (K) | 1350 |
| Liquidus temperature (K) | 1650 | |
| Ablation temperature (K) | 1500 | |
| Fraction H2O liberated | 0.68 | |
| Fraction CO2 liberated | 1.00 |
| S.No | Constituent | Case-1 | Case-2 | Case-3 | Case-4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mass (kg) | Percentage | Mass (kg) | Percentage | Mass (kg) | Percentage | Mass (kg) | Percentage | ||
| 1 | UO2 | 242.5 | 60.6 | 211.4 | 56.3 | 169.4 | 56.5 | 332.3 | 56.3 |
| 2 | ZrO2 | 99.6 | 24.9 | 86.8 | 23.1 | 64.5 | 21.5 | 136.5 | 23.1 |
| 3 | SiO2 | 13.6 | 3.4 | 41.9 | 11.2 | 12.2 | 4.1 | 65.9 | 11.2 |
| 4 | Al2O3 | 1.6 | 0.4 | 2.4 | 0.6 | 1.5 | 0.5 | 3.8 | 0.6 |
| 5 | MgO | 4.6 | 1.1 | 0.5 | 0.1 | 4.1 | 1.4 | 0.7 | 0.1 |
| 6 | CaO | 12.5 | 3.1 | 8.3 | 2.2 | 11.2 | 3.7 | 13.0 | 2.2 |
| 7 | Cr | 25.6 | 6.4 | 24.1 | 6.4 | 14.1 | 4.7 | 37.8 | 6.4 |
| 8 | Zr | 0.0 | 0.0 | 0.0 | 0.0 | 13.8 | 4.6 | 0.0 | 0.0 |
| 9 | Fe | 0.0 | 0.0 | 0.0 | 0.0 | 9.0 | 3.0 | 0.0 | 0.0 |
| Total | 400.0 | 100.0 | 375.4 | 100.0 | 300 | 100.0 | 590.0 | 1.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, I.; Afgan, I.; Addad, Y. Thermochemical Modeling of Metal Composition and Its Impact on the Molten Corium–Concrete Interaction: New Insights with Sensitivity Analysis. Energies 2022, 15, 3387. https://doi.org/10.3390/en15093387
Khurshid I, Afgan I, Addad Y. Thermochemical Modeling of Metal Composition and Its Impact on the Molten Corium–Concrete Interaction: New Insights with Sensitivity Analysis. Energies. 2022; 15(9):3387. https://doi.org/10.3390/en15093387
Chicago/Turabian StyleKhurshid, Ilyas, Imran Afgan, and Yacine Addad. 2022. "Thermochemical Modeling of Metal Composition and Its Impact on the Molten Corium–Concrete Interaction: New Insights with Sensitivity Analysis" Energies 15, no. 9: 3387. https://doi.org/10.3390/en15093387






