Metal–Organic Frameworks and Gas Hydrate Synergy: A Pandora’s Box of Unanswered Questions and Revelations
Abstract
:1. Introduction
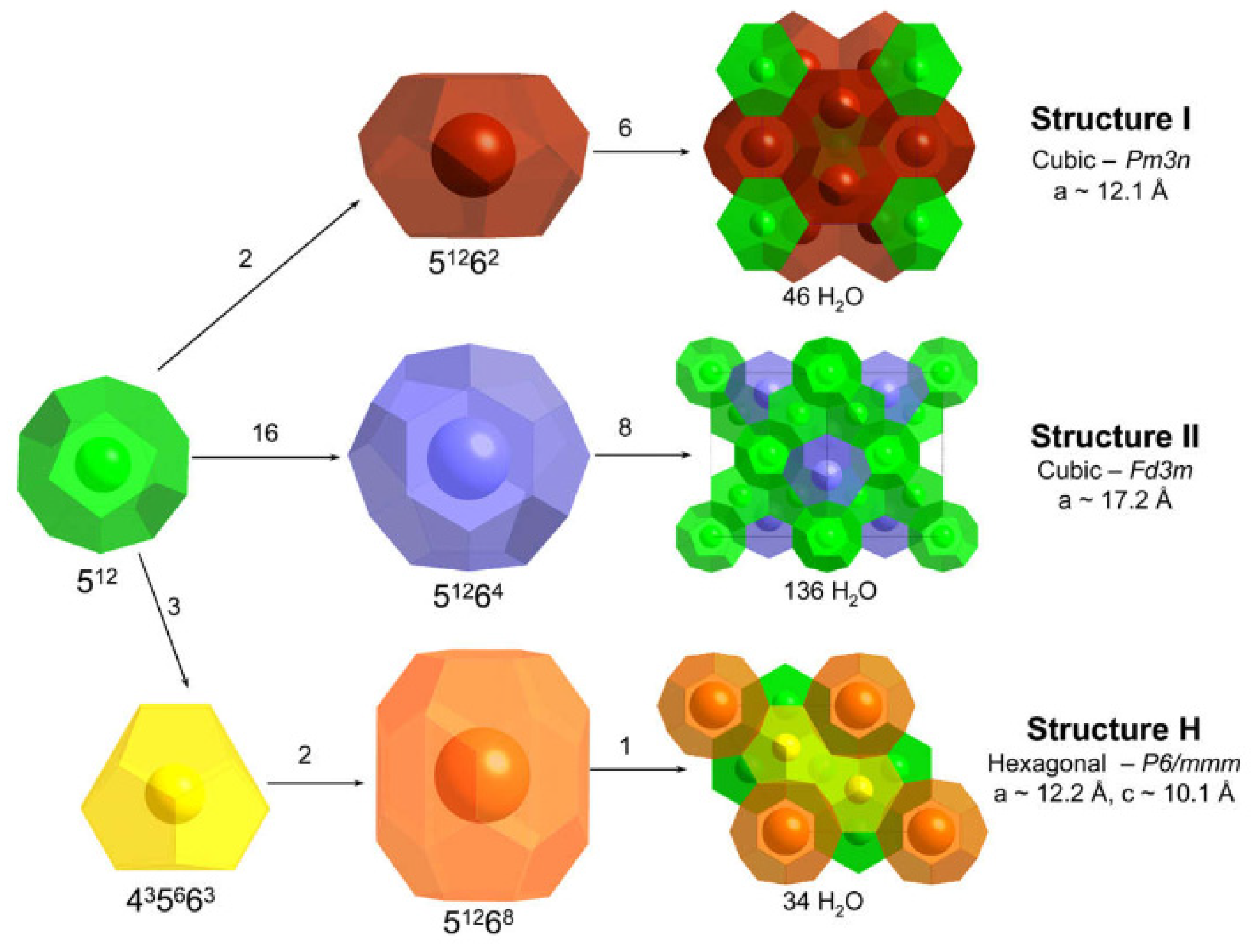
2. Gas Hydrates Introduction
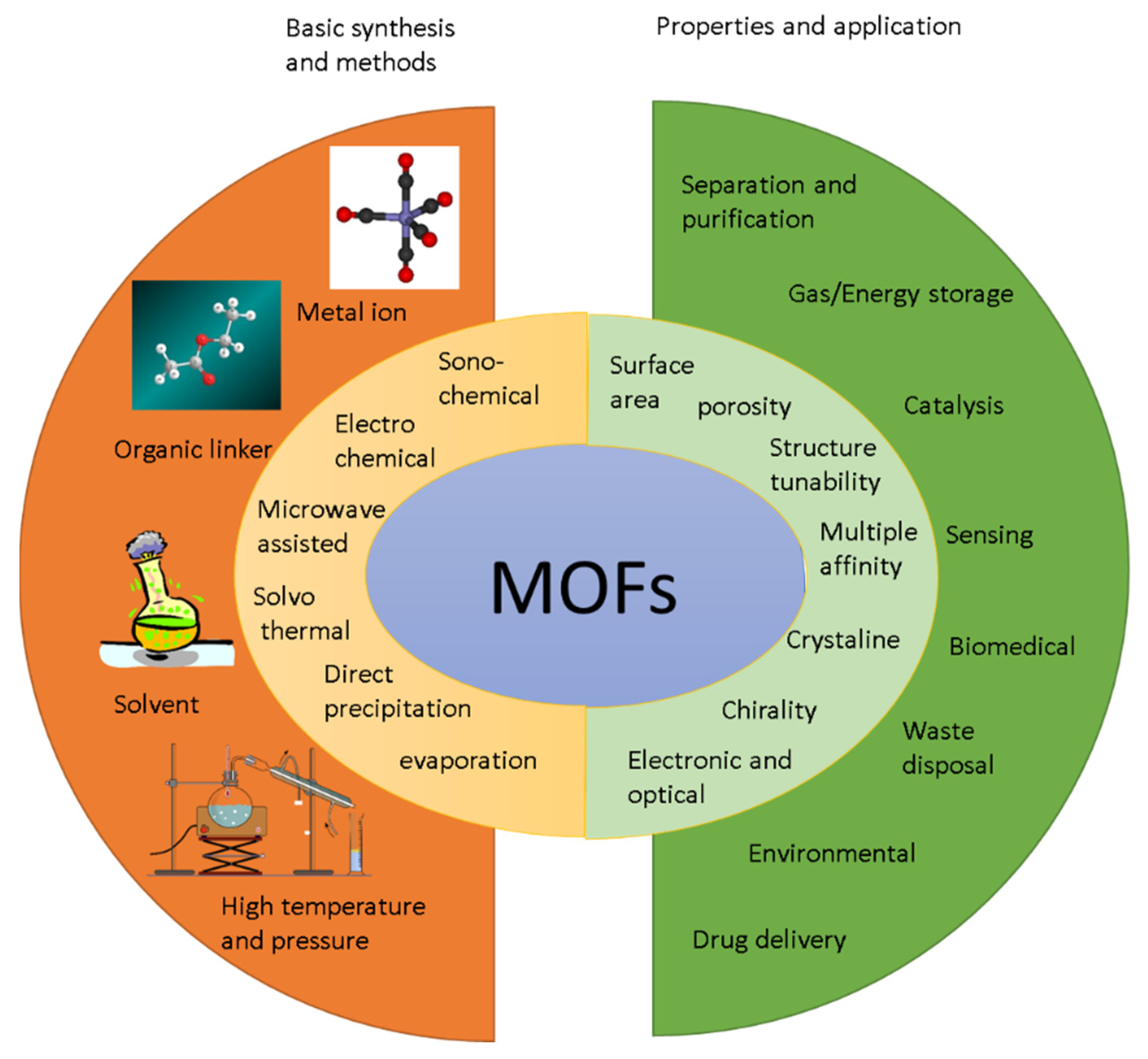
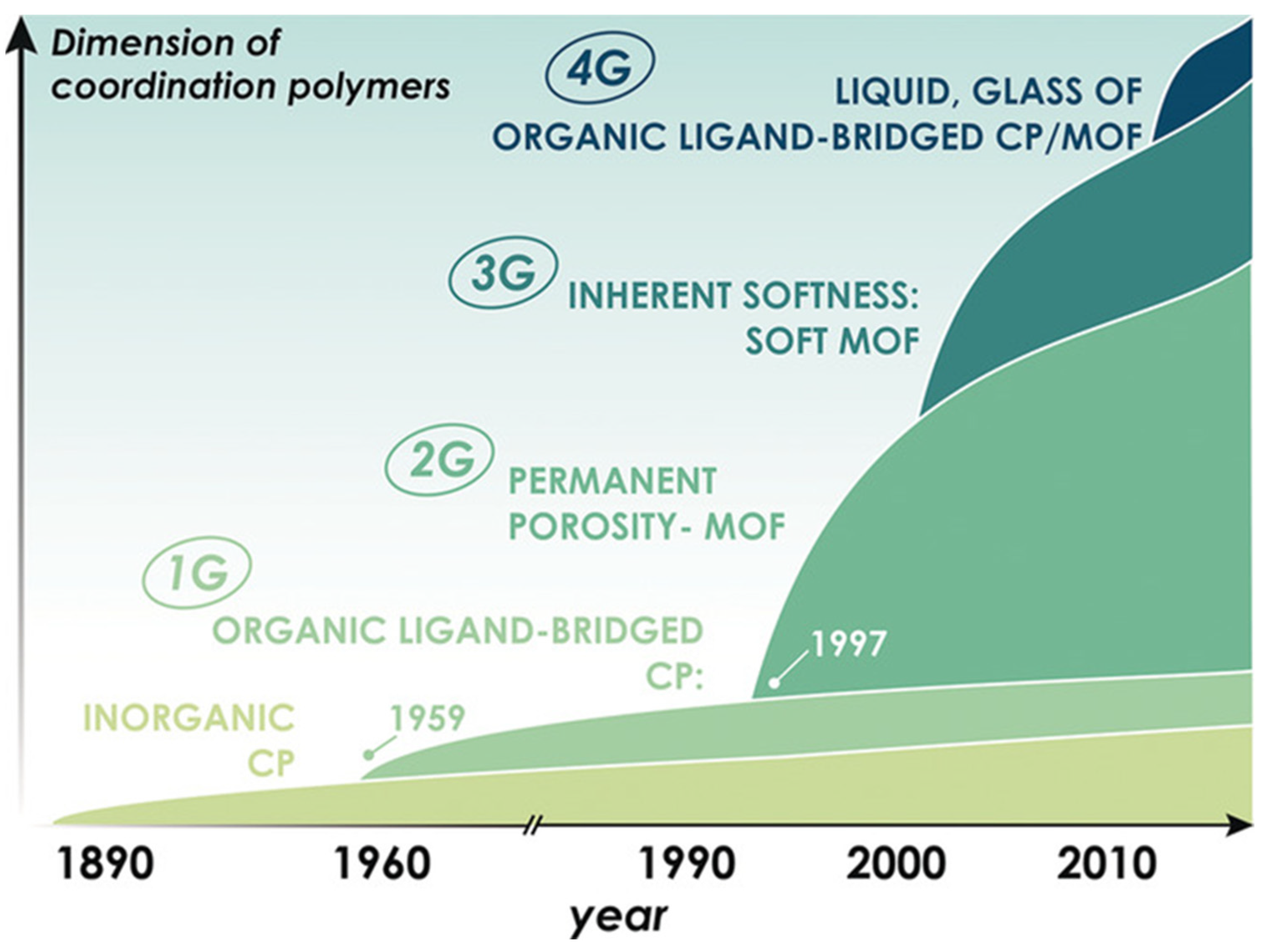
3. Metal–Organic Framework
3.1. Introduction
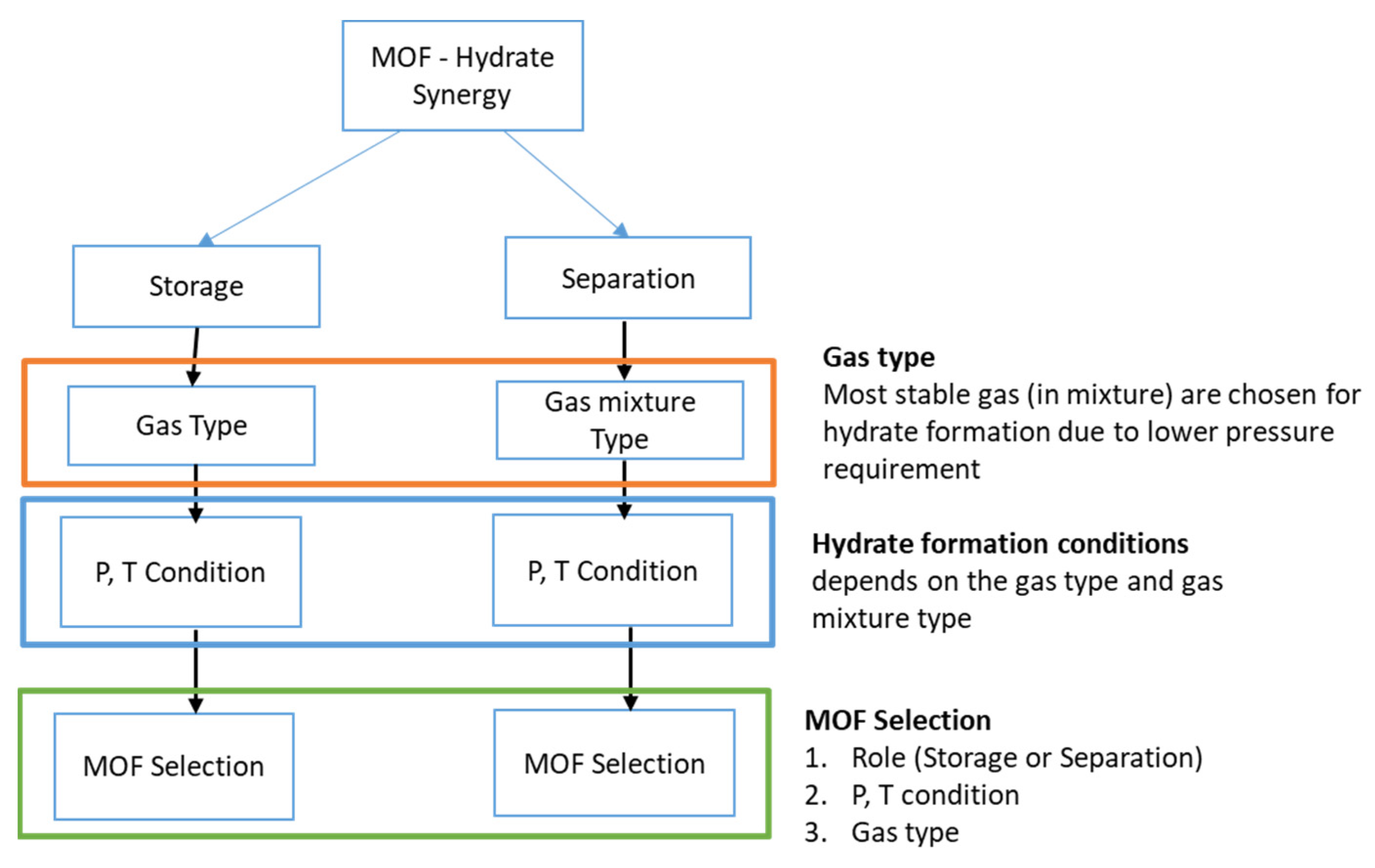
3.2. MOF–Hydrate Potential Synergy
4. MOF–Hydrate Synergy: Current State of the Art
4.1. MOF Material Characterization
4.2. MOF–Hydrate Synergy for CH4 Storage
| MOF Properties | |||
|---|---|---|---|
| Gas Name + MOF Reference Paper/Wettability | Pore Diameter (nm) and Specific Surface Area in m2/g | Pore Volume (cm3/g) | P (Max), T Condition/Key Observation |
| CH4-MIL-53 [76] (Hydrophilic) | 0.6, >10 nm (1500) | 0.18 (micro), 0.49 (Meso) | p = 94 bar T = 276 − 285 K Phase line for bulk and confined hydrates WC = 30% |
| CH4-HKUST-1 [62] (Hydrophilic) | 1.0 (1000) | p = 80 bar, T = −10 °C to 30 °C n = 8.1 mmol/gram at Rw = 1.02, Cmax = 87.2%, Td (Cmax) = 10.7 °C | |
| CH4-MIL-53 (Al) [77] (Hydrophilic) CH4-HKUST–1 (Hydrophilic) CH4-ZIF-8 (Hydrophobic) | 0.85 0.39, 0.9 1.16 | 0.59 0.79 0.65 | p = 120 bar, T = 1 °C WC = 0–35% MIL-53, n = 0.77 (wet) HKUST-1, n = 1.26 (wet) For ZIF-8, n = 8 (wet) |
| CH4-MIL-100 (Fe) [57] (Hydrophilic) CH4-ZIF-8 (Hydrophobic) | 2.4–2.9 (1476) 1.2 (1565) | 0.87 0.72 | T = 2 °C p = 100 bar Rw = 0–0.56 (MIL-100 (Fe)) nCH4 (max wt%) = 8% (Rw = 0). MIL-100 (Fe) Rw = 0–0.6 (ZIF-8) nCH4 (max wt%) = 16% (Rw = 0.6) |
| CH4-Cr-soc-MOF-1 [78] (Hydrophilic) CH4-Y-shp-MOF-5 (Hydrophilic) | 1.5, 1.7 (4500) 1.2 (1550) | 0.63 | T = 1.85 °C (275 K) Rw = 0–4 (MOF-1) nCH4 (max wt%) = 24% (Rw = 3.4) p = 70–80 bar Rw = 0–0.75 (MOF-5) nCH4 (max wt%) = 12% (Rw = 0) p = 60–70 bar |
| CH4-ZIF-8 [79] (Hydrophobic) | (1273) | 0.61 | T = −4 °C and 1 °C p = 110 bars Wc = 0–35.1% nCH4 = 9.304 mmol/g at T = 269.15 K and p = 28.5 bar (WC = 35.1%) |
| CH4-ZIF-8 (Zinc-based) [63] (Hydrophobic) CH4-ZIF-67 (Cobalt-based) (Hydrophobic) | 1.2 (1052) 1.2 (1585) | 0.6 | p = 80 bar, T = −15 °C to 30 °C Rw = 0–1.01, Cmax = 80% (ZIF-8) n = 7.9 mmol/g at Rw = 1.01 Rw = 0–0.82, Cmax = 83.6% (ZIF-67) n = 8.1 mmol/g at Rw = 0.82 |
| CH4-MIL-101 [80] (Simulation study) | 2.9–3.4 (nm) | p = 500 bar, T = 275 K Rw = 0, 0.68, 9.46 | |
| CH4-ZIF-8 [81] Hydrophobic (Simulation study) | 1.16 (nm) | p = 100 bar, T = 275 K Wc = 30% | |
| CH4-UIO-66-NH2 [82] Hydrophobic (Flow assurance study) | n/a | n/a | T = 272–285 K p = 100 bar |
4.3. MOF–Hydrate Synergy for CO2 Storage and/or Separation
5. MOF–Hydrate Synergy: Physical Properties
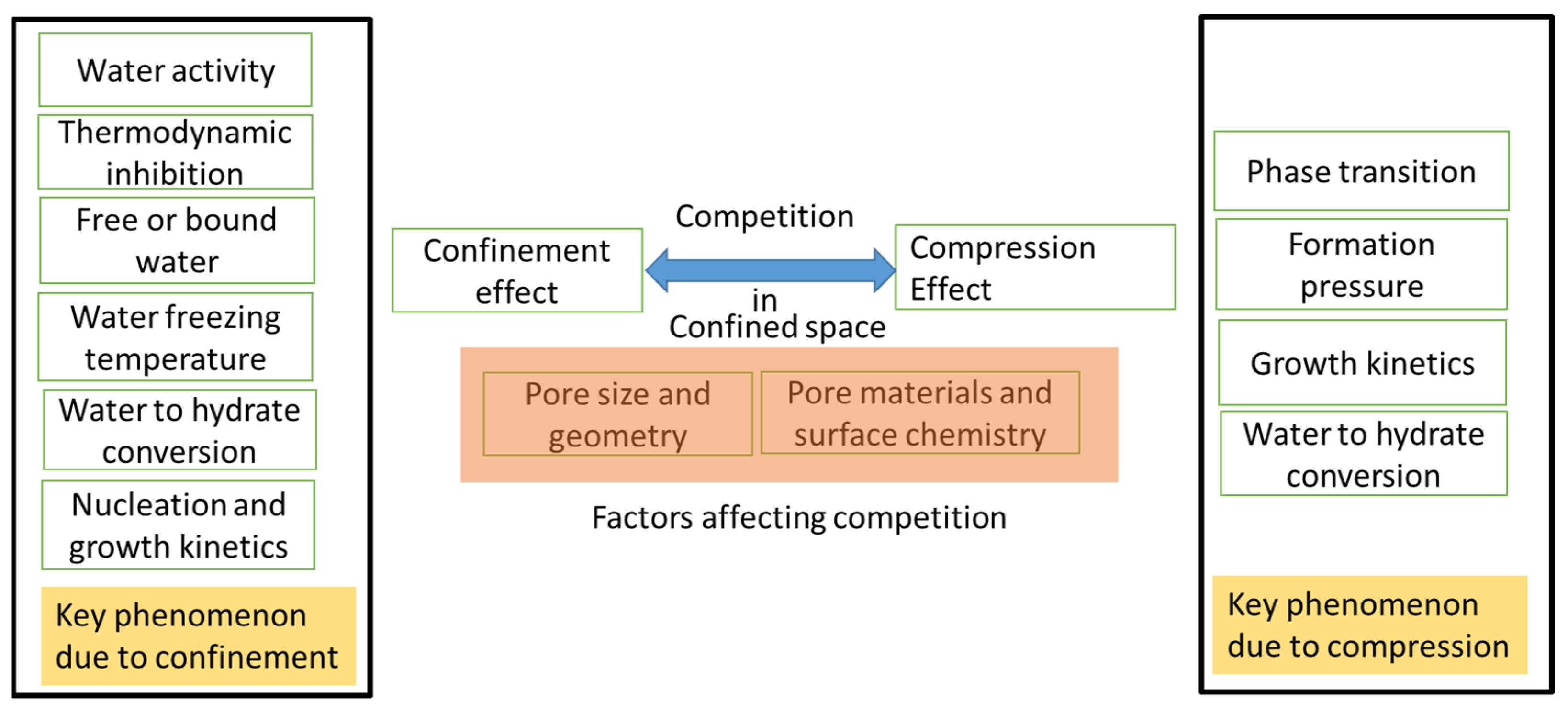
5.1. Confinement–Compression Effect in Nanopores
5.2. Pore Size Effect
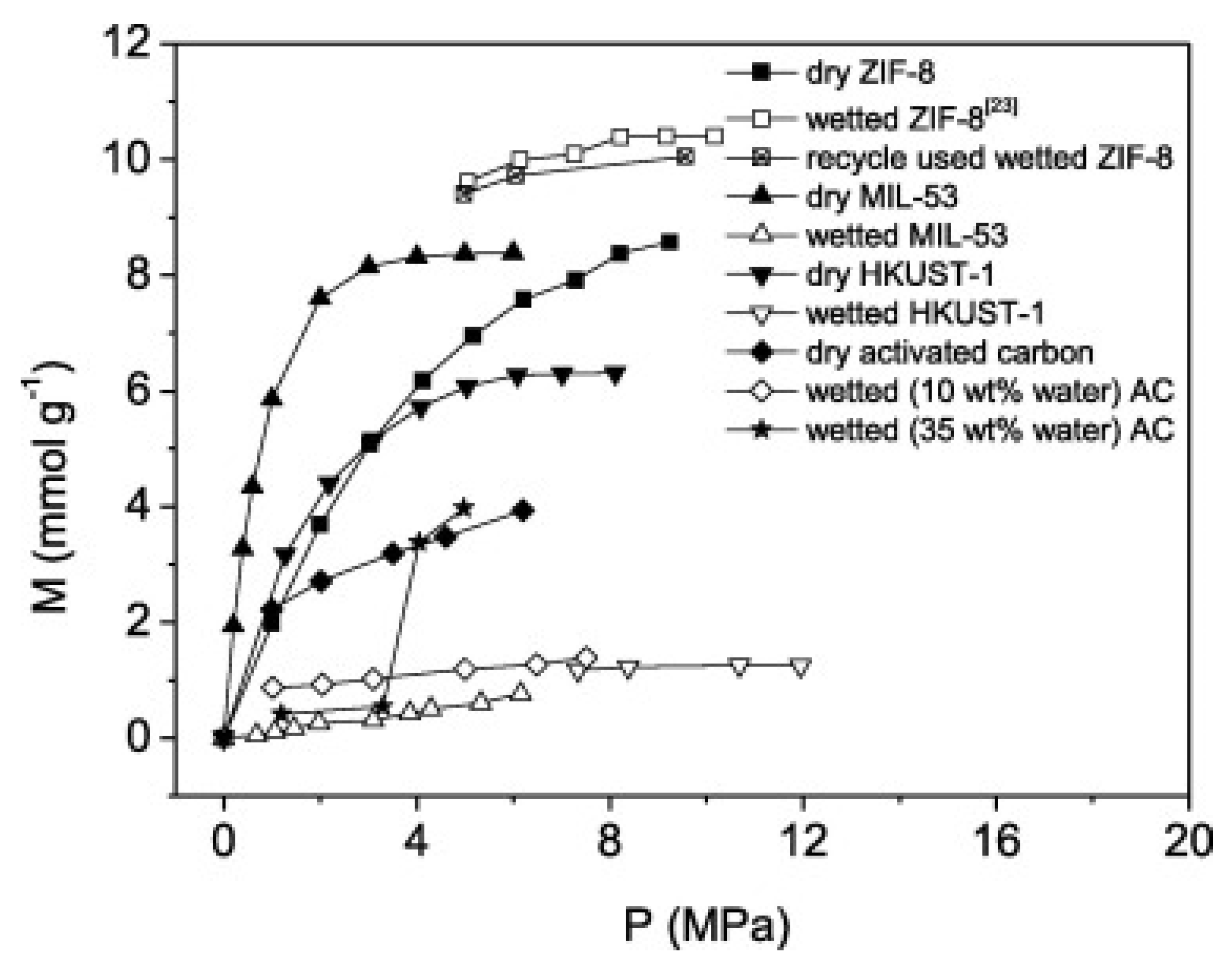
5.3. Hydrophobicity
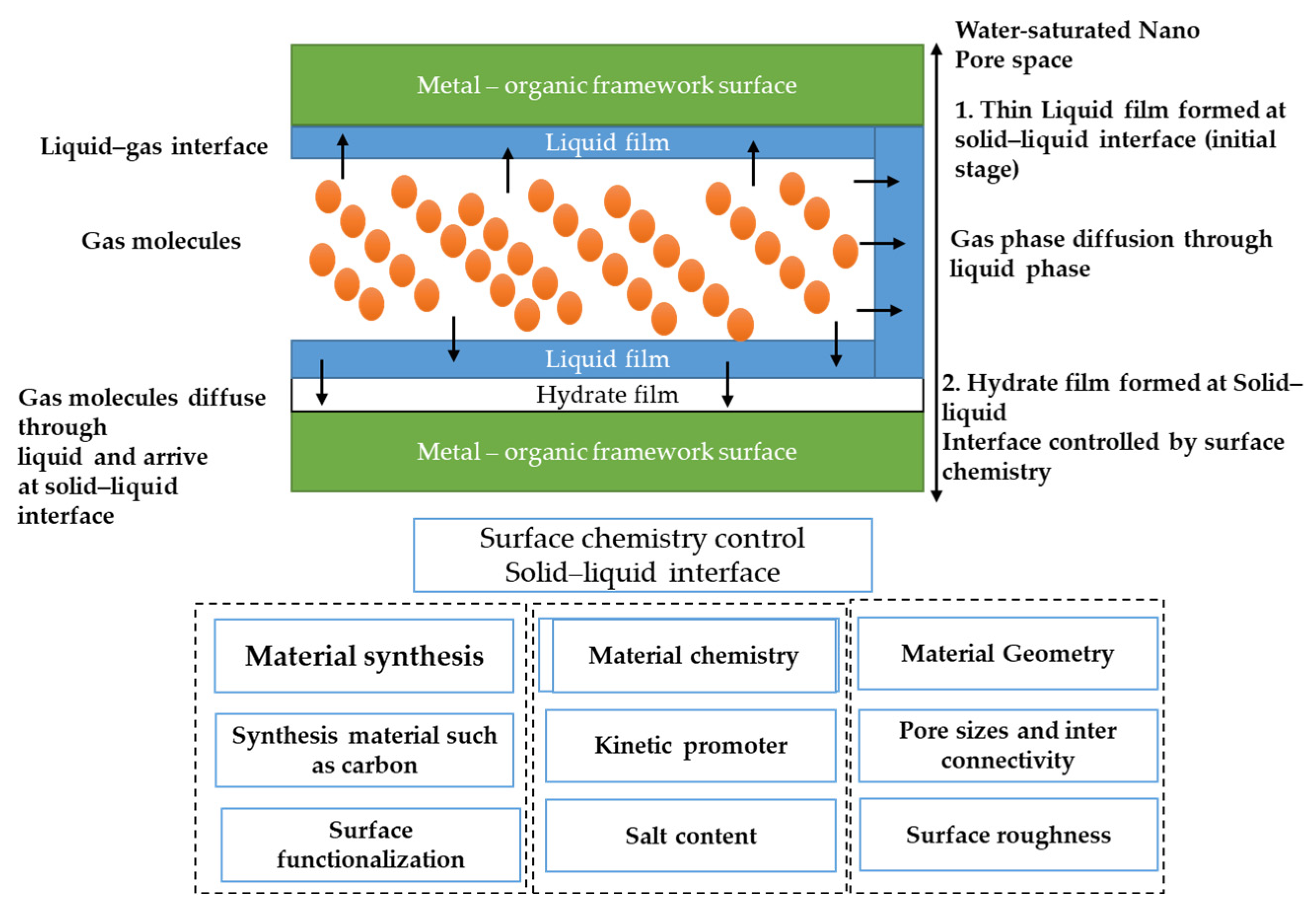
5.4. MOF Chemistry and Surface Functionalization
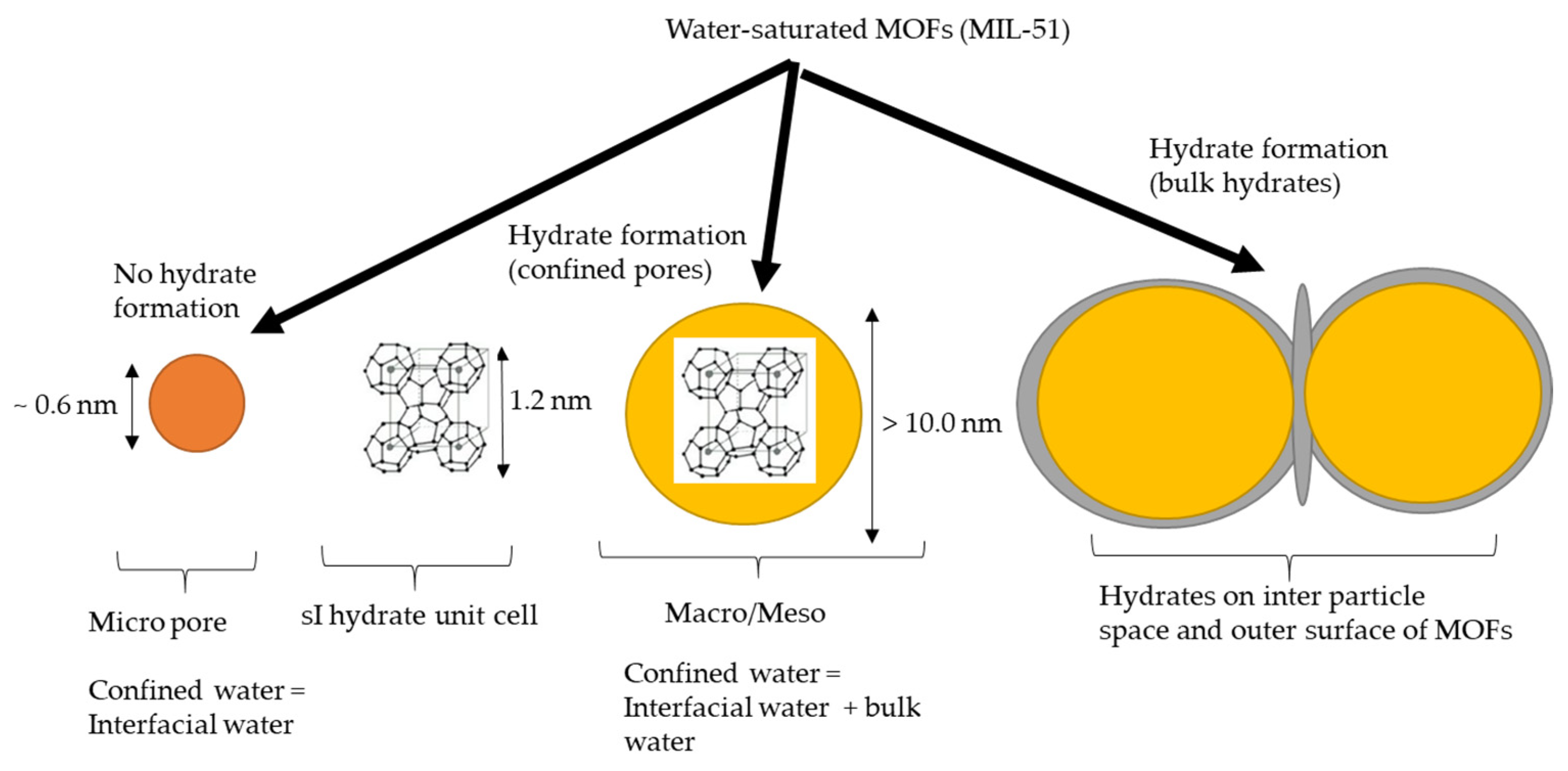
5.5. Water Properties in Confined Spaces
6. MOF–Hydrate Synergy: Metal–Organic Framework Stability in Water
7. Practical Implications
8. Conclusions and the Way Forward
- The authors believe that the initial results are promising and that research should be further expanded, focusing on gases such as H2 [54] and H2-rich natural gas [3,155] and on CO2-enriched gas mixtures, such as CO2/N2, CO2/H2 and CO2/CH4. There is sufficient comprehensible literature on these gas mixtures in the field of gas hydrates that can serve as a starting point.
- Another research area where the application of MOF–hydrate synergies should be investigated is desalination. It is common practice to explore various porous media to enhance hydrate formation, and much of the emerging research is limited to silica-based porous materials. For example, hydrate-based desalination using CO2 hydrate formation may be one of the most promising areas. Current research is focused on using liquid or gaseous co-promoters (propane or cyclopentane) and porous silica systems to improve the separation of hydrates formed and water release [156,157]. Although MOFs have been studied for desalination by membranes [158,159], the role of MOFs in desalination through gas hydrates has not been explored.
- The synergy between MOFs and gas hydrates also raises many research questions. For example, the behavior of water in nano space under high pressures and low temperatures, the effects of pore sizes and degrees of water saturation in nanopores, the competition between high compression and the inhibition effect and its correlation with pore size, water intrusion and hydrophilic pores, and the effects of surface chemistry on hydrate nucleation and growth kinetics are some topics that need further investigation.
- A prerequisite for technological maturity is the cyclic nature of hydrate formation and dissociation and long-term stability during storage and transport. However, most of the available MOF–hydrate synergy studies have focused on gas hydrate formation studies, and there are very few studies on dissociation, total gas recovery and the self-preservation of gas hydrates in the presence of MOFs. Therefore, the authors recommend further investigation in this research direction.
- Preliminary research shows that hydrophobic MOFs are promising candidates with high water–hydrate conversion. Research also shows that the key factors affecting the nucleation and growth kinetics of gas hydrates in the presence of MOFs are (1) pore size distribution, (2) surface chemistry within the pores and (3) growth conditions. Available research indicates that the selection of MOFs depends on the gas type and MOF material, and the end application controls the chemistry and design of MOFs, as different MOFs need to be tailored and functionalized depending on the end application. The authors believe that MOFs with high thermal conductivity, lower production costs and better scaling-up feasibility should be considered.
- Laboratory-scale studies are limited to the microscale (volume < 1 cm3 and sample size < 1 mg). Various phenomena and synergies between hydrates and MOFs must be studied in large reactors to scale up the system. Hydrates in large volumes and metal–organic frameworks can use their self-preservation properties to remain stable during transport and storage at sub-freezing temperatures and at atmospheric pressure, compared with MOFs in the dry state under high-pressure conditions. Low MOF contents dispersed in various packaging materials should be further investigated under high-volume conditions.
- Previous studies have been limited to the use of pure water and saltwater in the study of MOF–hydrate synergies, ignoring the role of the kinetic promoter for further improvements. Therefore, further studies using environmentally friendly kinetic promoters (e.g., amino acids) to study hydrate nucleation and growth kinetics in MOFs would be interesting.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon Capture and Storage Update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Wang, P.; Li, K.; Yang, J.; Zhu, J.; Zhao, Y.; Teng, Y. Experimental and Theoretical Study on Dissociation Thermodynamics and Kinetics of Hydrogen-Propane Hydrate. Chem. Eng. J. 2021, 426, 131279. [Google Scholar] [CrossRef]
- Pandey, J.S.; Hansen, J.L.; von Solms, N. Hydrogen-Rich Natural Gas Hydrates Formation Kinetics in the Presence of Promoters. Chem. Eng. J. 2022, 432, 134295. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.-G.G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; Van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Ren, B.; Li, H.-Y.; Yang, Y.-Z.; Wang, Z.-Q.; Wang, B.; Xu, J.-C.; Agarwal, R. CO2 Storage with Enhanced Gas Recovery (CSEGR): A Review of Experimental and Numerical Studies. Pet. Sci. 2022, 19, 594–607. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.M. CO2 Capture by Hydrate Crystallization—A Potential Solution for Gas Emission of Steelmaking Industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; von Solms, N. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes 2020, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, S.; Huang, Y.; McIlveen-Wright, D.; Hewitt, N.; Mondol, J.D. Comparative Assessment of Coal Fired IGCC Systems with CO2 Capture Using Physical Absorption, Membrane Reactors and Chemical Looping. Fuel 2009, 88, 2463–2472. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The Path towards Sustainable Energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven Chemical Separations to Change the World. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging Trends in Porous Materials for CO2 Capture and Conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Snurr, R.Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, B.; Morini, E.; Filipponi, M.; Nicolini, A.; Palombo, M.; Cotana, F.; Rossi, F. Clathrate Hydrates for Thermal Energy Storage in Buildings: Overview of Proper Hydrate-Forming Compounds. Sustainability 2014, 6, 6815–6829. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Zheng, J.; Kim, H.; Seo, Y.; Linga, P. Hydrates for Cold Energy Storage and Transport: A Review. Adv. Appl. Energy 2021, 2, 100022. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a Gas Hydrate Absorption for Energy Storage and Gas Separation—Proof of Concept Based on Natural Gas. Energy Procedia 2019, 158, 5367–5373. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the Synergistic Effect between Metal–Organic Frameworks and Gas Hydrates for CH4 Storage and CO2 Separation Applications. Renew. Sustain. Energy Rev. 2022, 167, 112807. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A. V “Nanoreactors” for Boosting Gas Hydrate Formation toward Energy Storage Applications. ACS Nano 2022, 16, 11504–11515. [Google Scholar] [CrossRef]
- Both, A.K.; Gao, Y.; Zeng, X.C.; Cheung, C.L. Gas Hydrates in Confined Space of Nanoporous Materials: New Frontier in Gas Storage Technology. Nanoscale 2021, 13, 7447–7470. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Albero, J. Clathrate-Mediated Gas Storage in Nanoporous Materials. In Green Energy and Technology; Springer: Singapore, 2019; pp. 383–403. ISBN 9789811335037. [Google Scholar]
- Sloan, E.D., Jr.; Koh, C.A.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429129148. [Google Scholar]
- Sloan, E.D. Fundamental Principles and Applications of Natural Gas Hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Strobel, T.A.; Hester, K.C.; Koh, C.A.; Sum, A.K.; Sloan, E.D. Properties of the Clathrates of Hydrogen and Developments in Their Applicability for Hydrogen Storage. Chem. Phys. Lett. 2009, 478, 97–109. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Goh, M.N.; Arumuganainar, S.E.K.K.; Zhang, Y.; Linga, P. Ultra-Rapid Uptake and the Highly Stable Storage of Methane as Combustible Ice. Energy Environ. Sci. 2020, 13, 4946–4961. [Google Scholar] [CrossRef]
- Adeyemo, A.; Kumar, R.; Linga, P.; Ripmeester, J.; Englezos, P. Capture of Carbon Dioxide from Flue or Fuel Gas Mixtures by Clathrate Crystallization in a Silica Gel Column. Int. J. Greenh. Gas Control 2010, 4, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Lee, Y.; Seo, D.; Lee, S.; Hong, S.; Ahn, Y.-H.; Park, Y. Critical Hydrogen Concentration of Hydrogen-Natural Gas Blends in Clathrate Hydrates for Blue Hydrogen Storage. Renew. Sustain. Energy Rev. 2021, 141, 110789. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Vasheghani Farahani, M.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas Hydrates in Sustainable Chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef]
- Xu, C.-G.; Li, X.-S. Research Progress on Methane Production from Natural Gas Hydrates. RSC Adv. 2015, 5, 54672–54699. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of Metal–Organic Frameworks and Coordination Polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A Historical Overview of the Activation and Porosity of Metal–Organic Frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lim, H.-K.; Ro, H.; Kim, H.; Lee, H. Unexpected Carbon Dioxide Inclusion in Water-Saturated Pores of Metal-Organic Frameworks with Potential for Highly Selective Capture of CO2. Chem. Eur. J. 2015, 21, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand-Lenoir, E.; Vagner, C.; Yoon, J.W.; Bazin, P.; Ragon, F.; Hwang, Y.K.; Serre, C.; Chang, J.-S.; Llewellyn, P.L. How Water Fosters a Remarkable 5-Fold Increase in Low-Pressure CO2 Uptake within Mesoporous MIL-100(Fe). J. Am. Chem. Soc. 2012, 134, 10174–10181. [Google Scholar] [CrossRef]
- Yazaydın, A.Ö.; Benin, A.I.; Faheem, S.A.; Jakubczak, P.; Low, J.J.; Willis, R.R.; Snurr, R.Q. Enhanced CO2 Adsorption in Metal-Organic Frameworks via Occupation of Open-Metal Sites by Coordinated Water Molecules. Chem. Mater. 2009, 21, 1425–1430. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Benin, A.I.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. CO2/H2O Adsorption Equilibrium and Rates on Metal−Organic Frameworks: HKUST-1 and Ni/DOBDC. Langmuir 2010, 26, 14301–14307. [Google Scholar] [CrossRef]
- De Toni, M.; Jonchiere, R.; Pullumbi, P.; Coudert, F.-X.X.; Fuchs, A.H. How Can a Hydrophobic MOF Be Water-Unstable? Insight into the Hydration Mechanism of IRMOFs. ChemPhysChem 2012, 13, 3497–3503. [Google Scholar] [CrossRef] [Green Version]
- Horike, S.; Nagarkar, S.S.; Ogawa, T.; Kitagawa, S. A New Dimension for Coordination Polymers and Metal–Organic Frameworks: Towards Functional Glasses and Liquids. Angew. Chem. Int. Ed. 2020, 59, 6652–6664. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: Status and Challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef]
- Bastin, L.; Bárcia, P.S.; Hurtado, E.J.; Silva, J.A.C.; Rodrigues, A.E.; Chen, B. A Microporous Metal−Organic Framework for Separation of CO2/N2 and CO2/CH4 by Fixed-Bed Adsorption. J. Phys. Chem. C 2008, 112, 1575–1581. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H. Phase Behavior of Gas Hydrates in Nanoporous Materials: Review. Korean J. Chem. Eng. 2016, 33, 1977–1988. [Google Scholar] [CrossRef]
- Angelini, P.; Armstrong, T.; Counce, R.; Griffith, W.; Klasson, T.; Muralidharan, G.; Closset, G.; Keller, G.; Watson Disclaimer, J. Materials for Separation Technologies: Energy and Emission Reduction Opportunities; BCS Inc.: Laurel, MD, USA, 2005. [Google Scholar] [CrossRef]
- Stroganov, Y.N.; Khakimov, R.T.; Ognev, O.G.; Kulev, M.V.; Belinskaia, I.V. Influence of Natural Gas Composition on Working Process of Gas Power Plant Efficiency. AIP Conf. Proc. 2022, 2456, 030039. [Google Scholar]
- Mimachi, H.; Takeya, S.; Yoneyama, A.; Hyodo, K.; Takeda, T.; Gotoh, Y.; Murayama, T. Natural Gas Storage and Transportation within Gas Hydrate of Smaller Particle: Size Dependence of Self-Preservation Phenomenon of Natural Gas Hydrate. Chem. Eng. Sci. 2014, 118, 208–213. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsunvigit, P.; Linga, P. Rapid Methane Hydrate Formation to Develop a Cost Effective Large Scale Energy Storage System. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Wang, W.; Bray, C.L.; Adams, D.J.; Cooper, A.I. Methane Storage in Dry Water Gas Hydrates. J. Am. Chem. Soc. 2008, 130, 11608–11609. [Google Scholar] [CrossRef]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef]
- Farrando-Perez, J.; Balderas-Xicohtencatl, R.; Cheng, Y.; Daemen, L.; Cuadrado-Collados, C.; Martinez-Escandell, M.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Rapid and Efficient Hydrogen Clathrate Hydrate Formation in Confined Nanospace. Nat. Commun. 2022, 13, 5953. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, L.; Casco, M.E.; Silvestre-Albero, J. Methane Hydrate in Confined Spaces: An Alternative Storage System. ChemPhysChem 2018, 19, 1298–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casco, M.E.; Silvestre-Albero, J.; Ramírez-Cuesta, A.J.; Rey, F.; Jordá, J.L.; Bansode, A.; Urakawa, A.; Peral, I.; Martínez-Escandell, M.; Kaneko, K.; et al. Methane Hydrate Formation in Confined Nanospace Can Surpass Nature. Nat. Commun. 2015, 6, 6432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casco, M.E.; Rey, F.; Jordá, J.L.; Rudić, S.; Fauth, F.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. Paving the Way for Methane Hydrate Formation on Metal–Organic Frameworks (MOFs). Chem. Sci. 2016, 7, 3658–3666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchardt, L.; Nickel, W.; Casco, M.; Senkovska, I.; Bon, V.; Wallacher, D.; Grimm, N.; Krause, S.; Silvestre-Albero, J. Illuminating Solid Gas Storage in Confined Spaces—Methane Hydrate Formation in Porous Model Carbons. Phys. Chem. Chem. Phys. 2016, 18, 20607–20614. [Google Scholar] [CrossRef] [Green Version]
- Casco, M.E.; Jordá, J.L.; Rey, F.; Fauth, F.; Martinez-Escandell, M.; Rodríguez-Reinoso, F.; Ramos-Fernández, E.V.; Silvestre-Albero, J. High-Performance of Gas Hydrates in Confined Nanospace for Reversible CH4/CO2 Storage. Chem. A Eur. J. 2016, 22, 10028–10035. [Google Scholar] [CrossRef]
- Chari, V.D.; Prasad, P.S.R.; Murthy, S.R. Structural Stability of Methane Hydrates in Porous Medium: Raman Spectroscopic Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 636–641. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Lee, Y.; Seo, Y. Thermodynamic and 13C NMR Spectroscopic Verification of Methane–Carbon Dioxide Replacement in Natural Gas Hydrates. Chem. Eng. J. 2013, 225, 636–640. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Metal–Organic Framework HKUST-1 Promotes Methane Hydrate Formation for Improved Gas Storage Capacity. ACS Appl. Mater. Interfaces 2020, 12, 53510–53518. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Methane Hydrate Growth Promoted by Microporous Zeolitic Imidazolate Frameworks ZIF-8 and ZIF-67 for Enhanced Methane Storage. ACS Sustain. Chem. Eng. 2021, 9, 9001–9010. [Google Scholar] [CrossRef]
- Denning, S.; Lucero, J.M.; Majid, A.A.A.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Porous Organic Cage CC3: An Effective Promoter for Methane Hydrate Formation for Natural Gas Storage. J. Phys. Chem. C 2021, 125, 20512–20521. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Crawford, J.M.; Wells, J.D.; Carreon, M.A.; Koh, C.A. Methane Storage Scale-up Using Hydrates & Metal Organic Framework HKUST-1 in a Packed Column. Fuel 2022, 325, 124920. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of Metal-Organic Frameworks by Water Adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Bordiga, S.; Regli, L.; Bonino, F.; Groppo, E.; Lamberti, C.; Xiao, B.; Wheatley, P.S.; Morris, R.E.; Zecchina, A. Adsorption Properties of HKUST-1 toward Hydrogen and Other Small Molecules Monitored by IR. Phys. Chem. Chem. Phys. 2007, 9, 2676. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Latroche, M.; Serre, C.; Millange, F.; Loiseau, T.; Percheron-Guégan, A. Hydrogen Adsorption in the Nanoporous Metal-Benzenedicarboxylate M(OH)(O2C–C6H4–CO2) (M = Al3+, Cr3+), MIL-53. Chem. Commun. 2003, 3, 2976–2977. [Google Scholar] [CrossRef]
- Babaei, H.; DeCoster, M.E.; Jeong, M.; Hassan, Z.M.; Islamoglu, T.; Baumgart, H.; McGaughey, A.J.H.; Redel, E.; Farha, O.K.; Hopkins, P.E.; et al. Observation of Reduced Thermal Conductivity in a Metal-Organic Framework Due to the Presence of Adsorbates. Nat. Commun. 2020, 11, 4010. [Google Scholar] [CrossRef]
- Gunatilleke, W.D.C.B.; Wei, K.; Niu, Z.; Wojtas, L.; Nolas, G.; Ma, S. Thermal Conductivity of a Perovskite-Type Metal–Organic Framework Crystal. Dalt. Trans. 2017, 46, 13342–13344. [Google Scholar] [CrossRef]
- Cui, B.; Audu, C.O.; Liao, Y.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T.; Grayson, M. Thermal Conductivity of ZIF-8 Thin-Film under Ambient Gas Pressure. ACS Appl. Mater. Interfaces 2017, 9, 28139–28143. [Google Scholar] [CrossRef]
- Huang, B.L.; Ni, Z.; Millward, A.; McGaughey, A.J.H.; Uher, C.; Kaviany, M.; Yaghi, O. Thermal Conductivity of a Metal-Organic Framework (MOF-5): Part II. Measurement. Int. J. Heat Mass Transf. 2007, 50, 405–411. [Google Scholar] [CrossRef]
- Dongliang, L.; Hao, P.; Deqing, L. Thermal Conductivity Enhancement of Clathrate Hydrate with Nanoparticles. Int. J. Heat Mass Transf. 2017, 104, 566–573. [Google Scholar] [CrossRef]
- Wei, R.; Shi, K.; Guo, X.; Wang, T.; Lv, X.; Li, Q.; Zhang, Y.; Zhao, J.; Yang, L. Evolving Thermal Conductivity upon Formation and Decomposition of Hydrate in Natural Marine Sediments. Fuel 2021, 302, 121141. [Google Scholar] [CrossRef]
- Li, X.-Y.; Feng, J.-C.; Li, X.-S.; Wang, Y.; Hu, H.-Q. Experimental Study of Methane Hydrate Formation and Decomposition in the Porous Medium with Different Thermal Conductivities and Grain Sizes. Appl. Energy 2022, 305, 117852. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, Y.-H.; Lee, H. Phase Equilibria of CO2 and CH4 Hydrates in Intergranular Meso/Macro Pores of MIL-53 Metal Organic Framework. J. Chem. Eng. Data 2015, 60, 2178–2185. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, S.; Guo, P.; Fan, S.; Zhang, S. Understanding the Characteristic of Methane Hydrate Equilibrium in Materials and Its Potential Application. Chem. Eng. J. 2018, 349, 775–781. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Mouchaham, G.; Daemen, L.; Cheng, Y.; Ramirez-Cuesta, A.; Aggarwal, H.; Missyul, A.; Eddaoudi, M.; Belmabkhout, Y.; Silvestre-Albero, J. Quest for an Optimal Methane Hydrate Formation in the Pores of Hydrolytically Stable Metal–Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 13391–13397. [Google Scholar] [CrossRef]
- Mu, L.; Liu, B.; Liu, H.; Yang, Y.; Sun, C.; Chen, G. A Novel Method to Improve the Gas Storage Capacity of ZIF-8. J. Mater. Chem. 2012, 22, 12246. [Google Scholar] [CrossRef]
- He, Z.; Zhang, K.; Jiang, J. Formation of CH 4 Hydrate in a Mesoporous Metal–Organic Framework MIL-101: Mechanistic Insights from Microsecond Molecular Dynamics Simulations. J. Phys. Chem. Lett. 2019, 10, 7002–7008. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Chen, S.; Fu, Y.; Zhang, Y.; Wang, D.; Pei, J.; Liu, D. Molecular Insights into Hybrid CH4 Physisorption-Hydrate Growth in Hydrophobic Metal–Organic Framework ZIF-8: Implications for CH4 Storage. Chem. Eng. J. 2022, 430, 132901. [Google Scholar] [CrossRef]
- Lee, D.; Jeoung, S.; Moon, H.R.; Seo, Y. Recoverable and Recyclable Gas Hydrate Inhibitors Based on Magnetic Nanoparticle-Decorated Metal–Organic Frameworks. Chem. Eng. J. 2020, 401, 126081. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Erkartal, M.; Erkilic, U.; Tam, B.; Usta, H.; Yazaydin, O.; Hupp, J.T.; Farha, O.K.; Sen, U. From 2-Methylimidazole to 1,2,3-Triazole: A Topological Transformation of ZIF-8 and ZIF-67 by Post-Synthetic Modification. Chem. Commun. 2017, 53, 2028–2031. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Matzger, A.J. Water Stability of Microporous Coordination Polymers and the Adsorption of Pharmaceuticals from Water. Langmuir 2010, 26, 17198–17202. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wannapaiboon, S.; Khaletskaya, K.; Fischer, R.A. Engineering Zeolitic-Imidazolate Framework (ZIF) Thin Film Devices for Selective Detection of Volatile Organic Compounds. Adv. Funct. Mater. 2015, 25, 4470–4479. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Zhang, C.; Koros, W.J.; Chance, R.R. Investigating the Intrinsic Ethanol/Water Separation Capability of ZIF-8: An Adsorption and Diffusion Study. J. Phys. Chem. C 2013, 117, 7214–7225. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cladek, B.R.; Everett, S.M.; McDonnell, M.T.; Tucker, M.G.; Keffer, D.J.; Rawn, C.J. Guest–Host Interactions in Mixed CH4–CO2 Hydrates: Insights from Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 19575–19583. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, B.J.; Yun, T.S.; Lee, J.; Cho, G.C. Review on the Gas Hydrate Development and Production as a New Energy Resource. KSCE J. Civ. Eng. 2011, 15, 689–696. [Google Scholar] [CrossRef]
- Xu, C.-G.; Chen, Z.-Y.; Cai, J.; Li, X.-S. Study on Pilot-Scale CO2 Separation from Flue Gas by the Hydrate Method. Energy Fuels 2014, 28, 1242–1248. [Google Scholar] [CrossRef]
- Kumar, R.; Englezos, P.; Moudrakovski, I.; Ripmeester, J.A. Structure and Composition of CO2/H2 and CO2/H2/C3H8 Hydrate in Relation to Simultaneous CO2 Capture and H2 Production. AIChE J. 2009, 55, 1584–1594. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3 (TMA)2(H2O)3] N. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting Linear Chains of Sulphur inside Carbon Nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urita, K.; Shiga, Y.; Fujimori, T.; Iiyama, T.; Hattori, Y.; Kanoh, H.; Ohba, T.; Tanaka, H.; Yudasaka, M.; Iijima, S.; et al. Confinement in Carbon Nanospace-Induced Production of KI Nanocrystals of High-Pressure Phase. J. Am. Chem. Soc. 2011, 133, 10344–10347. [Google Scholar] [CrossRef] [PubMed]
- Alcoutlabi, M.; McKenna, G.B. Effects of Confinement on Material Behaviour at the Nanometre Size Scale. J. Phys. Condens. Matter 2005, 17, R461–R524. [Google Scholar] [CrossRef]
- Derouane, E. Surface Curvature Effects in Physisorption and Catalysis by Microporous Solids and Molecular Sieves. J. Catal. 1988, 110, 58–73. [Google Scholar] [CrossRef]
- Sastre, G.; Corma, A. The Confinement Effect in Zeolites. J. Mol. Catal. A Chem. 2009, 305, 3–7. [Google Scholar] [CrossRef]
- Handa, Y.P.; Stupin, D.Y. Thermodynamic Properties and Dissociation Characteristics of Methane and Propane Hydrates in 70-.ANG.-Radius Silica Gel Pores. J. Phys. Chem. 1992, 96, 8599–8603. [Google Scholar] [CrossRef]
- Handa, Y.P.; Zakrzewski, M.; Fairbridge, C. Effect of Restricted Geometries on the Structure and Thermodynamic Properties of Ice. J. Phys. Chem. 1992, 96, 8594–8599. [Google Scholar] [CrossRef]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of Confinement on Freezing and Melting. J. Phys. Condens. Matter 2006, 18, R15–R68. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, D.W.; Lim, H.-K.; Jeon, J.; Kim, H.; Jung, H.-T.; Lee, H. Inhibited Phase Behavior of Gas Hydrates in Graphene Oxide: Influences of Surface and Geometric Constraints. Phys. Chem. Chem. Phys. 2014, 16, 22717–22722. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, S.; Cha, I.; Lee, J.D.; Kang, S.P. Phase Equilibria of Ethane and Propane Hydrates in Porous Silica Gels. Chem. Eng. Trans. 2009, 17, 1479–1484. [Google Scholar] [CrossRef]
- Anderson, R.; Llamedo, M.; Tohidi, B.; Burgass, R.W. Experimental Measurement of Methane and Carbon Dioxide Clathrate Hydrate Equilibria in Mesoporous Silica. J. Phys. Chem. B 2003, 107, 3507–3514. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Majid, A.A.A.; Martínez-Escandell, M.; Daemen, L.L.; Missyul, A.; Koh, C.; Silvestre-Albero, J. Freezing/Melting of Water in the Confined Nanospace of Carbon Materials: Effect of an External Stimulus. Carbon N. Y. 2020, 158, 346–355. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Galib, M.; Nguyen, A.V. Critical Review on Gas Hydrate Formation at Solid Surfaces and in Confined Spaces—Why and How Does Interfacial Regime Matter? Energy Fuels 2020, 34, 6751–6760. [Google Scholar] [CrossRef]
- Mohammadi, A.A.H.; Manteghian, M.; Haghtalab, A.; Mohammadi, A.A.H.; Rahmati-Abkenar, M. Kinetic Study of Carbon Dioxide Hydrate Formation in Presence of Silver Nanoparticles and SDS. Chem. Eng. J. 2014, 237, 387–395. [Google Scholar] [CrossRef]
- Jacobson, L.C.; Hujo, W.; Molinero, V. Amorphous Precursors in the Nucleation of Clathrate Hydrates. J. Am. Chem. Soc. 2010, 132, 11806–11811. [Google Scholar] [CrossRef]
- Miyawaki, J.; Kanda, T.; Suzuki, T.; Okui, T.; Maeda, Y.; Kaneko, K. Macroscopic Evidence of Enhanced Formation of Methane Nanohydrates in Hydrophobic Nanospaces. J. Phys. Chem. B 1998, 102, 2187–2192. [Google Scholar] [CrossRef]
- Perrin, A.; Celzard, A.; Marêché, J.F.; Furdin, G. Methane Storage within Dry and Wet Active Carbons: A Comparative Study. Energy Fuels 2003, 17, 1283–1291. [Google Scholar] [CrossRef]
- Müller, E.A.; Rull, L.F.; Vega, L.F.; Gubbins, K.E. Adsorption of Water on Activated Carbons: A Molecular Simulation Study. J. Phys. Chem. 1996, 100, 1189–1196. [Google Scholar] [CrossRef]
- Casco, M.E.; Zhang, E.; Grätz, S.; Krause, S.; Bon, V.; Wallacher, D.; Grimm, N.; Többens, D.M.; Hauß, T.; Borchardt, L. Experimental Evidence of Confined Methane Hydrate in Hydrophilic and Hydrophobic Model Carbons. J. Phys. Chem. C 2019, 123, 24071–24079. [Google Scholar] [CrossRef]
- Andersen, R.; Llamedo, M.; Tohidi, B.; Burgass, R.W. Characteristics of Clathrate Hydrate Equilibria in Mesopores and Interpretation of Experimental Data. J. Phys. Chem. B 2003, 107, 3500–3506. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V.; Steel, K.M.; Dang, L.X.; Galib, M. Interfacial Gas Enrichment at Hydrophobic Surfaces and the Origin of Promotion of Gas Hydrate Formation by Hydrophobic Solid Particles. J. Phys. Chem. C 2017, 121, 3830–3840. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Ahmadpour, A.; Rashidi, H. Improving Methane Storage on Wet Activated Carbons at Various Amounts of Water. J. Fuel Chem. Technol. 2012, 40, 385–389. [Google Scholar] [CrossRef]
- Siangsai, A.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S.; Linga, P. Investigation on the Roles of Activated Carbon Particle Sizes on Methane Hydrate Formation and Dissociation. Chem. Eng. Sci. 2015, 126, 383–389. [Google Scholar] [CrossRef]
- Mukherjee, S.; Datta, K.K.R.; Fischer, R.A. Hydrophobicity: A Key Factor En Route to Applications of Metal–Organic Frameworks. Trends Chem. 2021, 3, 911–925. [Google Scholar] [CrossRef]
- Bai, D.; Chen, G.; Zhang, X.; Wang, W. Microsecond Molecular Dynamics Simulations of the Kinetic Pathways of Gas Hydrate Formation from Solid Surfaces. Langmuir 2011, 27, 5961–5967. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, W.; Pu, W.; Hu, J.; Zhao, J.; Zhang, L. CH 4 Nanobubbles on the Hydrophobic Solid–Water Interface Serving as the Nucleation Sites of Methane Hydrate. Langmuir 2018, 34, 10181–10186. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Lum, K.; Chandler, D.; Weeks, J.D. Hydrophobicity at Small and Large Length Scales. J. Phys. Chem. B 1999, 103, 4570–4577. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Deng, S.; Zhao, R.; Nie, X.; Liu, Y. Effect of Nanobubble Evolution on Hydrate Process: A Review. J. Therm. Sci. 2019, 28, 948–961. [Google Scholar] [CrossRef]
- Le Donne, A.; Tinti, A.; Amayuelas, E.; Kashyap, H.K.; Camisasca, G.; Remsing, R.C.; Roth, R.; Grosu, Y.; Meloni, S. Intrusion and Extrusion of Liquids in Highly Confining Media: Bridging Fundamental Research to Applications. Adv. Phys. X 2022, 7, 2052353. [Google Scholar] [CrossRef]
- Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Energetic Performances of the Metal–Organic Framework ZIF-8 Obtained Using High Pressure Water Intrusion–Extrusion Experiments. Phys. Chem. Chem. Phys. 2013, 15, 4888. [Google Scholar] [CrossRef] [PubMed]
- Khay, I.; Chaplais, G.; Nouali, H.; Marichal, C.; Patarin, J. Water Intrusion–Extrusion Experiments in ZIF-8: Impacts of the Shape and Particle Size on the Energetic Performances. RSC Adv. 2015, 5, 31514–31518. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.; Yee, D.; Linga, P.; Palmer, A.; Khoo, B.C.; Tan, T.S.; Rangsunvigit, P. Morphology of Methane Hydrate Formation in Porous Media. Energy Fuels 2013, 27, 3364–3372. [Google Scholar] [CrossRef]
- Jung, J.W.; Santamarina, J.C. Hydrate Formation and Growth in Pores. J. Cryst. Growth 2012, 345, 61–68. [Google Scholar] [CrossRef]
- Yan, L.; Chen, G.; Pang, W.; Liu, J. Experimental and Modeling Study on Hydrate Formation in Wet Activated Carbon. J. Phys. Chem. B 2005, 109, 6025–6030. [Google Scholar] [CrossRef]
- Govindaraj, V.; Mech, D.; Pandey, G.; Nagarajan, R.; Sangwai, J.S. Kinetics of Methane Hydrate Formation in the Presence of Activated Carbon and Nano-Silica Suspensions in Pure Water. J. Nat. Gas Sci. Eng. 2015, 26, 810–818. [Google Scholar] [CrossRef]
- Ganji, H.; Manteghian, M.; Sadaghiani, K.; Omidkhah, M.R.; Mofrad, H.R. Effect of Different Surfactants on Methane Hydrate Formation Rate, Stability and Storage Capacity. Fuel 2007, 86, 434–441. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Von Solms, N. Methane Hydrate Formation, Storage and Dissociation Behavior InUnconsolidated Sediments in the Presence of Environment-FriendlyPromoters. In Proceedings of the SPE Europec Featured at 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 18–21 October 2021. [Google Scholar] [CrossRef]
- Shanker Pandey, J.; Jouljamal Daas, Y.; Paul Karcz, A.; Solms, N. Von Methane Hydrate Formation Behavior in the Presence of Selected Amino Acids. J. Phys. Conf. Ser. 2020, 1580, 012003. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Karcz, A.P.; von Solms, N. Enhanced Hydrate-Based Geological CO2 Capture and Sequestration as a Mitigation Strategy to Address Climate Change. Energies 2020, 13, 5661. [Google Scholar] [CrossRef]
- Celzard, A.; Marêché, J.F. Optimal Wetting of Active Carbons for Methane Hydrate Formation. Fuel 2006, 85, 957–966. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Guo, K.; Wang, R.; Fan, S. Formation and Dissociation of HFC134a Gas Hydrate in Nano-Copper Suspension. Energy Convers. Manag. 2006, 47, 201–210. [Google Scholar] [CrossRef]
- Casco, M.E.; Cuadrado-Collados, C.; Martínez-Escandell, M.; Rodríguez-Reinoso, F.; Silvestre-Albero, J. Influence of the Oxygen-Containing Surface Functional Groups in the Methane Hydrate Nucleation and Growth in Nanoporous Carbon. Carbon N. Y. 2017, 123, 299–301. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-C.; Chou, H.-J.; Lin, C.-Y.; Janmanchi, D.; Chung, P.-W.; Mou, C.-Y.; Yu, S.S.F.; Chan, S.I. The Oversolubility of Methane Gas in Nano-Confined Water in Nanoporous Silica Materials. Microporous Mesoporous Mater. 2020, 293, 109793. [Google Scholar] [CrossRef]
- Muñoz-Santiburcio, D.; Marx, D. Chemistry in Nanoconfined Water. Chem. Sci. 2017, 8, 3444–3452. [Google Scholar] [CrossRef] [Green Version]
- Findenegg, G.H.; Jähnert, S.; Akcakayiran, D.; Schreiber, A. Freezing and Melting of Water Confined in Silica Nanopores. ChemPhysChem 2008, 9, 2651–2659. [Google Scholar] [CrossRef]
- Mallamace, F.; Broccio, M.; Corsaro, C.; Faraone, A.; Majolino, D.; Venuti, V.; Liu, L.; Mou, C.-Y.; Chen, S.-H. Evidence of the Existence of the Low-Density Liquid Phase in Supercooled, Confined Water. Proc. Natl. Acad. Sci. USA 2007, 104, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.N.; Clauzier, S.; Schuurman, Y.; Farrusseng, D.; Coasne, B. Gas Uptake in Solvents Confined in Mesopores: Adsorption versus Enhanced Solubility. J. Phys. Chem. Lett. 2013, 4, 2274–2278. [Google Scholar] [CrossRef]
- Ho, L.N.; Schuurman, Y.; Farrusseng, D.; Coasne, B. Solubility of Gases in Water Confined in Nanoporous Materials: ZSM-5, MCM-41, and MIL-100. J. Phys. Chem. C 2015, 119, 21547–21554. [Google Scholar] [CrossRef]
- Coasne, B.; Farrusseng, D. Gas Oversolubility in Nanoconfined Liquids: Review and Perspectives for Adsorbent Design. Microporous Mesoporous Mater. 2019, 288, 109561. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef] [PubMed]
- Domán, A.; Czakkel, O.; Porcar, L.; Madarász, J.; Geissler, E.; László, K. Role of Water Molecules in the Decomposition of HKUST-1: Evidence from Adsorption, Thermoanalytical, X-Ray and Neutron Scattering Measurements. Appl. Surf. Sci. 2019, 480, 138–147. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Li, Y.; Xu, F.; Xiao, J.; Xia, Q.; Li, Y.; Li, Z. A Novel Mechanochemical Method for Reconstructing the Moisture-Degraded HKUST-1. Chem. Commun. 2015, 51, 10835–10838. [Google Scholar] [CrossRef] [PubMed]
- Majano, G.; Martin, O.; Hammes, M.; Smeets, S.; Baerlocher, C.; Pérez-Ramírez, J. Solvent-Mediated Reconstruction of the Metal-Organic Framework HKUST-1 (Cu3(BTC)2). Adv. Funct. Mater. 2014, 24, 3855–3865. [Google Scholar] [CrossRef]
- Lin, J.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-nik, O.; Marx, S.; et al. A Scalable Metal-Organic Framework as a Durable Physisorbent for Carbon Dioxide Capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Silvestre-Albero, J. Methane Storage on Nanoporous Carbons. In Green Energy and Technology; Springer Nature: Singapore, 2019; pp. 209–226. ISBN 9789811335044. [Google Scholar]
- Cracknell, R.F.; Gordon, P.; Gubbins, K.E. Influence of Pore Geometry on the Design of Microporous Materials for Methane Storage. J. Phys. Chem. 1993, 97, 494–499. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Koh, C.A. Stability and Growth of Methane Hydrates in Confined Media for Carbon Sequestration. J. Phys. Chem. C 2022, 126, 11800–11809. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Moon, S.; Koh, D.Y.; Hong, S.; Lee, H.; Lee, J.W.; Park, Y. One-Step Formation of Hydrogen Clusters in Clathrate Hydrates Stabilized via Natural Gas Blending. Energy Storage Mater. 2020, 24, 655–661. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual Behavior of Propane as a Co-Guest during Hydrate Formation in Silica Sand: Potential Application to Seawater Desalination and Carbon Dioxide Capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy-Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Barati Farimani, A. Water Desalination with Two-Dimensional Metal–Organic Framework Membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-Organic Frameworks (MOFs) in Water Filtration Membranes for Desalination and Other Applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]







| MOF Design Criteria for CO2 Capture/Storage | MOF Design Criteria for CH4 Storage | |
|---|---|---|
| Goal | To improve CO2 interaction with the framework | High uptake |
| Strategies | To have more open metal sites Pore size control Amine functionalization Sufficient binding sites | To have the large surface area To have a large pore volume To have a flexible MOF structure |
| Comments | Extremely high surface area and pore volume are not needed Regeneration cost Influence of water Presence of other impurities | Impurities in natural gas Thermal effects due to adsorption and desorption |
| MOF Properties | |||
|---|---|---|---|
| Gas Name + MOF Reference Paper/Wettability | Pore Diameter (nm)/Specific Surface Area in m2/g | Pore Volume (cm3/g) | p, T Conditions/Key Observation |
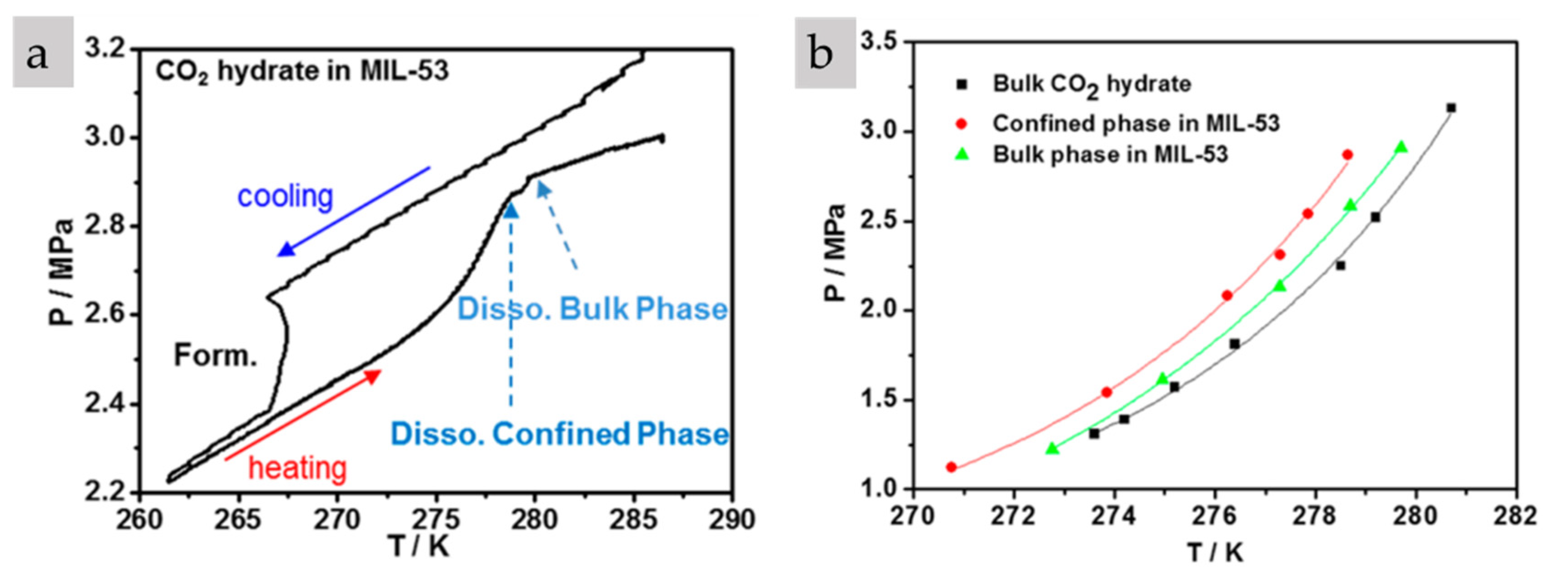
| CO2-MIL-53 [76] Hydrophilic | <2 (nm) (1500) | 0.67 0.18 (micro) 0.49 (meso) | p = 11–29 bar 270–279 K Phase equilibrium for bulk and confined |
| CO2-HKUST-1 [36] Hydrophilic | 0.5 (micro) 1.06, 1.24 (meso) (1091–1238) | n/a | p = 25 bar T = −30 °C WC = 36% n = 3 (at p = 23 bar) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, J.S.; von Solms, N. Metal–Organic Frameworks and Gas Hydrate Synergy: A Pandora’s Box of Unanswered Questions and Revelations. Energies 2023, 16, 111. https://doi.org/10.3390/en16010111
Pandey JS, von Solms N. Metal–Organic Frameworks and Gas Hydrate Synergy: A Pandora’s Box of Unanswered Questions and Revelations. Energies. 2023; 16(1):111. https://doi.org/10.3390/en16010111
Chicago/Turabian StylePandey, Jyoti Shanker, and Nicolas von Solms. 2023. "Metal–Organic Frameworks and Gas Hydrate Synergy: A Pandora’s Box of Unanswered Questions and Revelations" Energies 16, no. 1: 111. https://doi.org/10.3390/en16010111





