Application of Paraffin-Based Phase Change Materials for the Amelioration of Thermal Energy Storage in Hydronic Systems
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Setup
2.2. Thermal Model
3. Results and Discussion
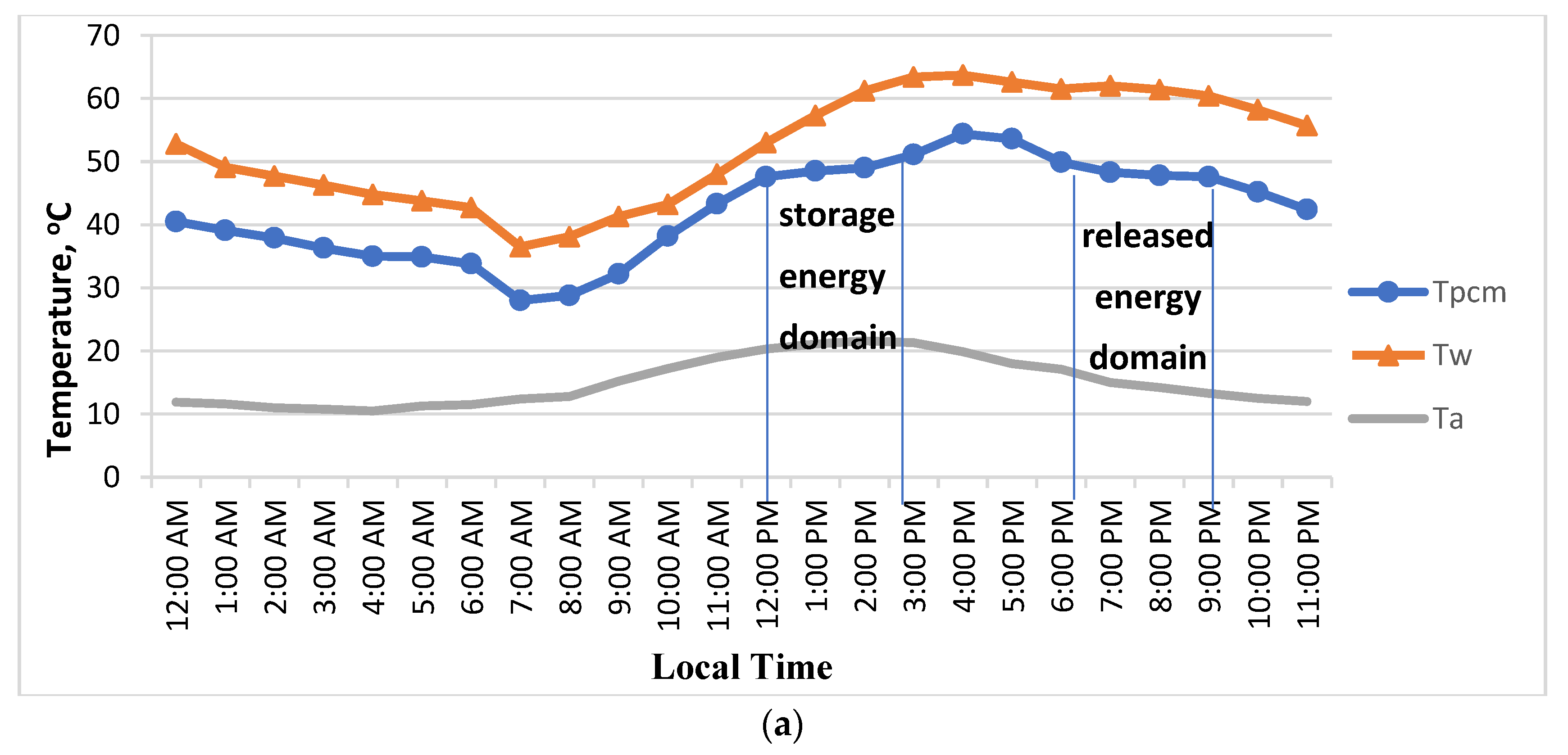
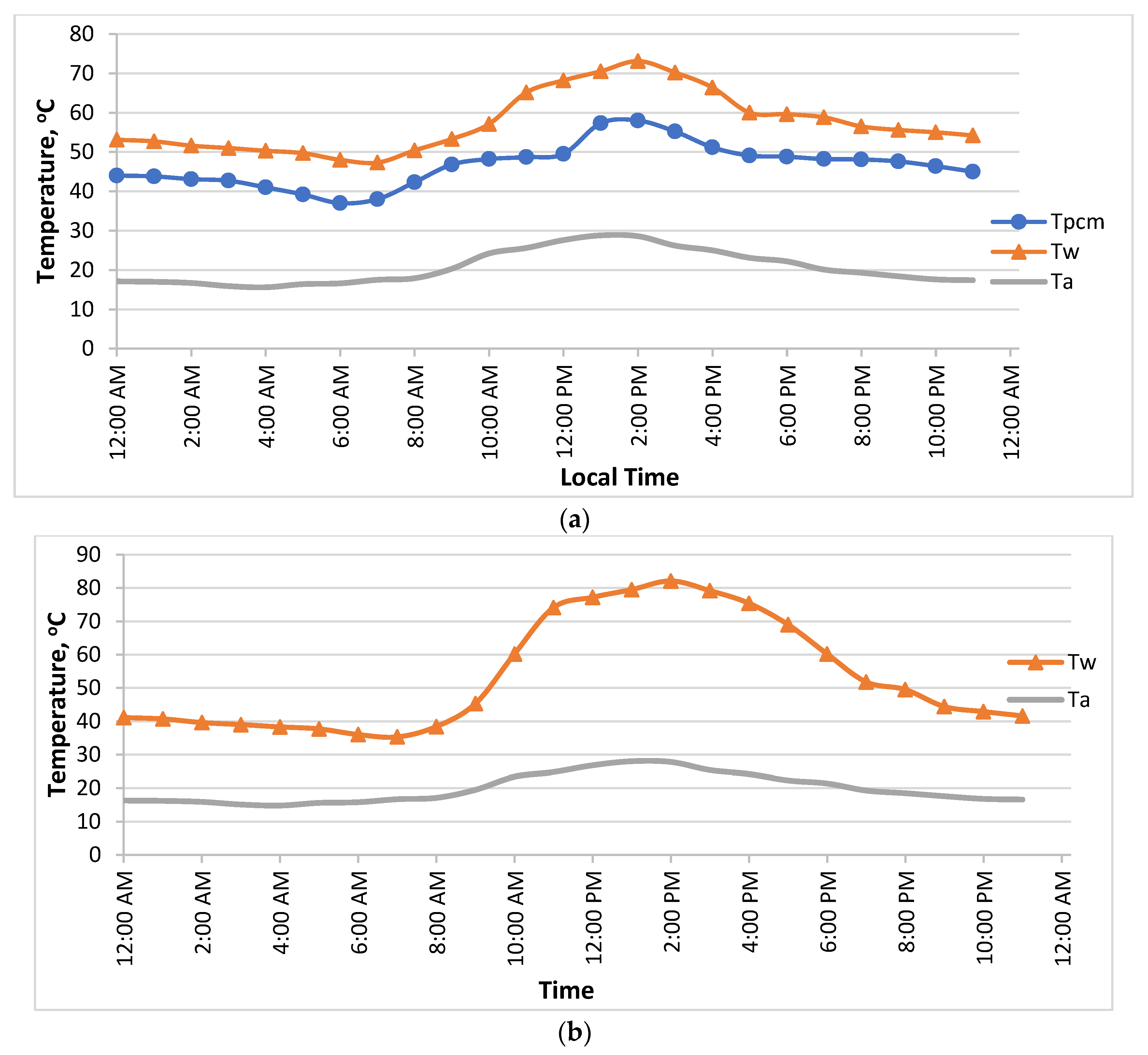
3.1. Temperature Distributions
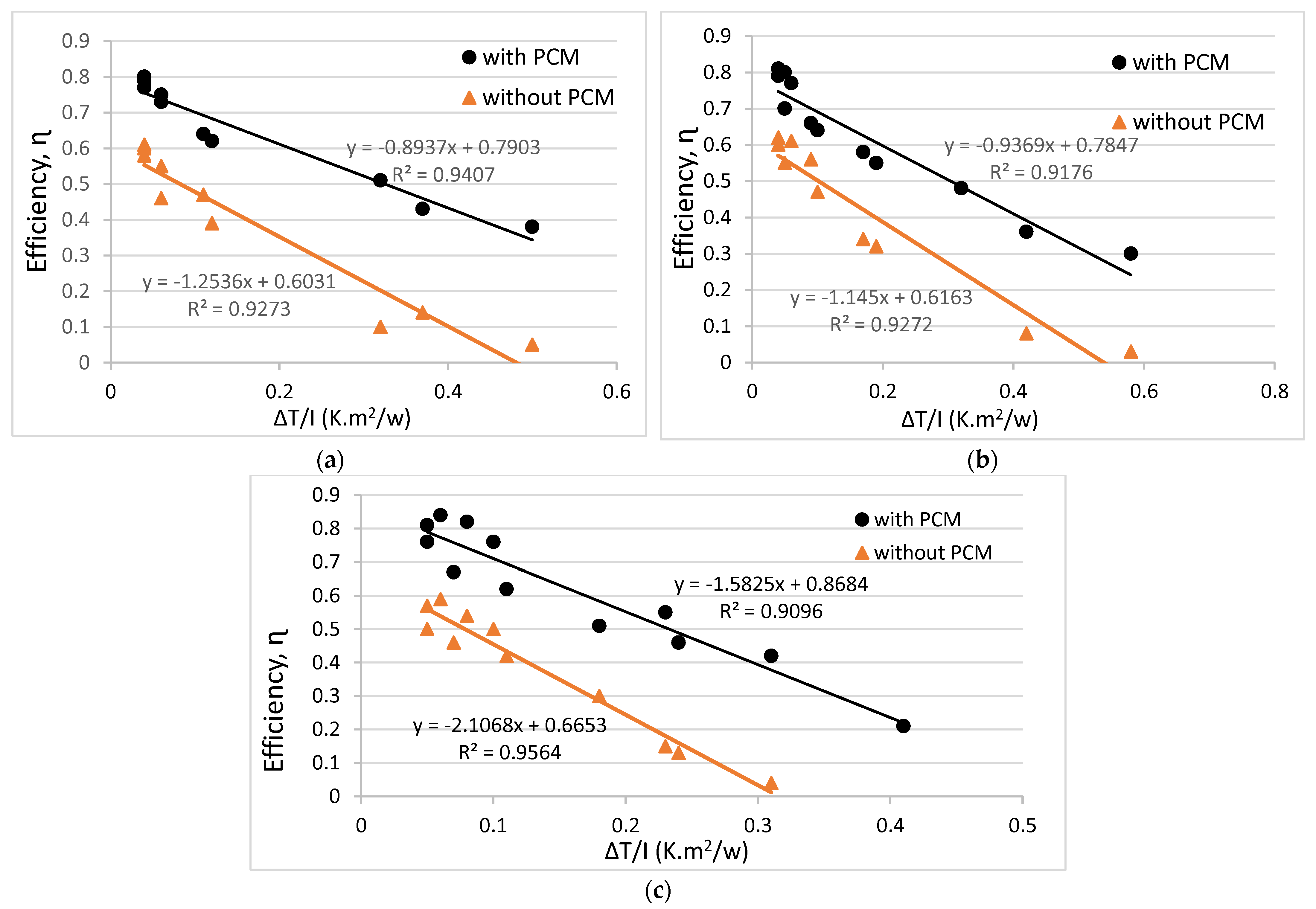
3.2. System Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| T | Temperature: K |
| Ac | Solar collector area, m2 |
| Cp | Specific heat, kJ/kg⋅K |
| I | Global solar irradiance, w/m2 |
| kθi | Incident angle modifier |
| m | Mass flow rate, kg/s |
| Phase change material energy, w | |
| Load energy, w | |
| energy losses, w | |
| Useful energy, w | |
| Over-all heat transfer coefficient of heat loss, W/m2⋅K | |
| rin | Inner radius of the hot water tank, m |
| ro | Outer radius of the hot water tank, m |
| (τα)eff | Effective transmissivity-absorptivity product coefficient |
| λ | Latent heat, kJ/kg |
| η | Thermal efficiency, % |
| Subscripts | |
| a | Ambient |
| w | water |
| tube | Evacuated tube solar collector |
| tank | Hot water tank |
| sys | system |
References
- Al Rabadi, S. Theoretical Investigations on The Optimal Topology of Crude Oil Desulfurization Process. In Proceedings of the 9th Jordan International Chemical Engineering Conference (JICHEC9), Online, 12–14 October 2021; pp. 225–238. Available online: https://jeaconf.org/UploadedFiles/AssetsManagement/JIChECIX/JIChECIX.pdf (accessed on 1 October 2022).
- Koželj, R.; Osterman, E.; Leonforte, F.; Del Pero, C.; Miglioli, A.; Zavrl, E.; Stropnik, R.; Aste, N.; Stritih, U. Phase-change materials in hydronic heating and cooling systems: A literature review. Materials 2020, 13, 2971. [Google Scholar] [CrossRef] [PubMed]
- Al Rabadi, S.; Al-Zboon, K.; Shawaqfah, M.; Damseh, R.; Al Zoubi, O. Biogas Production through Co-Digestion of Olive Mill with Municipal Sewage Sludge and Cow Manure. Environ. Nat. Resour. J. 2022, 20, 137–147. [Google Scholar] [CrossRef]
- Chiradeja, P.; Pothisarn, C.; Jettanasen, C.; Yoomak, S.; Songsukthawan, P.; Bunjongjit, S.; Leelajindakrairerk, M.; Ngaopitakkul, A. Solar water heating in residential building. Int. J. Smart Grid Clean Energy 2019, 8, 422–429. [Google Scholar] [CrossRef]
- Stropnik, R.; Stritih, U. Increasing the efficiency of PV panel with the use of PCM. Renew. Energy 2016, 97, 671–679. [Google Scholar] [CrossRef]
- Browne, M.C.; Norton, B.; McCormack, S.J. Phase change materials for photovoltaic thermal management. Renew. Sustain. Energy Rev. 2015, 47, 762–782. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase change materials application in battery thermal management system: A review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef]
- Yang, L.; nan Huang, J.; Zhou, F. Thermophysical properties and applications of nano-enhanced PCMs: An update review. Energy Convers. Manag. 2020, 214, 112876. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2021, 33, 101913. [Google Scholar] [CrossRef]
- Rezaie, B.; Rosen, M.A. District heating and cooling: Review of technology and potential enhancements. Appl. Energy 2012, 93, 2–10. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Wu, H.; Xu, T. Feasibility study of a PCM storage tank integrated heating system for outdoor swimming pools during the winter season. Appl. Therm. Eng. 2018, 134, 490–500. [Google Scholar] [CrossRef]
- Venegas-Troncoso, T.; Ugarte-Larraguibel, G.; Vasco, D.A.; Rouault, F.; Pérez, R.A. Feasibility study of the application of a cooling energy storage system in a chiller plant of an office building located in Santiago, Chile. Int. J. Refrig. 2019, 102, 142–150. [Google Scholar] [CrossRef]
- Mazarrón, F.R.; Porras-Prieto, C.J.; García, J.L.; Benavente, R.M. Feasibility of active solar water heating systems with evacuated tube collector at different operational water temperatures. Energy Convers. Manag. 2016, 113, 16–26. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Liu, K.; Yu, H.; Cheng, K. Design of high-performance water-in-glass evacuated tube solar water heaters by a high-throughput screening based on machine learning: A combined modeling and experimental study. Sol. Energy 2017, 142, 61–67. [Google Scholar] [CrossRef]
- Ge, R.Z.T.S.; Wang, Z.Y.; Xu, Q.W.; Pan, S.; Du, X.M.; Chen, T.; Ma, X.N.; Wu, X.L.; Sun, J.F.C. Solar heating and cooling: Present and future development. Renew. Energy 2018, 126, 1126–1140. [Google Scholar] [CrossRef]
- Buker, M.S.; Riffat, S.B. Building integrated solar thermal collectors-A review. Renew. Sustain. Energy Rev. 2015, 51, 327–346. [Google Scholar] [CrossRef]
- Sobhansarbandi, S.; Martinez, P.M.; Papadimitratos, A.; Zakhidov, A.; Hassanipour, F. Evacuated tube solar collector with multifunctional absorber layers. Sol. Energy 2017, 146, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Krishnavel, V.; Karthick, A.; Murugavel, K.K. Experimental analysis of concrete absorber solar water heating systems. Energy Build. 2014, 84, 501–505. [Google Scholar] [CrossRef]
- Malkawi, D.S.; Tamimi, A.I. Comparison of phase change material for thermal analysis of a passive hydronic solar system. J. Energy Storage 2021, 33, 102069. [Google Scholar] [CrossRef]
- Gürtürk, M.; Koca, A.; Öztop, H.F. Energy and exergy analysis of a heat storage tank with a novel eutectic phase change material layer of a solar heater system. Int. J. Green Energy 2017, 14, 1073–1080. [Google Scholar] [CrossRef]
- Manoj Kumar, P.; Mylsamy, K.; Alagar, K.; Sudhakar, K. Investigations on an evacuated tube solar water heater using hybrid-nano based organic phase change material. Int. J. Green Energy 2020, 17, 872–883. [Google Scholar] [CrossRef]
- Feliński, P.; Sekret, R. Effect of PCM application inside an evacuated tube collector on the thermal performance of a domestic hot water system. Energy Build. 2017, 152, 558–567. [Google Scholar] [CrossRef]
- Papadimitratos, A.; Sobhansarbandi, S.; Pozdin, V.; Zakhidov, A.; Hassanipour, F. Evacuated tube solar collectors integrated with phase change materials. Sol. Energy 2016, 129, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Pereira da Cunha, J.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Rousse, D.R.; Ben Salah, N.; Lassue, S. An overview of phase change materials and their implication on power demand. In Proceedings of the 2009 IEEE Electrical Power & Energy Conference (EPEC), Montreal, QC, Canada, 22–23 October 2009; pp. 1–6. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Manoj Kumar, P.; Anandkumar, R.; Mylsamy, K.; Prakash, K.B. Experimental investigation on thermal conductivity of nanoparticle dispersed paraffin (NDP). Mater. Today Proc. 2021, 45, 735–739. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature latent heat energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 20–27. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V. Long-term thermal and chemical reliability study of different organic phase change materials for thermal energy storage applications. J. Therm. Anal. Calorim. 2016, 124, 1357–1366. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Chopra, K.; Sharma, R.K.; Pandey, A.K.; Tyagi, S.K. Muhammad Shakeel Ahmad, Ahmet Sarı, R.K. A comprehensive review on phase change materials for heat storage applications: Development, characterization, thermal and chemical stability. Sol. Energy Mater. Sol. Cells 2022, 234, 111392. [Google Scholar] [CrossRef]
- Murali, G.; Mayilsamy, K.; Arjunan, T.V. An Experimental Study of PCM-Incorporated Thermosyphon Solar Water Heating System. Int. J. Green Energy 2015, 12, 978–986. [Google Scholar] [CrossRef]
- Rabady, R.I.; Malkawi, D.S. Thermal conductivity enhancement of sodium thiosulfate pentahydrate by adding carbon nano-tubes/graphite nano-particles. J. Energy Storage 2020, 27, 101166. [Google Scholar] [CrossRef]
- Mourad, A.; Abderrahmane, A.; Said, Z.; Younis, O.; Iqbal, M.; Alazzam, A. Recent advances on the applications of phase change materials for solar collectors, practical limitations, and challenges: A critical review. J. Energy Storage 2022, 49, 104186. [Google Scholar] [CrossRef]
- Jirawattanapanit, A.; Abderrahmane, A.; Mourad, A.; Guedri, K.; Younis, O.; Bouallegue, B.; Subkrajang, K.; Rajchakit, G.; Shah, N.A. A Numerical Investigation of a Melting Rate Enhancement inside a Thermal Energy Storage System of Finned Heat Pipe with Nano-Enhanced Phase Change Material. Nanomaterials 2022, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Abderrahmane, A.; Saeed, A.; Guedri, K.; Mourad, A.; Younis, O.; Botmart, T.; Shah, N. Melting enhancement of PCM in a finned tube latent heat thermal energy storage. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, M.; Karkri, M.; Albouchi, F.; Ben Nasrallah, S.; Fois, M.; Sobolciak, P. Thermal properties measurement and heat storage analysis of paraffin/graphite composite phase change material. Compos. Part B Eng. 2014, 66, 518–525. [Google Scholar] [CrossRef]
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2013; ISBN 9780470873663. [Google Scholar]
- Holman, V. Heat Transfer, 10th ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Azimi, M.; Mirjavadi, S.S.; Mohammadkarim, A. Simulation and Optimization of Vacuum Tube Solar Collector Water Heating System in Iran. J. Sci. Eng. 2015, 7, 001–019. [Google Scholar]
- Siuta-Olcha, A.; Cholewa, T.; Dopieralska-Howoruszko, K. Experimental studies of thermal performance of an evacuated tube heat pipe solar collector in Polish climatic conditions. Environ. Sci. Pollut. Res. 2021, 28, 14319–14328. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Kim, H.M.; Cho, H.H. Theoretical investigation of the thermal performance of evacuated heat pipe solar collector with optimum tilt angle under various operating conditions. J. Mech. Sci. Technol. 2016, 30, 903–913. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Number of tubes | 20 |
| Outer diameter | 0.058 m |
| Inner diameter | 0.047 m |
| Length | 1.8 m |
| Tube material | Borosilicate glass |
| Absorptivity coefficient | 95% |
| Emissivity coefficient | 5% |
| PCM | Melting Temperature [°C] | Latent Heat [kJ/kg] | Specific Heat [kJ/kg⋅°C] | Thermal Conductivity [W/m⋅°C] |
|---|---|---|---|---|
| Paraffin wax | 48 | 210 | 2.4 (liquid) 2.1 (Solid) | 0.24 |
| Local Time | Tw (K) | Ta (K) | I (w/m2) | Quseful (w) | Qloss, sys (w) | ∆T/I (K⋅m2/w) | ||
|---|---|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||||
| 7:00 AM | 313.65 | 285.55 | 42.09 | 201.25 | 92.41 | 0.37 | 0.43 | 0.14 |
| 8:00 AM | 311.25 | 285.95 | 206.47 | 527.41 | 82.98 | 0.12 | 0.62 | 0.39 |
| 9:00 AM | 314.45 | 288.35 | 449.72 | 1170.86 | 86.23 | 0.06 | 0.75 | 0.46 |
| 10:00 AM | 316.35 | 290.35 | 657.35 | 1730.45 | 86.37 | 0.04 | 0.77 | 0.58 |
| 11:00 AM | 321.15 | 292.15 | 660.99 | 2017.54 | 97.07 | 0.04 | 0.79 | 0.61 |
| 12:00 PM | 326.15 | 293.45 | 770.52 | 1540.62 | 110.55 | 0.04 | 0.8 | 0.6 |
| 1:00 PM | 330.45 | 294.25 | 592.19 | 924.34 | 123.29 | 0.06 | 0.73 | 0.55 |
| 2:00 PM | 334.35 | 294.75 | 361.18 | 311.50 | 135.52 | 0.11 | 0.64 | 0.47 |
| 3:00 PM | 336.55 | 294.45 | 131.38 | 194.21 | 104.64 | 0.32 | 0.51 | 0.1 |
| 4:00 PM | 336.15 | 293.05 | 88.21 | 141.14 | 92.41 | 0.5 | 0.38 | 0.05 |
| Local Time | Tw (K) | Ta (K) | I (w/m2) | Quseful (w) | Qloss, sys (w) | ∆T/I (K⋅m2/w) | ||
|---|---|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||||
| 7:00 AM | 312.15 | 290.65 | 67.82 | 162.47 | 63.84 | 0.32 | 0.48 | 0.17 |
| 8:00 AM | 314.35 | 291.05 | 121.32 | 302.60 | 69.39 | 0.19 | 0.55 | 0.32 |
| 9:00 AM | 317.35 | 293.45 | 239.80 | 615.55 | 71.69 | 0.1 | 0.64 | 0.47 |
| 10:00 AM | 321.35 | 297.35 | 465.14 | 1211.92 | 72.73 | 0.05 | 0.7 | 0.55 |
| 11:00 AM | 329.95 | 298.75 | 671.44 | 1751.12 | 95.73 | 0.05 | 0.8 | 0.55 |
| 12:00 PM | 333.95 | 300.75 | 795.03 | 2075.75 | 102.87 | 0.04 | 0.81 | 0.6 |
| 1:00 PM | 337.55 | 301.95 | 798.70 | 2082.36 | 111.08 | 0.04 | 0.79 | 0.62 |
| 2:00 PM | 340.55 | 301.75 | 681.87 | 1769.23 | 121.40 | 0.06 | 0.77 | 0.61 |
| 3:00 PM | 342.25 | 299.35 | 480.51 | 1231.29 | 134.41 | 0.09 | 0.66 | 0.56 |
| 4:00 PM | 342.55 | 298.15 | 256.79 | 636.88 | 119.08 | 0.17 | 0.58 | 0.34 |
| 5:00 PM | 343.15 | 296.25 | 111.33 | 249.13 | 96.66 | 0.42 | 0.36 | 0.08 |
| 6:00 PM | 339.45 | 295.35 | 89.32 | 190.98 | 67.18 | 0.58 | 0.3 | 0.03 |
| Local Time | Tw (K) | Ta (K) | I (w/m2) | Quseful (w) | Qloss, sys (w) | ∆T/I (K⋅m2/w) | ||
|---|---|---|---|---|---|---|---|---|
| Experimental | Theoretical | |||||||
| 7:00 AM | 320.45 | 290.65 | 124.60 | 305.87 | 101.62 | 0.41 | 0.21 | 0.04 |
| 8:00 AM | 323.55 | 291.05 | 138.30 | 339.54 | 104.03 | 0.24 | 0.46 | 0.13 |
| 9:00 AM | 326.45 | 293.45 | 145.10 | 356.24 | 104.86 | 0.23 | 0.55 | 0.15 |
| 10:00 AM | 330.25 | 297.35 | 401.15 | 1733.79 | 127.29 | 0.1 | 0.76 | 0.5 |
| 11:00 AM | 338.25 | 298.75 | 665.25 | 2138.88 | 132.21 | 0.08 | 0.82 | 0.54 |
| 12:00 PM | 341.35 | 300.75 | 820.88 | 2132.10 | 136.54 | 0.06 | 0.84 | 0.59 |
| 1:00 PM | 343.65 | 301.95 | 819.26 | 1719.74 | 145.85 | 0.05 | 0.81 | 0.57 |
| 2:00 PM | 346.25 | 301.75 | 664.39 | 1045.19 | 143.57 | 0.05 | 0.76 | 0.5 |
| 3:00 PM | 343.35 | 299.35 | 410.99 | 565.76 | 134.29 | 0.07 | 0.67 | 0.46 |
| 4:00 PM | 339.55 | 298.15 | 229.66 | 272.33 | 118.24 | 0.11 | 0.62 | 0.42 |
| 5:00 PM | 333.15 | 296.25 | 117.68 | 248.26 | 119.90 | 0.18 | 0.51 | 0.3 |
| 6:00 PM | 332.75 | 295.35 | 106.39 | 117.31 | 83.18 | 0.31 | 0.42 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkawi, D.S.; Rabady, R.I.; Malkawi, M.S.; Al Rabadi, S.J. Application of Paraffin-Based Phase Change Materials for the Amelioration of Thermal Energy Storage in Hydronic Systems. Energies 2023, 16, 126. https://doi.org/10.3390/en16010126
Malkawi DS, Rabady RI, Malkawi MS, Al Rabadi SJ. Application of Paraffin-Based Phase Change Materials for the Amelioration of Thermal Energy Storage in Hydronic Systems. Energies. 2023; 16(1):126. https://doi.org/10.3390/en16010126
Chicago/Turabian StyleMalkawi, Dua’a S., Rabi Ibrahim Rabady, Mosa’b S. Malkawi, and Said Jereis Al Rabadi. 2023. "Application of Paraffin-Based Phase Change Materials for the Amelioration of Thermal Energy Storage in Hydronic Systems" Energies 16, no. 1: 126. https://doi.org/10.3390/en16010126
APA StyleMalkawi, D. S., Rabady, R. I., Malkawi, M. S., & Al Rabadi, S. J. (2023). Application of Paraffin-Based Phase Change Materials for the Amelioration of Thermal Energy Storage in Hydronic Systems. Energies, 16(1), 126. https://doi.org/10.3390/en16010126







