Particle Size Distribution and Enrichment of Alkali and Heavy Metals in Fly Ash on Air and Oxy-Fuel Conditions from Sludge Combustion
Abstract
:1. Introduction
2. Test Facility and Experimental Methods
2.1. Test Facility and Fuel Characteristics
2.2. Sampling and Analysis
3. Results and Discussion
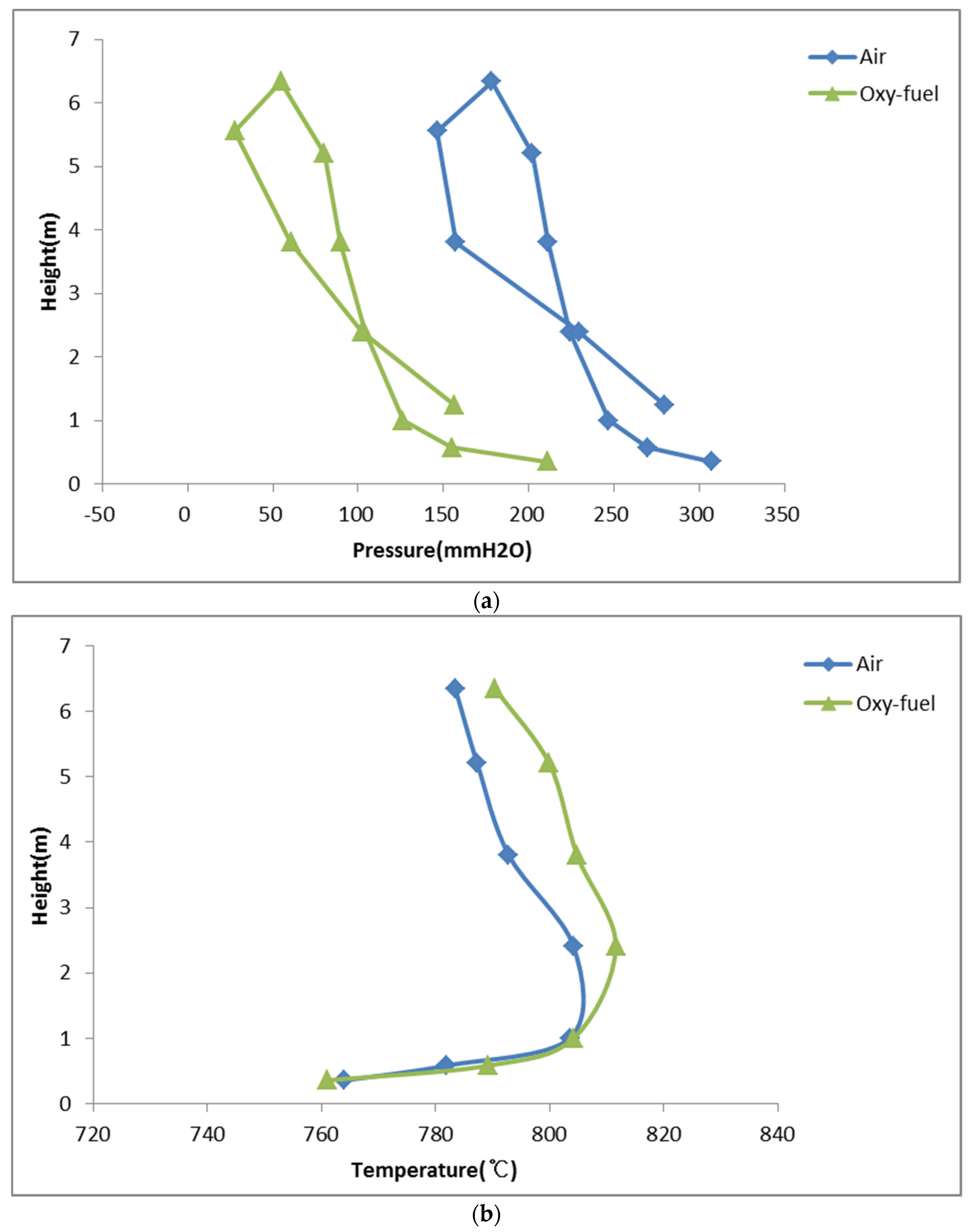
3.1. Combustion Surroundings and Flue Gas Composition
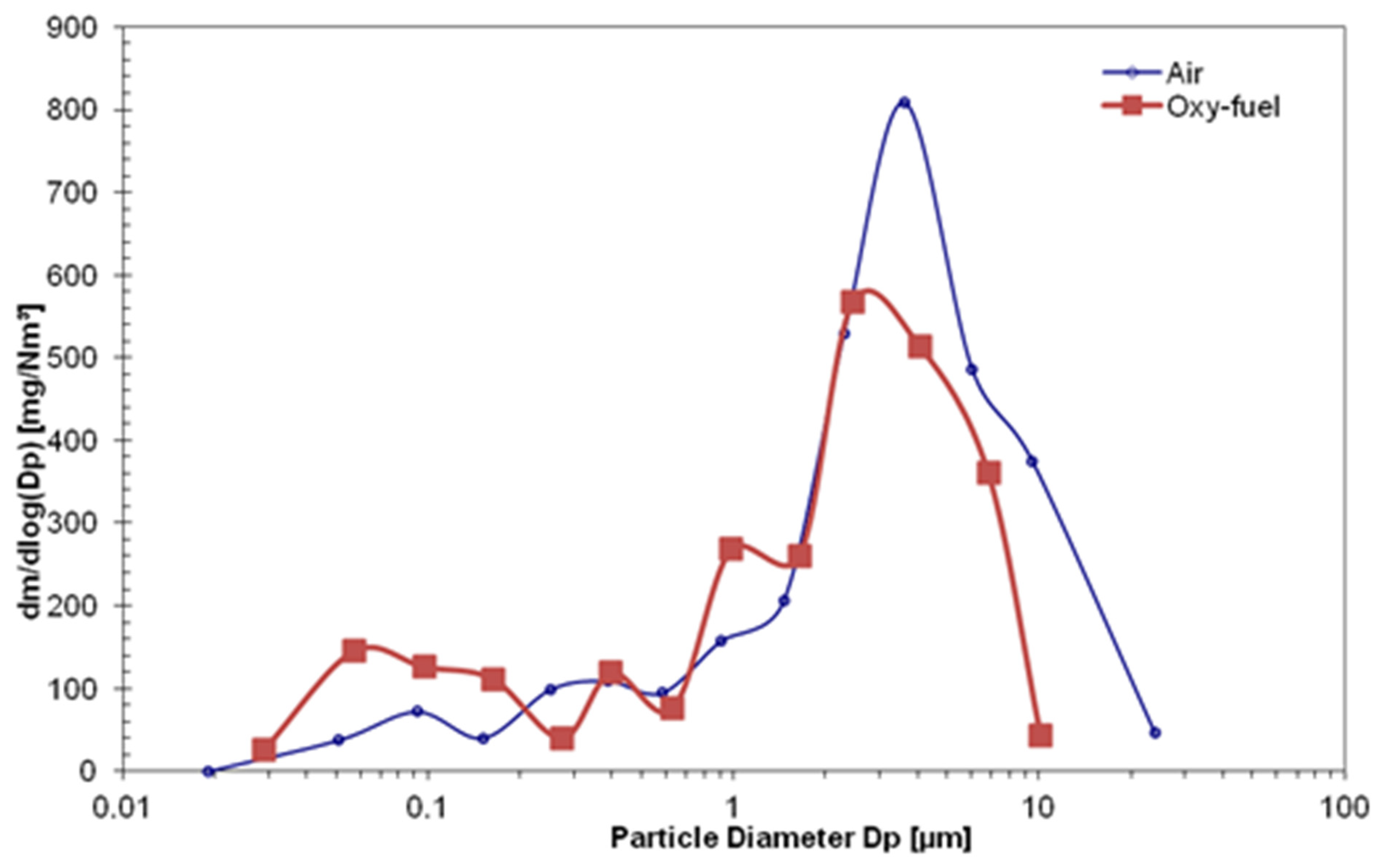
3.2. The Behavior of Ash and Heavy Metals
4. Conclusions
- Temperature and pressure profiles in air and oxy-fuel combustion of sewage sludge were different according to mixtures of O2/CO2 and O2/N2. It was indicated that combustion surroundings for CFB waste sludge combustion changed due to physical gas properties, such as kinematic viscosity, density, and heat capacity of nitrogen, oxygen, and carbon dioxide during air and oxy-fuel combustion.
- Based on flue gas and ash composition analysis in air and oxy-fuel combustion, the oxy-fuel combustion was more efficient than air combustion in terms of heat recovery, beneficial carbon dioxide capture, and economical long-term operation by mitigating agglomeration, fouling, and corrosion problems from sewage sludge combustion.
- The PSD in fly ash under the oxygen with nitrogen condition was mainly distributed as coarse particles over 2.5 μm, whereas that under the oxygen with carbon dioxide plotted each peak mode as ultra-fine particle below 1 μm and fine particle between 1 μm and 2.5 μm. The results were caused by each series of mechanisms by metal compounds under different circumstances in both combustion conditions.
- The portions of alkali metals under the oxygen with carbon dioxide condition were below 2.5 μm a bit larger than those under the oxygen with nitrogen condition. It is explained that the ignition time delay under the oxygen with carbon dioxide condition was more rapid than that under the oxygen with nitrogen condition. and fine particle formation from the metals was elevated by volatilization and condensation reactions.
- Fine particle formation from chrome, nickel, copper, and zinc was more intensively conducted under the oxygen with carbon dioxide condition than under the oxygen with nitrogen condition. This was because of the large amount of carbon dioxide, and the compounds vapor could be more intensively re-oxidized to generate fine particles under the oxygen with carbon dioxide condition than those under the oxygen with nitrogen condition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buhre, B.; Elliott, L.; Sheng, C.; Gupta, R.; Wall, T. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Glarborg, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Rink, K.K.; Kozinski, J.A.; Lighty, J.S. Biosludge incineration in FBCs: Behavior of ash particles. Combust. Flame 1995, 100, 121–130. [Google Scholar] [CrossRef]
- Cenni, R.; Frandsen, F.; Gerhardt, T.; Spliethoff, H.; Hein, K. Study on trace metal partitioning in pulverized combustion of bituminous coal and dry sewage sludge. Waste Manag. 1998, 18, 433–444. [Google Scholar] [CrossRef]
- Latva-Somppi, J.; Kauppinen, E.; Valmari, T.; Ahonen, P.; Gurav, A.S.; Kodas, T.T.; Johanson, B. The ash formation during co-combustion of wood and sludge in industrial fluidized bed boilers. Fuel Process. Technol. 1998, 54, 79–94. [Google Scholar] [CrossRef]
- Lopez, M.H.; Abelha, P.; Lapa, N.; Oliveira, J.S.; Cabrita, I.; Gulyurtlu, I. The behavior of ashes and heavy metals during the co-combustion of sewage sludge in a fluidized bed. Waste Manag. 2003, 23, 859–870. [Google Scholar]
- Marani, D.; Braguglia, C.M.; Mininni, G.; Maccioni, F. Behaviour of Cd, Cr, Mn, Ni, Pb, and Zn in sewage sludge incineration by fluidized bed furnace. Waste Manag. 2003, 23, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Åmand, L.-E.; Leckner, B. Metal emissions from co-combustion of sewage sludge and coal/wood in fluidized bed. Fuel 2004, 83, 1803–1821. [Google Scholar] [CrossRef]
- Elled, A.L.; Amand, L.E.; Leckner, B.; Andersson, B.A. Influence of phosphorus on sulphur capture during co-firing of sewage sludge with wood or bark in a fluidized bed. Fuel 2006, 85, 1671–1678. [Google Scholar] [CrossRef]
- Elled, A.L.; Amand, L.E.; Leckner, B.; Andersson, B.A. The fate of trace elements in fluidized bed combustion of sewage sludge and wood. Fuel 2007, 86, 843–852. [Google Scholar] [CrossRef]
- Van de Velden, M.; Dewil, R.; Baeyens, J.; Josson, L.; Lanssens, P. The distribution of heavy metals during fluidized bed combustion of sludge (FBSC). J. Hazard. Mater. 2008, 151, 96–102. [Google Scholar] [CrossRef]
- Barbosa, R.; Lapa, N.; Boavida, D.; Lopes, H.; Gulyurtlu, I.; Mendes, B. Co-combustion of coal and sewage sludge: Chemical and ecotoxicological properties of ashes. J. Hazard. Mater. 2009, 170, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Solimene, R.; Urciuolo, M.; Cammarota, A.; Chirone, R.; Salatino, P.; Damonte, G.; Donati, C.; Puglisi, G. Devolatilization and ash comminution of two different sewage sludges under fluidized bed combustion conditions. Exp. Therm. Fluid Sci. 2010, 34, 387–395. [Google Scholar] [CrossRef]
- Lopez, H.; Gulyurtlu, I.; Abelha, P.; Crujeira, T.; Salema, D.; Freire, M.; Pereira, R.; Cabrita, I. Particulate and PCDD/F emissions from coal co-firing with solid biofuels in a bubbling fluidized bed reactor. Fuel 2009, 88, 2373–2384. [Google Scholar] [CrossRef]
- Urciuolo, M.; Solimene, R.; Chirone, R.; Salatino, P. Fluidized bed combustion and fragmentation of wet sewage sludge. Exp. Therm. Fluid Sci. 2012, 43, 97–104. [Google Scholar] [CrossRef]
- Jang, H.-N.; Kim, J.-H.; Back, S.-K.; Sung, J.-H.; Yoo, H.-M.; Choi, H.S.; Seo, Y.-C. Combustion characteristics of waste sludge at air and oxy-fuel combustion conditions in a circulating fluidized bed reactor. Fuel 2015, 170, 92–99. [Google Scholar] [CrossRef]
- Fryda, L.; Sobrino, C.; Cieplik, M.; van de Kamp, W. Study on ash deposition under oxyfuel combustion of coal/biomass blends. Fuel 2010, 89, 1889–1902. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, C.; Tan, Y.; Jia, L.; Anthony, E.J. Characterization of ashes from a 100 kWth pilot-scale circulating fluidized bed with oxy-fuel combustion. Appl. Energy 2011, 88, 2940–2948. [Google Scholar] [CrossRef]
- Fryda, L.; Sobrino, C.; Glazer, M.; Bertrand, C.; Cieplik, M. Study of ash deposition during coal combustion under oxyfuel conditions. Fuel 2012, 92, 308–317. [Google Scholar] [CrossRef]



| Design Factor | Value |
|---|---|
| Bed diameter (m) | 0.15 |
| Fuel feeding rate (kg/h) | 13 |
| Solid fuel mixing rate (%) | 0~30 |
| Oxygen injection rate (%) | 23 |
| Combustion temperature (°C) | 800 |
| Flow rate (L/min) | 900 |
| Proximate Analysis (wt, %) | Element Analysis (wt, %) | ||
|---|---|---|---|
| Moisture | 7.32 | Carbon | 28.14 |
| Volatile | 45.11 | Hydrogen | 4.74 |
| Fixed carbon | 12.25 | Nitrogen | 4.43 |
| Ash | 35.04 | Oxygen | 23.90 |
| - | - | Sulfur | 0.43 |
| Calorific value (kcal/kg) | 3008 | Chloride | 0.053 |
| Selected metals analysis | |||
| Alkali metals analysis (ppm) | Toxic heavy metals analysis (ppm) | ||
| Al | 21,700.0 | Zn | 635.4 |
| Ca | 11,204.7 | Cu | 305.2 |
| K | 8249.0 | Cr | 42.5 |
| - | - | Ni | 30.8 |
| H2O | O2 | N2 | CO2 | Ratio, CO2/N2 | |
|---|---|---|---|---|---|
| Density (ρ) [kg/m3] | 0.157 | 0.278 | 0.244 | 0.383 | 1.6 |
| Thermal conductivity (k) [W/m∙k] | 0.136 | 0.087 | 0.082 | 0.097 | 1.2 |
| Specific heat capacity (cp) [J/mol∙°C] | 45.67 | 36.08 | 34.18 | 57.83 | 1.7 |
| Kinematic viscosity (m2/s) | 3.20 | 2.09 × 10−4 | 2.00 × 10−4 | 1.31 × 10−4 | 0.7 |
| Test Condition | O2 (%) | CO2 (%) | CO (%) | Temp (°C) |
|---|---|---|---|---|
| Air | 5.1 | 15.3 | 0.9 | 697.4 |
| Oxy-fuel | 6.4 | 82.9 | 1.5 | 736.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-N.; Yoo, H.-M.; Choi, H.S. Particle Size Distribution and Enrichment of Alkali and Heavy Metals in Fly Ash on Air and Oxy-Fuel Conditions from Sludge Combustion. Energies 2023, 16, 145. https://doi.org/10.3390/en16010145
Jang H-N, Yoo H-M, Choi HS. Particle Size Distribution and Enrichment of Alkali and Heavy Metals in Fly Ash on Air and Oxy-Fuel Conditions from Sludge Combustion. Energies. 2023; 16(1):145. https://doi.org/10.3390/en16010145
Chicago/Turabian StyleJang, Ha-Na, Heung-Min Yoo, and Hang Seok Choi. 2023. "Particle Size Distribution and Enrichment of Alkali and Heavy Metals in Fly Ash on Air and Oxy-Fuel Conditions from Sludge Combustion" Energies 16, no. 1: 145. https://doi.org/10.3390/en16010145







