Effect of the Design Parameters of the Combustion Chamber on the Efficiency of a Thermal Oxidizer
Abstract
:1. Introduction
1.1. Classification of Burners
1.2. Turbulence–Chemistry Interaction Model
1.3. Porous Media Model for a Flame Arrestor
2. Numerical Simulation
2.1. Geometry and Mesh
2.2. Governing Equations
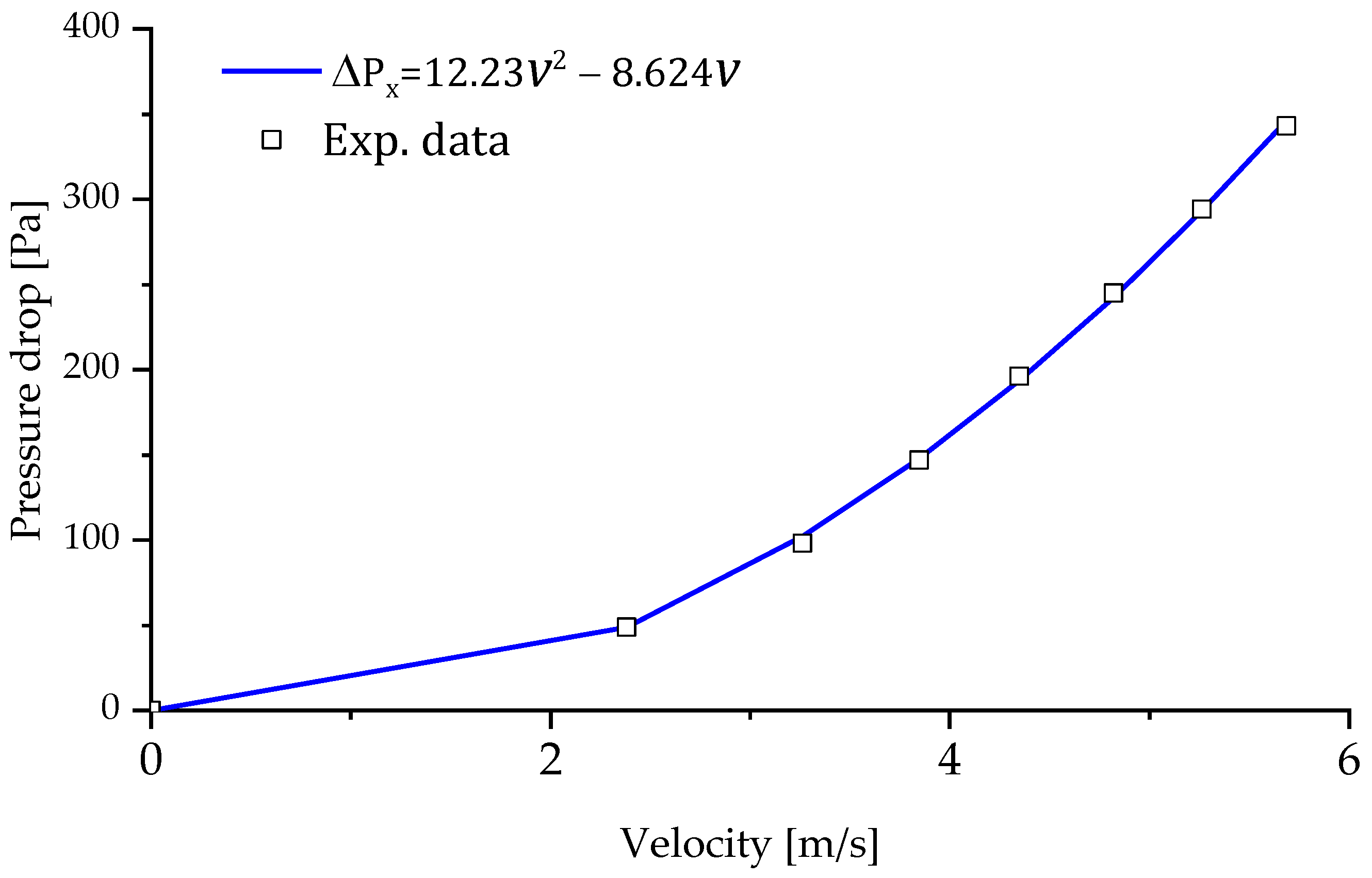
2.3. Validation of the Numerical Procedure
2.4. Boundary Conditions
2.5. Grid Independence Test
3. Result and Discussion
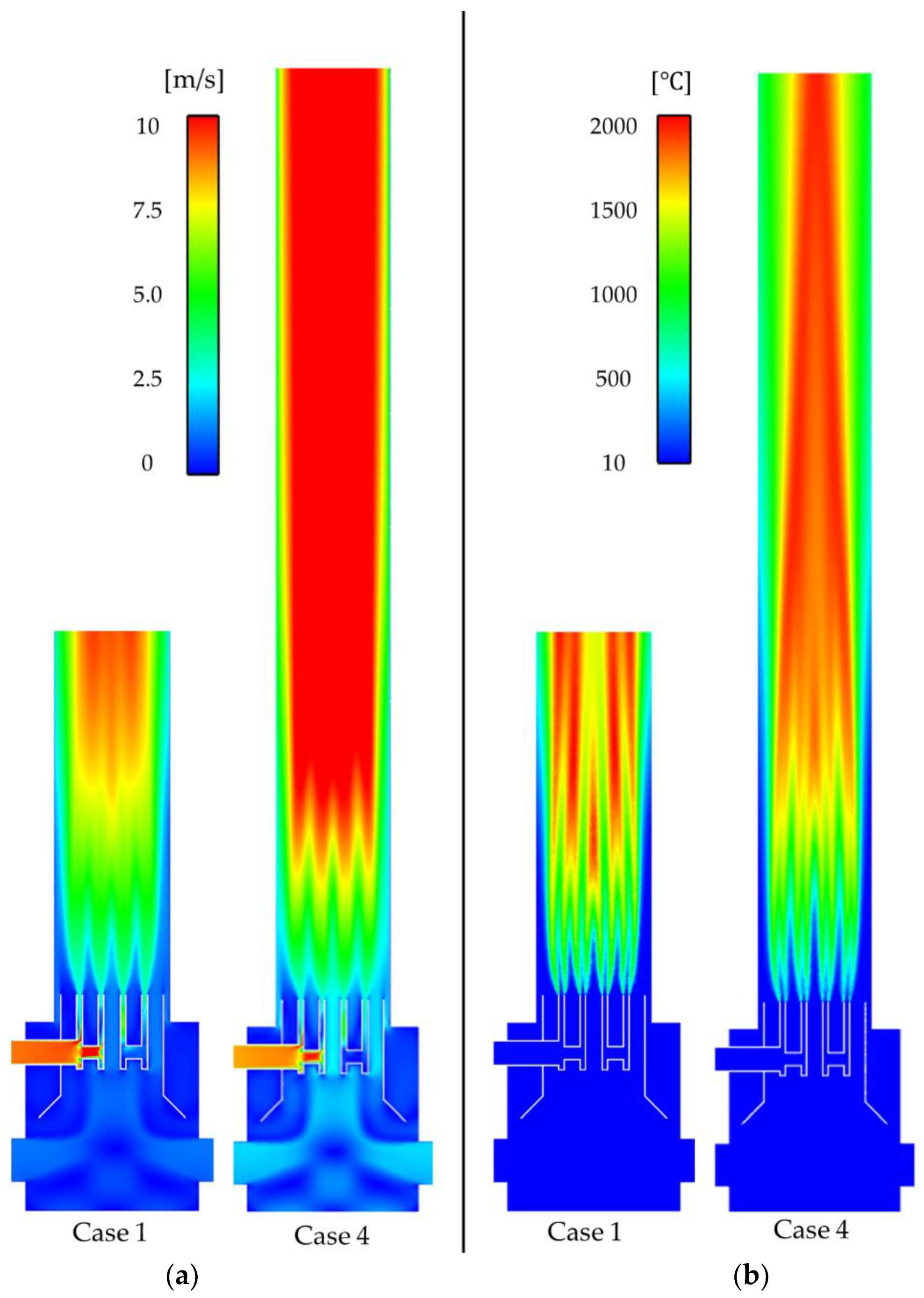
3.1. Effect of a Flame Arrestor
3.2. Effect of Combustor Design Parameters on the Combustion Performance
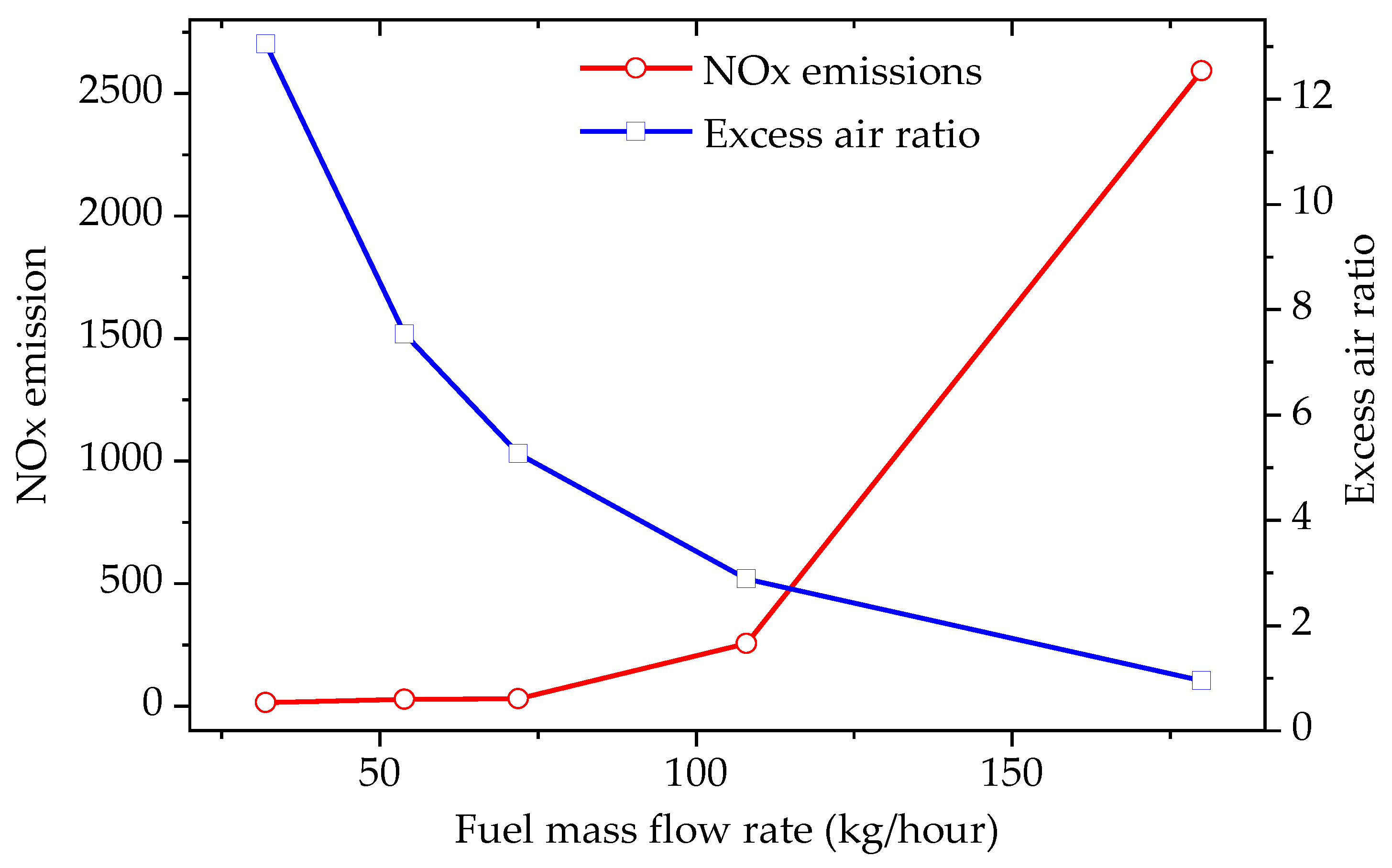
3.3. Effect of the Fuel Mass Flow Rate (FMR) on Combustion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | 3D | 3-dimensional | |
| EDM | Eddy dissipation model | ||
| FMR | Fuel mass flow rate (kg/h) | ||
| g | Gravitational acceleration (m/s2) | ||
| IDF | Inverse diffusion flame | ||
| NDF | Normal diffusion flame | ||
| SST | Shear stress transport | Velocity (m/s) | |
| t | Time (s) | Turbulent viscosity (Pa·s) | |
| VOCs | Volatile organic compounds | Specific dissipation (1/s) | |
| 1D | 1-dimensional | Direction component (m) | |
| V | Magnitude velocity (m/s) | Viscosity (Pa.) | |
| Pressure drop (Pa) | k | ||
| Length of porous media (m) | Stress tensor (N) | ||
| P | Pressure (Pa) | Velocity vector (m/s) | |
| H | Energy flux of species j (W) | ||
| E | |||
References
- Muir, B.; Sobczyk, M.; Bajda, T. Fundamental features of mesoporous functional materials influencing the efficiency of removal of VOCs from aqueous system. Sci. Total Environ. 2021, 784, 147121. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Chandramohan, V.P. Effect of chimney height and collector roof angle on flow parameters of solar updraft tower (SUT) plant. J. Therm. Anal. Calorim. 2019, 136, 133–145. [Google Scholar] [CrossRef]
- Pundle, A.; Sullivan, B.; Means, P.; Posner, J.D.; Kramlich, J.C. Predicting and analyzing the performance of biomass-burning natural draft rocket cookstoves using computational fluid dynamics. Biomass Bioenergy 2019, 131, 105402. [Google Scholar] [CrossRef]
- Lee, C.C.; Tran, M.V.; Nurmukan, D.; Tan, B.T.; Chong, C.T.; Scribano, G. Investigation of flame and stabilization characteristics of palm-methyl-esters diffusion flames. Fuel 2022, 313, 123034. [Google Scholar] [CrossRef]
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity evaluation and emission characteristics of poultry litter co-combustion process. Appl. Sci. 2019, 9, 4416. [Google Scholar] [CrossRef] [Green Version]
- Al-Arkawazi, S.A.F. Analyzing and predicting the relation between air–fuel ratio (AFR), lambda (λ) and the exhaust emissions percentages and values of gasoline-fueled vehicles using versatile and portable emissions measurement system tool. SN Appl. Sci. 2019, 1, 1370. [Google Scholar] [CrossRef] [Green Version]
- Kassem, H.I.; Sqar, K.M.; Aly, H.S.; Sies, M.M.; Wahid, M.A. Implementation of the eddy dissipation model of turbulent non-premixed combustion in OpenFoam. Int. Commun. Heat Mass Transf. 2011, 38, 363–367. [Google Scholar] [CrossRef]
- Murugan, P.C.; Prasad, G.A.; Sekhar, S.J. Implementation of species-transport model in numerical simulation to predict the performance of downdraft gasifier with biomass blends. Energy Res. J. 2019, 10, 36–47. [Google Scholar]
- Brookes, S.J.; Moss, B.J. Measurements of soot and thermal radiation from confined turbulent jet diffusion flames of methane. Combust. Flame 1999, 116, 49–61. [Google Scholar] [CrossRef]
- Yilmaz, H.; Cam, O.; Tangoz, O.; Yimaz, I. Effect of different turbulence models on combustion and emission characteristics of hydrogen/air flames. Int. J. Hydrog. Energy 2017, 42, 25744–25755. [Google Scholar] [CrossRef]
- Jiaqiang, E. Numerical investigation on hydrogen/air non-premixed combustion in a three-dimensional micro combustor. Energy Convers. Manag. 2016, 134, 427–438. [Google Scholar]
- Halouane, H.; Dehbi, A. CFD simulations of premixed hydrogen combustion using the Eddy Dissipation and the Turbulent Flame Closure models. Int. J. Hydrogen Energy 2017, 42, 21990–22004. [Google Scholar] [CrossRef]
- Yilmaz, H.; Kareyen, S.; Tepe, A.U.; Bruggemann, D. Colorless distributed combustion characteristics of hydrogen/air mixtures in a micro combustor. Fuel 2022, 332, 126163. [Google Scholar] [CrossRef]
- Zhen, H.S.; Wei, Z.L.; Liu, Z.H.; Wang, X.C.; Huang, Z.H.; Leung, C.W. A state-of-the-art review of lab-scale inverse diffusion burners & flames: Form laminar to turbulent. Fuel Process. Technol. 2021, 222, 106940. [Google Scholar]
- Artemov, V.; Beale, S.B.; de Vahl Davis, G.; Escudier, M.P.; Fueyo, N.; Launder, B.E.; Leonardi, E.; Malin, M.R.; Minkowycz, W.J.; Patankar, S.V.; et al. A tribute to D.B. Spalding and his contributions in science and engineering. Int. J. Heat Mass Transf. 2009, 52, 3884–3905. [Google Scholar] [CrossRef] [Green Version]
- Farah, H.D. Numerical Study of Detonation Flame Arrestor Performance and Detonation Interaction with the Arrestor Element. Ph.D. Thesis, The University of Texas at Arlington, Arlington, TX, USA, 2019. [Google Scholar]
- Menter, F.R. Two-equation eddy-viscosity turbulence models for engineering applications. AIAA J. 1994, 32, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Burtt, D.C.; Corbin, D.J.; Armitage, J.R.; Crosland, B.M.; Jefferson, A.M.; Kopp, G.A.; Kostiuk, L.W.; Johnson, M.R. A methodology for quantifying combustion efficiencies and species emission rates of flares subjected to crosswind. J. Energy Inst. 2022, 104, 124–132. [Google Scholar] [CrossRef]










| Design Model | ||
|---|---|---|
| M1 | 2.0 | |
| M2 | 2.0 | |
| M3 | 4.0 |
| Constants | |||||
|---|---|---|---|---|---|
| Values | 1.0 | 1.3 | 1.44 | 1.92 | 0.09 |
| Fuel Velocity (m/s) | (K) | Oxidizer Velocity (m/s) | Fraction O2 | (K) |
|---|---|---|---|---|
| 20 | 290 | 0.5 | 0.23 | 300 |
| Case | Design Model | Fuel Inlet | Air Inlet |
|---|---|---|---|
| Fuel (CO) Mass Flow Rate (kg/h) | Pressure (Pa), Air Mass Flow Rate (kg/h) | ||
| 1 | M1 | ||
| 2 | M2 | ||
| 3 | M1 | (by a fan) | |
| 4 | M3 | ||
| 5 | M3 | ||
| 6 | M3 | ||
| 7 | M3 |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Fuel mass flow rate, (kg/h) | 180 | 180 | 180 |
| Air mass flow rate, (kg/h) | 103 | 106 | 720 |
| Combustion efficiency, (%) | 63 | 64 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Q.H.; Lee, S.-W. Effect of the Design Parameters of the Combustion Chamber on the Efficiency of a Thermal Oxidizer. Energies 2023, 16, 170. https://doi.org/10.3390/en16010170
Cao QH, Lee S-W. Effect of the Design Parameters of the Combustion Chamber on the Efficiency of a Thermal Oxidizer. Energies. 2023; 16(1):170. https://doi.org/10.3390/en16010170
Chicago/Turabian StyleCao, Quang Hat, and Sang-Wook Lee. 2023. "Effect of the Design Parameters of the Combustion Chamber on the Efficiency of a Thermal Oxidizer" Energies 16, no. 1: 170. https://doi.org/10.3390/en16010170




