Hybrid Anionic Electrolytes for the High Performance of Aqueous Zinc-Ion Hybrid Supercapacitors
Abstract
:1. Introduction
2. Methods and Methods
2.1. Preparation of AZHS Electrodes
2.2. Formulation of AZHS Electrolytes
2.3. Material Characterization
2.4. Electrochemical Performance Testing of AZHS Electrodes
3. Results and Discussion
3.1. Comparison of the Performances of Different Electrolytes
3.2. Characterization Testing of Zinc Anodes
3.3. AC Characterization Test of AC Cathode
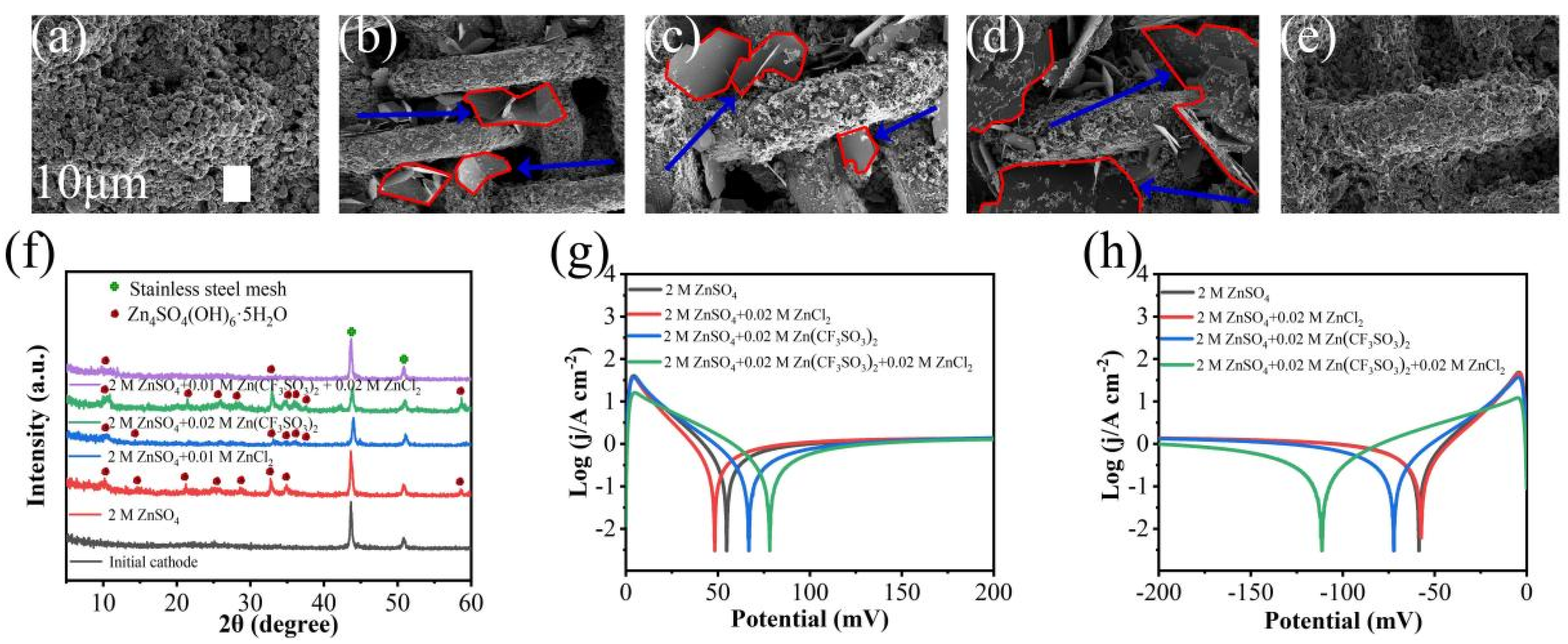
3.4. The 2 M ZnSO4 Electrolyte Doped with ZnCl2
3.5. The 2 M ZnSO4 Electrolyte Doped with Zn(CF3SO3)2
3.6. The 2 M ZnSO4 Electrolyte co-Doped with ZnCl2 and Zn(CF3SO3)2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.G.; Zhang, M.F.; Zou, P.M.; Sun, B.Y.; Tao, S.W. Historical development and novel concepts on electrolytes for aqueous rechargeable batteries. Energy Environ. Sci. 2022, 15, 1805–1839. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.-K.; Zheng, Y.-M.; Zhao, Q.-B.; Yu, H.-Q. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Fu, M.; Lv, R.; Lei, Y.; Terrones, M. Ultralight Flexible Electrodes of Nitrogen-Doped Carbon Macrotube Sponges for High-Performance Supercapacitors. Small 2021, 17, 2004827. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cao, Z.; Wang, S.; Sun, L.; Zhou, L.; Xue, H.; Ming, J. Bio-inspired heteroatom-doped hollow aurilave-like structured carbon for high-performance sodium-ion batteries and supercapacitors. J. Power Sources 2020, 461, 228128. [Google Scholar] [CrossRef]
- Xu, X.L.; Chen, Y.; Zheng, D.; Ruan, P.C.; Cai, Y.H.; Dai, X.J.; Cao, X.H. Ultra-Fast and Scalable Saline Immersion Strategy Enabling Uniform Zn Nucleation and Deposition for High-Performance Zn-Ion Batteries. Small 2021, 17, e2101901. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.Y.; Tang, Y.G.; Ji, X.B.; Wang, H.Y. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Edit. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Getie, F.A.; Ayele, D.W.; Habtu, N.G.; Yihun, F.A.; Yemata, T.A. Development of electrolytes for rechargeable zinc-air batteries: Current progress, challenges, and future outlooks. SN Appl. Sci. 2022, 4, 270. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Li, Z.W.; Bai, Z.Y.; Mi, H.Y.; Ji, C.C.; Pang, H.; Qiu, J.S. High energy-power Zn-ion hybrid supercapacitors enabled by layered B/N co-doped carbon cathode. Nano Energy 2019, 66, 104132. [Google Scholar] [CrossRef]
- Tian, Y.H.; Amal, R.; Wang, D.W. An Aqueous Metali-ion Capacitor with Oxidized Carbon Nanotubes and Metalicn Zinc Electrodes. Front. Energy Res. 2016, 4, 34. [Google Scholar] [CrossRef]
- Sun, G.; Yang, H.; Zhang, G.; Gao, J.; Jin, X.; Zhao, Y.; Qu, L. A capacity recoverable zinc-ion micro-supercapacitor. Energy Environ. Sci. 2018, 11, 3367–3374. [Google Scholar] [CrossRef]
- Wu, S.L.; Chen, Y.T.; Jiao, T.P.; Zhou, J.; Cheng, J.Y.; Liu, B.; Zhang, W.J. An Aqueous Zn-Ion Hybrid Supercapacitor with High Energy Density and Ultrastability up to 80000 Cycles. Adv. Energy Mater. 2019, 9, 1902915. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y. A novel zinc-ion hybrid supercapacitor for long-life and low-cost energy storage applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Zhang, K.; Kamruzzaman, M.; Zhi, C.; Zapien, J.A. A flexible solid-state zinc ion hybrid supercapacitor based on co-polymer derived hollow carbon spheres. J. Mater. Chem. A 2019, 7, 7784–7790. [Google Scholar] [CrossRef]
- Dong, L.B.; Ma, X.P.; Li, Y.; Zhao, L.; Liu, W.B.; Cheng, J.Y.; Kang, F.Y. Extremely safe, high-rate and ultralong-life zinc-ion hybrid supercapacitors. Energy Storage Mater. 2018, 13, 96–102. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Chai, Y.H.; Ismail, I.S.; Othman, M.F.H.; Yusup, S. Biomass as activated carbon precursor and potential in supercapacitor applications. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Zhang, T.S.; Tang, Y.; Guo, S.; Cao, X.X.; Pan, A.Q.; Fang, G.Z.; Liang, S.Q. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Gong, X.; Chen, J.; Lee, P.S. Zinc-Ion Hybrid Supercapacitors: Progress and Future Perspective. Batter. Supercaps 2021, 4, 1529–1546. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.L.; Dai, X.; Wang, X.Y.; Niu, Z.Q.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.L.; Shao, Y.Y.; Yan, P.F.; Cheng, Y.W.; Han, K.S.; Nie, Z.M.; Liu, J. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Shen, Y.H.; Liu, B.; Liu, X.R.; Liu, J.; Ding, J.; Zhong, C.; Hu, W.B. Water-in-salt electrolyte for safe and high-energy aqueous battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Wang, G.Q.; Liu, J.Q.; Dong, W.N.; Yan, C.; Zhang, W. Nitrogen/sulfur co-doped porous carbon nanosheets and its electrochemical performance. Acta Phys. Sin. 2018, 67. [Google Scholar]
- Zhang, L.; Liu, Z.; Wang, G.; Feng, J.; Ma, Q. Developing high voltage Zn(TFSI)2/Pyr14TFSI/AN hybrid electrolyte for a carbon-based Zn-ion hybrid capacitor. Nanoscale 2021, 13, 17068–17076. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, L.; Feng, Y.; Cao, M.; Zhang, P.; Zhang, X.-F.; Yao, J. Zinc ion trapping in a cellulose hydrogel as a solid electrolyte for a safe and flexible supercapacitor. J. Mater. Chem. 2020, 8, 12314–12318. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Jiang, D.; Wu, S.; Yi, W.; Sun, X.; Li, J. Organohydrogel electrolyte-based flexible zinc-ion hybrid supercapacitors with dendrite-free anode, broad temperature adaptability and ultralong cycling life. J. Power Sources 2022, 528, 231210. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, H.L.; Wang, S.J.; Zhao, X.; Kong, F.G. Regulatory pore structure of biomass-based carbon for supercapacitor applications. Microporous Mesoporous Mater. 2020, 297, 110032. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, T.; Zhao, M.; Geng, S.; Ivey, D.G. Ethylene Glycol as an Antifreeze Additive and Corrosion Inhibitor for Aqueous Zinc-Ion Batteries. Batter. Supercaps 2022, 5, e202100420. [Google Scholar]
- Chen, M.F.; Chen, J.Z.; Zhou, W.J.; Han, X.; Yao, Y.G.; Wong, C.P. Realizing an All-Round Hydrogel Electrolyte toward Environmentally Adaptive Dendrite-Free Aqueous Zn-MnO2 Batteries. Adv. Mater. 2021, 33, 2007559. [Google Scholar] [CrossRef]
- Tie, Z.W.; Liu, L.J.; Deng, S.Z.; Zhao, D.B.; Niu, Z.Q. Proton Insertion Chemistry of a Zinc-Organic Battery. Angew. Chem. Int. Edit. 2020, 59, 4920–4924. [Google Scholar] [CrossRef]
- Huang, J.H.; Wang, Z.; Hou, M.Y.; Dong, X.L.; Liu, Y.; Wang, Y.G.; Xia, Y.Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef] [Green Version]
- Biesheuvel, P.M.; Bazant, M.Z. Nonlinear dynamics of capacitive charging and desalination by porous electrodes. Phys. Rev. E 2010, 81, 031502. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, B.; He, J.; Sun, Y.; Li, S.; Li, J. Hybrid Anionic Electrolytes for the High Performance of Aqueous Zinc-Ion Hybrid Supercapacitors. Energies 2023, 16, 248. https://doi.org/10.3390/en16010248
Xie B, He J, Sun Y, Li S, Li J. Hybrid Anionic Electrolytes for the High Performance of Aqueous Zinc-Ion Hybrid Supercapacitors. Energies. 2023; 16(1):248. https://doi.org/10.3390/en16010248
Chicago/Turabian StyleXie, Bin, Junjie He, Yuchen Sun, Senlin Li, and Jing Li. 2023. "Hybrid Anionic Electrolytes for the High Performance of Aqueous Zinc-Ion Hybrid Supercapacitors" Energies 16, no. 1: 248. https://doi.org/10.3390/en16010248





