Promising Hydrate Formation Promoters Based on Sodium Sulfosuccinates of Polyols
Abstract
:1. Introduction
2. Materials and Methods
3. Results
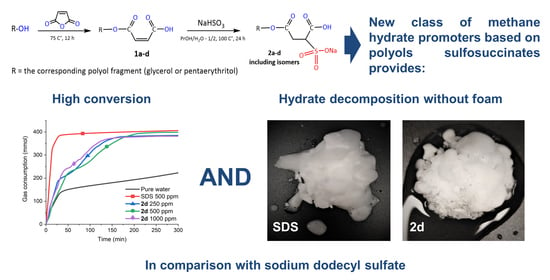
3.1. Evaluation of Hydrate Promoting Effect
3.2. Foam Formation
4. Discussion
4.1. Evaluation of Hydrate Promoting Effect
4.2. Foam Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EIA. Carbon Dioxide Emissions Coefficients [EB/OL]. Available online: https://www.eia.gov/environment/emissions/state/analysis/ (accessed on 2 November 2022).
- China Petroleum Pipeline Engineering. An Overview of the World Pipeline, 1st ed.; Petroleum Industry Press: Beijing, China, 2004; pp. 1–302. [Google Scholar]
- Khan, M.I.; Yasmin, T.; Shakoor, A. Technical overview of compressed natural gas (CNG) as a transportation fuel. Renew. Sustain. Energy Rev. 2015, 51, 785–797. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Wang, X.; Economides, M. Liquefied Natural Gas (LNG). In Natural Gas Processing; Gulf Professional Publishing Company: Houston, TX, USA, 2010; pp. 59–113. [Google Scholar]
- Wu, Z.J.; Wee, V.; Ma, X.B.; Zhao, D. Adsorbed Natural Gas Storage for Onboard Applications. Adv. Sustain. Syst. 2021, 5, 2000200. [Google Scholar] [CrossRef]
- Li, B.; Li, X.S.; Li, G.; Feng, J.C.; Wang, Y. Depressurization induced gas production from hydrate deposits with low gas saturation in a pilot-scale hydrate simulator. Appl. Energy 2014, 129, 274–286. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–752. [Google Scholar]
- Sloan, D.; Koh, C.A.; Sum, A.K.; Ballard, A.L.; McMullen, N.D.; Shoup, G.; Ballard, A.L.; Palermo, T. Chapter Eight—Conclusion. In Natural Gas Hydrates in Flow Assurance; Sloan, E.D., Ed.; Gulf Professional Publishing Company: Houston, TX, USA, 2010; pp. 163–170. [Google Scholar]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy–Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, D.L.; Li, Z.; Li, J.B. Application of tetra-n-butyl ammonium bromide semi-clathrate hydrate for CO2 capture from unconventional natural gases. Energy 2020, 197, 117209. [Google Scholar] [CrossRef]
- Kiyokawa, H.; Horiia, S.; Alavib, S.; Ohmura, R. Improvement of continuous hydrate-based CO2 separation by forming structure II hydrate in the system of H2 + CO2 + H2O + Tetrahydropyran (THP). Fuel 2020, 278, 118330. [Google Scholar] [CrossRef]
- Xie, Y.M.; Li, G.; Liu, D.; Liu, N.; Qi, Y.X.; Liang, D.Q.; Guo, K.H.; Fan, S.S. Experimental study on a small scale of gas hydrate cold storage apparatus. Appl. Energy 2010, 87, 3340–3346. [Google Scholar] [CrossRef]
- Chaturvedi, E.; Prasad, N.; Mandal, A. Enhanced formation of methane hydrate using a novel synthesized anionic surfactant for application in storage and transportation of natural gas. J. Nat. Gas Sci. Eng. 2018, 56, 246–257. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Atilhan, M.; Altamash, T.; Aparicio, S.; Aminnaji, M.; Tohidi, B. High-pressure gas hydrate autoclave hydraulic experiments and scale-up modeling on the effect of stirring RPM effect. J. Nat. Gas Sci. Eng. 2017, 38, 50–58. [Google Scholar] [CrossRef]
- Partoon, B.; Sabil, K.M.; Lau, K.K.; Lal, B.; Nasrifar, K. Production of gas hydrate in a semi-batch spray reactor process as a means for separation of carbon dioxide from methane. Chem. Eng. Res. Des. 2018, 138, 168–175. [Google Scholar] [CrossRef]
- Wang, W.X.; Bray, C.L.; Adams, D.J.; Cooper, A.I. Methane Storage in Dry Water Gas Hydrates. J. Am. Chem. Soc. 2008, 130, 11608–11609. [Google Scholar] [CrossRef] [PubMed]
- Benmesbah, F.D.; Ruffine, L.; Clain, P.; Osswald, V.; Fandino, O.; Fournaison, L.; Delahaye, A. Methane Hydrate Formation and Dissociation in Sand Media: Effect of Water Saturation, Gas Flowrate and Particle Size. Energies 2020, 13, 5200. [Google Scholar] [CrossRef]
- Zaripova, Y.; Yarkovoi, V.; Varfolomeev, M.; Kadyrov, R.; Stoporev, A. Influence of Water Saturation, Grain Size of Quartz Sand and Hydrate-Former on the Gas Hydrate Formation. Energies 2021, 14, 1272. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Sowjanya, Y.; Shiva Prasad, K. Micro-Raman investigations of mixed gas hydrates. Vib. Spectrosc. 2009, 50, 319–323. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, S.; Kumar, R.; Rangsunvigit, P.; Linga, P. Enhanced clathrate hydrate formation kinetics at near ambient temperatures and moderate pressures: Application to natural gas storage. Fuel 2016, 182, 907–919. [Google Scholar] [CrossRef]
- Perez-Lopez, H.I.; Elizalde-Solis, O.; Avendano-Gomez, J.R.; Zuniga-Moreno, A.; Sanchez-Minero, F. Methane Hydrate Formation and Dissociation in Synperonic PE/F127, CTAB, and SDS Surfactant Solutions. J. Chem. Eng. Data 2018, 63, 2477–2485. [Google Scholar] [CrossRef]
- Liu, C.C.; Chou, H.J.; Lin, C.Y.; Janmanchi, D.; Chung, P.W.; Mou, C.Y.; Yu, S.S.F.; Chan, S.I. The oversolubility of methane gas in nano-confined water in nanoporous silica materials. Microporous Mesoporous Mater. 2020, 293, 109793. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane Storage in a Hydrated Form as Promoted by Leucines for Possible Application to Natural Gas Transportation and Storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.; Abdelhay, Z.; Emelianov, D.; Delaunay, A.; Dalmazzone, D. Accelerated Methane Hydrate Formation by Ethylene Diamine Tetraacetamide As an Efficient Promoter for Methane Storage without Foam Formation. Ind. Eng. Chem. Res. 2019, 58, 7752–7760. [Google Scholar] [CrossRef]
- Pavelyev, R.S.; Gainullin, S.E.; Semenov, M.E.; Zaripova, Y.F.; Yarkovoi, V.V.; Luneva, A.I.; Farhadian, A.; Varfolomeev, M.A. Dual Promotion–Inhibition Effects of Novel Ethylenediaminetetraacetic Acid Bisamides on Methane Hydrate Formation for Gas Storage and Flow Assurance Applications. Energy Fuels 2021, 36, 290–297. [Google Scholar] [CrossRef]
- Farhadian, A.; Stoporev, A.S.; Varfolomeev, M.A.; Zaripova, Y.F.; Yarkovoi, V.V.; Semenov, M.E.; Kiiamov, A.G.; Pavelyev, R.S.; Aimaletdinov, A.M.; Mohammad, T. Sulfonated Castor Oil as an Efficient Biosurfactant for Improving Methane Storage in Clathrate Hydrates. ACS Sustain. Chem. Eng. 2022, 10, 9921–9932. [Google Scholar] [CrossRef]
- Pandey, G.; Bhattacharjee, G.; Veluswamy, H.P.; Kumar, R.; Sangwai, J.S.; Linga, P. Alleviation of foam formation in a surfactant driven gas hydrate system: Insights via a detailed morphological study. ACS Appl. Energy Mater. 2018, 1, 6899–6911. [Google Scholar] [CrossRef]
- Qin, J.; Kuhs, W.F. Quantitative analysis of gas hydrates using Raman spectroscopy. AIChE J. 2013, 59, 2155–2167. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P.D.; Bishnoi, P.R. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Farhadian, A.; Heydari, A.; Maddah, M.; Hosseini, M.; Sadeh, E.; Varaminian, F. Renewable biosurfactants for energy-efficient storage of methane: An experimental and computational investigation. Chem. Eng. J. 2021, 427, 131723. [Google Scholar] [CrossRef]





| Sample | Concentration (ppm) | Methane Hydrate Conversion (%) | Maximum Gas Consumption (mmol) | Kinetic Constant at 1/2 of the Process Time (K1/2) | Kinetic Constant at 2/3 of the Process Time (K2/3) |
|---|---|---|---|---|---|
| Water | - | 62 ± 5 | 285 ± 6 | 0.0024 | 0.0031 |
| SDS | 500 | 88 ± 6 | 406 ± 5 | 0.0493 | 0.0762 |
| 2a | 250 | 52 ± 2 | 241 ± 4 | 0.0056 | 0.0068 |
| 500 | 86 ± 4 | 398 ± 4 | 0.0048 | 0.0082 | |
| 1000 | 85 ± 5 | 392 ± 3 | 0.0032 | 0.0119 | |
| 2b | 250 | 79 ± 4 | 367 ± 4 | 0.0055 | 0.0079 |
| 500 | 84 ± 1 | 389 ± 3 | 0.0039 | 0.0051 | |
| 1000 | 81 ± 3 | 373 ± 3 | 0.0071 | 0.0119 | |
| 2c | 250 | 81 ± 5 | 373 ± 3 | 0.0091 | 0.0151 |
| 500 | 84 ± 3 | 387 ± 3 | 0.0048 | 0.0082 | |
| 1000 | 83 ± 3 | 384 ± 4 | 0.0068 | 0.0131 | |
| 2d | 250 | 83 ± 4 | 385 ± 3 | 0.0077 | 0.0128 |
| 500 | 86 ± 4 | 398 ± 2 | 0.0057 | 0.0093 | |
| 1000 | 82 ± 4 | 381 ± 2 | 0.0103 | 0.0143 |
| Sample | Concentration (ppm) | Foam Rate | Half-Life (s) |
|---|---|---|---|
| 2a | 500 | - | - |
| 2b | 500 | - | - |
| 2c | 500 | - | - |
| 2d | 500 | - | - |
| SDS | 500 | 3.7 ± 0.2 | 229 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirkova, Y.F.; Mirzakimov, U.Z.; Semenov, M.E.; Pavelyev, R.S.; Varfolomeev, M.A. Promising Hydrate Formation Promoters Based on Sodium Sulfosuccinates of Polyols. Energies 2023, 16, 359. https://doi.org/10.3390/en16010359
Chirkova YF, Mirzakimov UZ, Semenov ME, Pavelyev RS, Varfolomeev MA. Promising Hydrate Formation Promoters Based on Sodium Sulfosuccinates of Polyols. Energies. 2023; 16(1):359. https://doi.org/10.3390/en16010359
Chicago/Turabian StyleChirkova, Yulia F., Ulukbek Zh. Mirzakimov, Matvei E. Semenov, Roman S. Pavelyev, and Mikhail A. Varfolomeev. 2023. "Promising Hydrate Formation Promoters Based on Sodium Sulfosuccinates of Polyols" Energies 16, no. 1: 359. https://doi.org/10.3390/en16010359