Biomass and Coal Ash Sintering—Thermodynamic Equilibrium Modeling versus Pressure Drop Test and Mechanical Test
Abstract
:1. Introduction
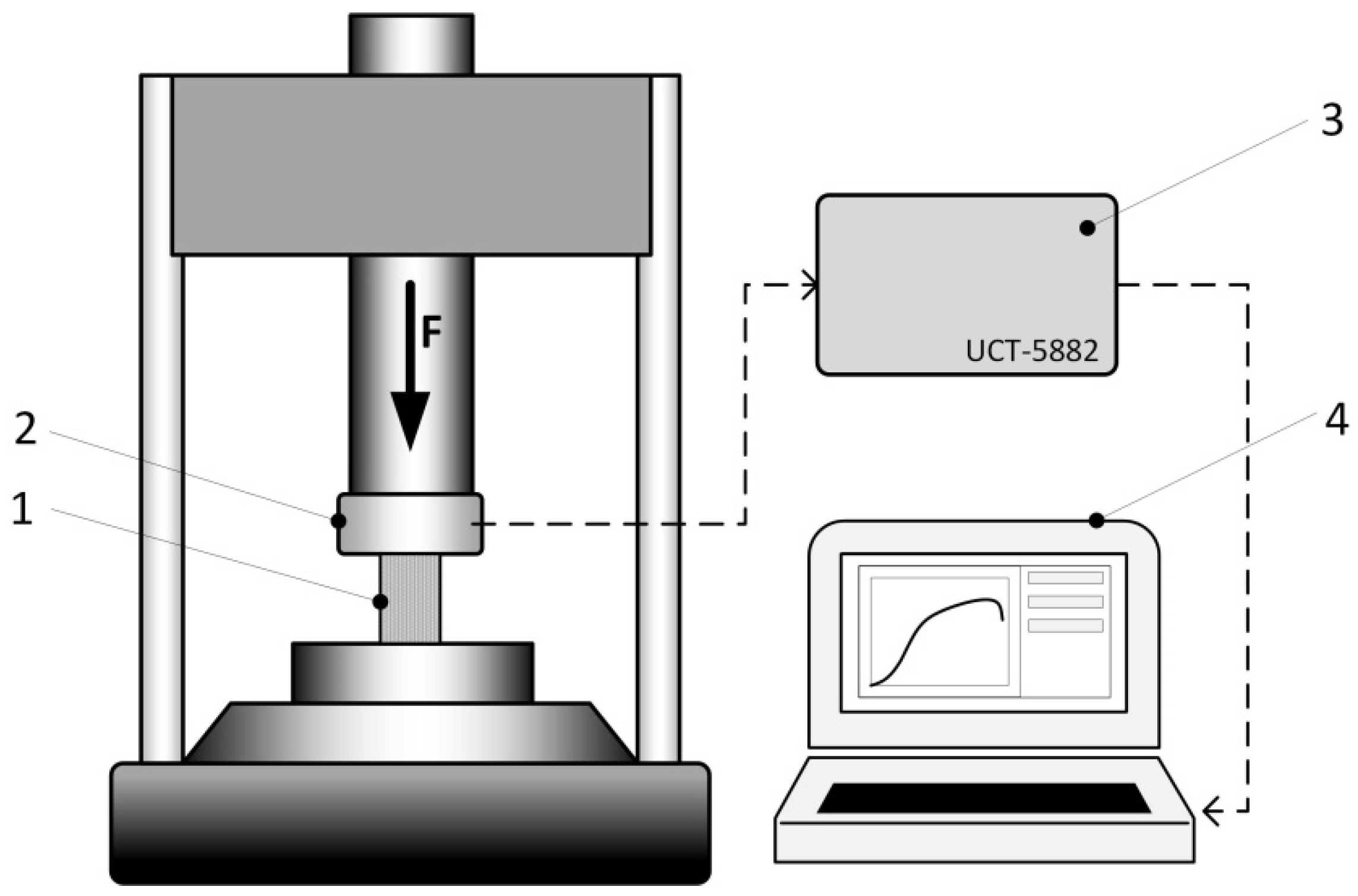
2. Materials and Methods
3. Results and Discussion
3.1. Samples Analsyis
3.2. FactSage Analysis versus Pressure Drop Test and Mechanical Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Regulation (EU) 2021/1119 of the European Parliament and of the Council of 30 June 2021 Establishing the Framework for Achieving Climate Neutrality and Amending Regulations (EC) No 401/2009 and (EU) 2018/1999 (‘European Climate Law’). Available online: http://data.europa.eu/eli/reg/2021/1119/oj (accessed on 25 November 2022).
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behavior of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Tchapda, A.H.; Pisupati, S.V. A Review of Thermal Co-Conversion of Coal and Biomass/Waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.; Matias, J.; Catalão, J. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Tan, H.Z.; Hụi, S.E. Ash related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization and related countermeasures. Prog. Energ. Combust. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Pophali, A.; Emami, B.; Bussmann, M.; Tran, H. Studies on sootblower jet dynamics and ash deposit removal in industrial boilers. Fuel Process. Technol. 2013, 105, 69–76. [Google Scholar] [CrossRef]
- Kotelnikova, A.; Rogova, O.; Karpukhina, E.; Solopov, A.; Levin, I.; Levkina, V.; Proskurnin, M.; Volkov, D. Assessment of the structure, composition, and agrochemical properties of fly ash and ash-and-slug waste from coal-fired power plants for their possible use as soil ameliorants. J. Clean. Prod. 2021, 333, 130088. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, J.; Hao, B.; Li, X.; Yan, M.; Liu, K. Feasible synthesis of coal fly ash based porous composites with multiscale pore structure and its application in Congo red adsorption. Chemosphere 2022, 298, 134136. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, J.; Wang, Z.; Ji, M.; Zhang, M. Effect of walnut shell ash on pore structure characteristics during Zhundong coal sintering. Fuel Process. Technol. 2021, 221, 106923. [Google Scholar] [CrossRef]
- Romdhane, L.; Ebinezer, L.B.; Panozzo, A.; Barion, G.; Cortivo, C.D.; Radhouane, L.; Vamerali, T. Effects of Soil Amendment With Wood Ash on Transpiration, Growth, and Metal Uptake in Two Contrasting Maize (Zea mays L.) Hybrids to Drought Tolerance. Front. Plant Sci. 2021, 12, 905. [Google Scholar] [CrossRef]
- Hariana; Prabowo; Hilmawan, E.; Kuswa, F.M.; Darmawan, A.; Aziz, M. A comprehensive evaluation of cofiring biomass with coal and slagging-fouling tendency in pulverized coal-fired boilers. Ain Shams Eng. J. 2022, 102001. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Elliott, L.K.; Wall, T.F.; Moghtaderi, B. Measurement of the Sintering Kinetics of Coal Ash. Energy Fuels 2000, 14, 994–1001. [Google Scholar] [CrossRef]
- Jing, N.; Wang, Q.; Luo, Z.; Cen, K. Effect of different reaction atmospheres on the sintering temperature of Jincheng coal ash under pressurized conditions. Fuel 2011, 90, 2645–2651. [Google Scholar] [CrossRef]
- Shi, W.; Bai, J.; Kong, L.; Li, H.; Bai, Z.; Vassilev, S.V.; Li, W. An overview of the coal ash transition process from solid to slag. Fuel 2020, 287, 119537. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J. Optimizing ash deposit removal system to maximize biomass recycling as renewable energy for CO2 reduction. Renew. Energy 2022, 190, 1006–1017. [Google Scholar] [CrossRef]
- Raask, E. Mineral Impurities in Coal Combustion: Behavior, Problems and Remedial Measures; Hemisphere Publishing Corporation: Washington, DC, USA, 1985; Chapter 10; pp. 137–160. [Google Scholar]
- Luan, C.; You, C.; Zhang, D. Composition and sintering characteristics of ashes from co–firing of coal and biomass in a la-boratory-scale drop tube furnace. Energy 2014, 69, 562–570. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Gądek, W.; Pronobis, M.; Kalisz, S. Investigation of ash deposition in PF boiler during combustion of torrefied biomass. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Krakow, Poland, 14–17 November 2017; Volume 214, p. 012080. [Google Scholar]
- Magdziarz, A.; Wilk, M.; Gajek, M.; Nowak-Woźny, D.; Kopia, A.; Kalemba-Rec, I.; Koziński, J. Properties of ash genarated during sewage sludge combustion: A multifaceded analysis. Energy 2016, 113, 85–94. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass-Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Pronobis, M. The influence of biomass co-combustion on boiler fouling and efficiency. Fuel 2006, 85, 474–480. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Kitano, K.; Takeda, S.; Tsurue, T. Influence of mineral and chemical composition of coal ashes on their fusibility. Fuel Process. Technol. 1995, 45, 27–51. [Google Scholar] [CrossRef]
- Polish Standard PN-ISO 540:2001 Solid Fuels—Determination of Ash Fusibility at High Temperature Using the Pipe Method. Available online: https://sklep.pkn.pl/pn-iso-540-2001p.html (accessed on 19 February 2021).
- Du, S.; Yang, H.; Qian, K.; Wang, X.; Chen, H. Fusion and transformation properties of the inorganic components in biomass ash. Fuel 2014, 117, 1281–1287. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Meng, A.; Li, L.; Li, G. Study on ash fusion temperature using original and simulated biomass ashes. Fuel Process. Technol. 2013, 107, 107–112. [Google Scholar] [CrossRef]
- Król, K.; Nowak-Woźny, D. Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash. Energies 2021, 14, 1126. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Bryant, G.W.; Elliott, L.K.; Skrifvars, B.J.; Hupa, M.; Wall, T.F. Experimental Options for Determining theTemperature for the Onset of Sintering of Coal Ash. Energy Fuels 2000, 14, 227–233. [Google Scholar] [CrossRef]
- Jung, B.; Schobert, H.H. Viscous sintering of coal ashes. 2. Sintering behavior at short residence times in a drop tube furnace. Energy Fuels 1992, 6, 59–68. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behavior of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 393–449. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash formation and deposition in coal and biomass fired combustion systems: Progress and challenges in the field of ash particle sticking and rebound behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Sefidari, H.; Lindblom, B.; Wiinikka, H.; Nordin, L.-O.; Mouzon, J.; Bhuiyan, I.U.; Öhman, M. The effect of disintegrated iron-ore pellet dust on deposit formation in a pilot-scale pulverized coal combustion furnace. Part I: Characterization of process gas particles and deposits. Fuel Process. Technol. 2018, 177, 283–298. [Google Scholar] [CrossRef]
- Sefidari, H.; Lindblom, B.; Wiinikka, H.; Nordin, L.-O.; Lennartsson, A.; Mouzon, J.; Bhuiyan, I.U.; Öhman, M. The effect of disintegrated iron-ore pellet dust on deposit formation in a pilot-scale pulverized coal combustion furnace. Part II: Thermochemical equilibrium calculations and viscosity estimations. Fuel Process. Technol. 2018, 180, 189–206. [Google Scholar] [CrossRef]
- Sefidari, H.; Wiinikka, H.; Lindblom, B.; Nordin, L.; Wu, G.; Yazhenskikh, E.; Müller, M.; Ma, C.; Öhman, M. Comparison of high-rank coals with respect to slagging/deposition tendency at the transfer-chute of iron-ore pelletizing grate-kiln plants: A pilot-scale experimental study accompanied by thermochemical equilibrium modeling and viscosity estimations. Fuel Process. Technol. 2019, 193, 244–262. [Google Scholar] [CrossRef]
- Sefidari, H. Mechanisms of Deposit Formation in the Grate-Kiln Process. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2018. Available online: https://scholar.google.com/scholar_lookup?title=Mechanisms%20of%20Deposit%20Formation%20in%20the%20Grate-Kiln%20Process&publication_year=2018&author=H.%20Sefidari (accessed on 25 November 2022).
- Atallah, E.; Defoort, F.; Pisch, A.; Dupont, C. Thermodynamic equilibrium approach to predict the inorganic interactions of ash from biomass and their mixtures: A critical assessment. Fuel Process. Technol. 2022, 235, 107369. [Google Scholar] [CrossRef]
- Daley, P.; Reinmöller, M.; Williams, O.; Pang, C.H.; Lester, E. The influence of mineral addition on the Optimised Advanced Ash Fusion Test (OAAFT) and its thermochemical modelling and prediction. J. Energy Inst. 2022, 105, 121–132. [Google Scholar] [CrossRef]
- Magdziarz, A.; Gajek, M.; Nowak-Woźny, D.; Wilk, M. Mineral phase transformation of biomass ashes–Experimental and thermochemical calculations. Renew. Energy 2018, 128, 446–459. [Google Scholar] [CrossRef]
- Kaniowski, W.; Taler, J.; Wang, X.; Kalemba-Rec, I.; Gajek, M.; Mlonka-Mędrala, A.; Nowak-Woźny, D.; Magdziarz, A. Investigation of biomass, RDF and coal ash-related problems: Impact on metallic heat exchanger surfaces of boilers. Fuel 2022, 326, 125122. [Google Scholar] [CrossRef]
- Nowak-Woźny, D.; Ferens, W.; Wach, J. Using dissipation factor method in testing the ash sintering process of cereal pellet and coal fuels. Energy 2022, 250, 123718. [Google Scholar] [CrossRef]
- CEN/TS 14775:2004 Solid Biofuels-Method for the Determination of Ash Content. Available online: https://standards.iteh.ai/catalog/standards/sist/7a423bc5-65a2-40ae-90f7-319c45968a59/sist-ts-cen-ts-14775-2004 (accessed on 25 November 2022).
- Polish Standard PN-G-04502:2014-11; Hard and Brown Coals—Sampling and Preparation of Samples for the Laboratory Tests—Primary Methods. ISO: London, UK, 2014.
- Polish Standard PN-EN ISO 18134-1:2015-11; Solid Biofuels—Determination of Moisture Content—Oven Dry Meth-od—Part 1: Total Moisture—Reference Method. ISO: London, UK, 2015.
- Polish Standard PN-EN ISO 18123:2016-01; Solid Biofuels—Determination of the Content of Volatile Matter. ISO: London, UK, 2015.
- Polish Standard PN-EN ISO 18122:2016-01; Solid Biofuels—Determination of Ash Content. ISO: London, UK, 2015.
- Polish Standard PN-ISO 1928:2020-05; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calo-rimetric Method and Calculation of Net Calorific Value. ISO: London, UK, 2020.
- Sato, R.; Kadoma, T.; Fujimoto, Y.; Ogata, N.; Yabuuchi, K.; Ninomiya, Y.; Horio, M. Clinker Formation Behavior in a Co-current Up-flowing Moving Bed Gasifier Fueled with Japanese Cedar Pellets. J. Jpn. Inst. Energy 2021, 100, 236–244. [Google Scholar] [CrossRef]
- Růžičková, J.; Raclavská, H.; Juchelková, D.; Šafář, M.; Kucbel, M.; Švédová, B.; Slamová, K.; Grobelak, A. The use of polymer compounds in the deposits from the combustion of briquettes in domestic heating as an identifier of fuel quality. Environ. Sci. Pollut. Res. 2021, 1–19. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, K.; Moroń, W.; Nowak-Woźny, D. Biomass and Coal Ash Sintering—Thermodynamic Equilibrium Modeling versus Pressure Drop Test and Mechanical Test. Energies 2023, 16, 362. https://doi.org/10.3390/en16010362
Król K, Moroń W, Nowak-Woźny D. Biomass and Coal Ash Sintering—Thermodynamic Equilibrium Modeling versus Pressure Drop Test and Mechanical Test. Energies. 2023; 16(1):362. https://doi.org/10.3390/en16010362
Chicago/Turabian StyleKról, Karol, Wojciech Moroń, and Dorota Nowak-Woźny. 2023. "Biomass and Coal Ash Sintering—Thermodynamic Equilibrium Modeling versus Pressure Drop Test and Mechanical Test" Energies 16, no. 1: 362. https://doi.org/10.3390/en16010362
APA StyleKról, K., Moroń, W., & Nowak-Woźny, D. (2023). Biomass and Coal Ash Sintering—Thermodynamic Equilibrium Modeling versus Pressure Drop Test and Mechanical Test. Energies, 16(1), 362. https://doi.org/10.3390/en16010362







