Innovative Experimental Design for the Evaluation of Nanofluid-Based Solvent as a Hybrid Technology for Optimizing Cyclic Steam Stimulation Applications
Abstract
1. Introduction
2. Methodology
2.1. Nanotechnology for EOR Thermal Process
- Reducing the decomposition temperature of asphaltenes so that the aquathermolysis reaction can occur;
- Less effective activation energy is necessary for the reaction;
- Decomposition of large hydrocarbon chains into lighter fractions with lower molecular weight implies a reduction in viscosity and improvement in mobility in the production of extra-heavy oils.
2.2. Aquathermolysis in Cyclic Steam Injection Processes
- Decrease in the content of the heavy fraction in the crude oil matrix;
- Increase in the H/C ratio;
- Improvement of oil quality;
- Decrease in viscosity.
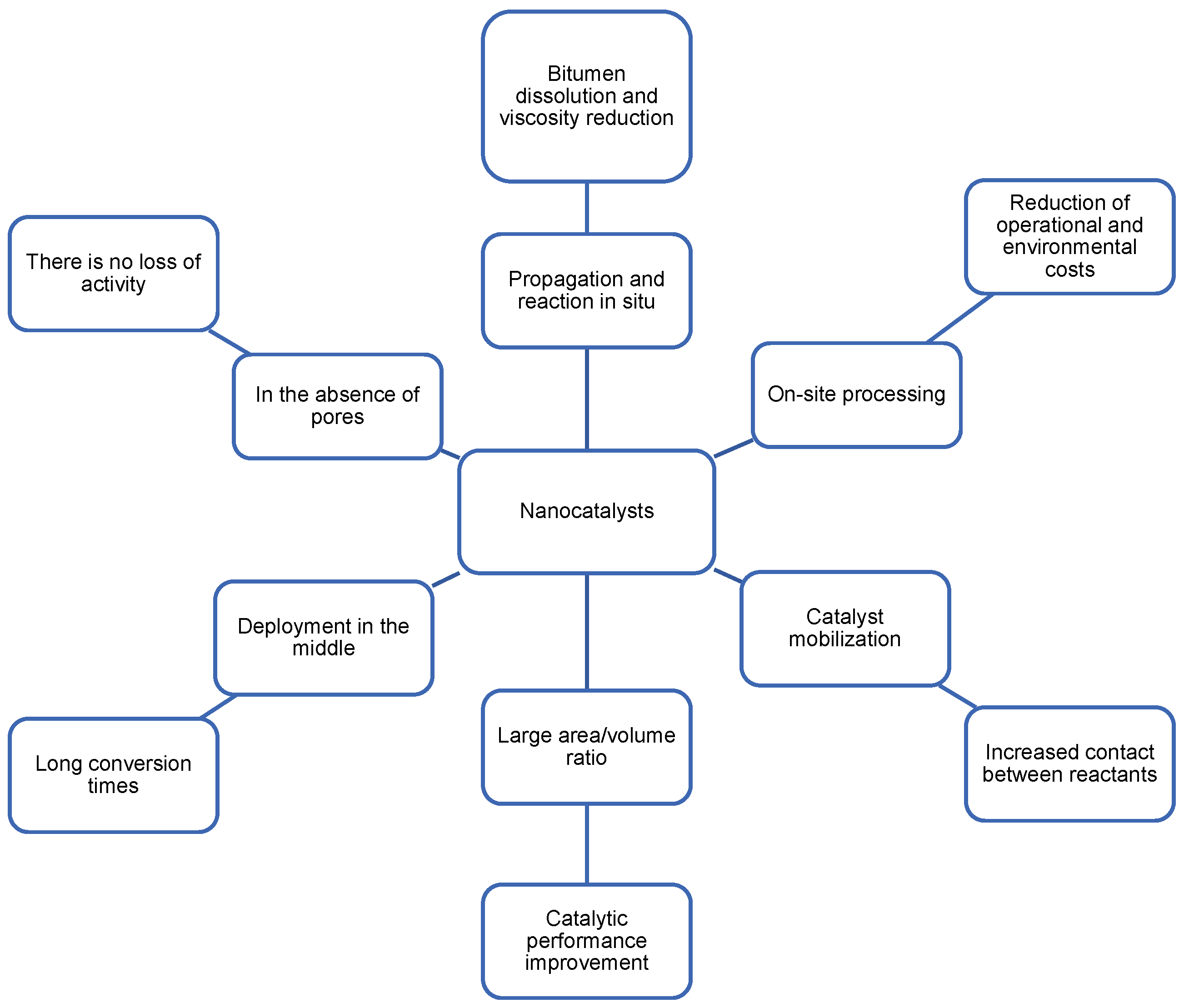
2.3. Nanocatalizers for Cyclic Steam Injection
2.4. Factors That Affect Nanocatalysts in EOR Processes
2.4.1. Type, Size, and Concentration of Nanoparticles
2.4.2. Heat Transfer
2.4.3. Crude Oil Composition
2.4.4. Porous Medium
2.4.5. Formation Damage Inhibition
2.5. Environmental Influences of Nanoparticles in Steam Injection Processes
2.5.1. Decrease in Heat Consumption
2.5.2. Sulfur Removal
2.5.3. Greenhouse Gases
3. Results
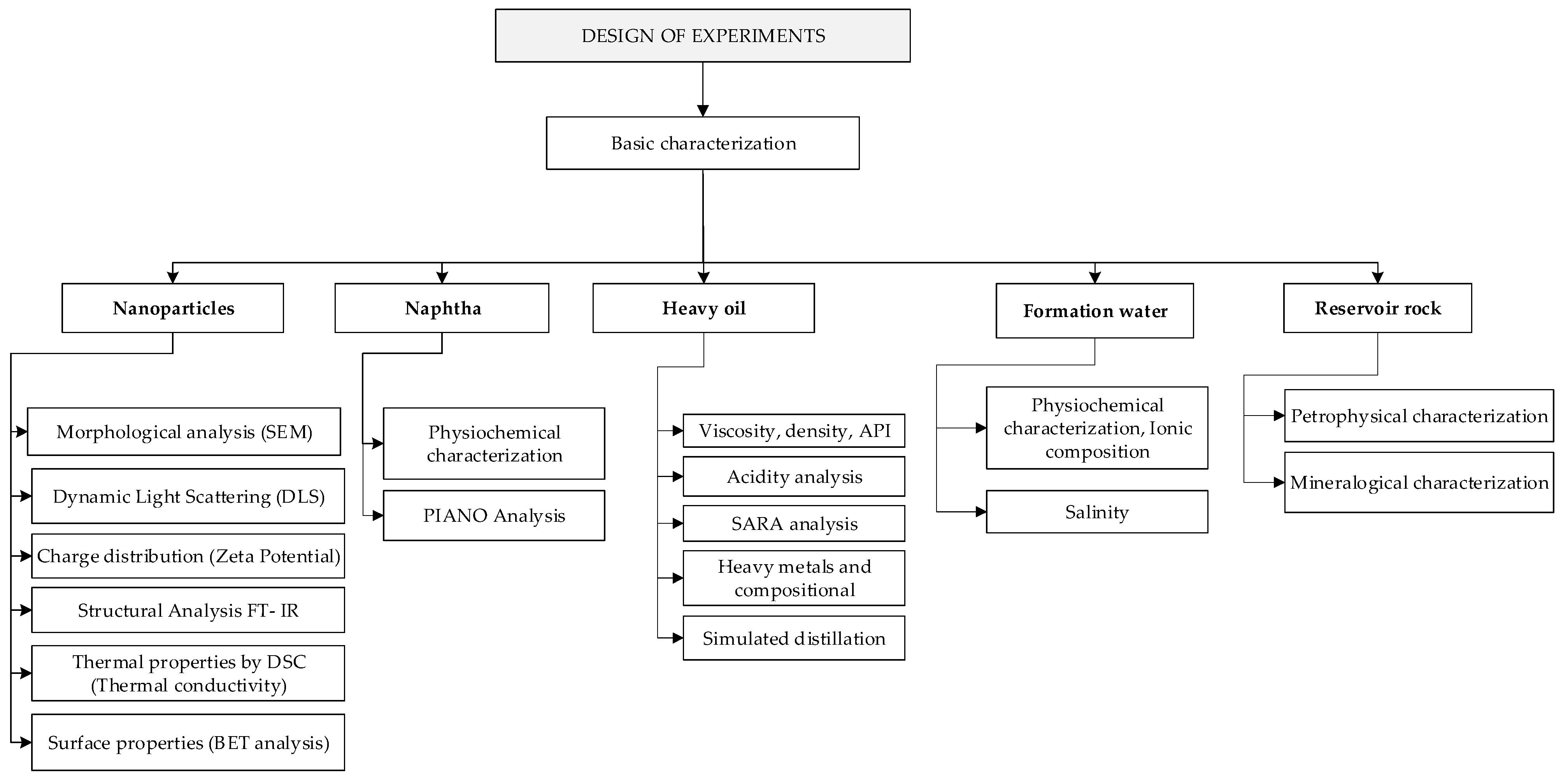
3.1. Basic Characterization
3.2. Naphtha Improved with Nanoparticles Formulation
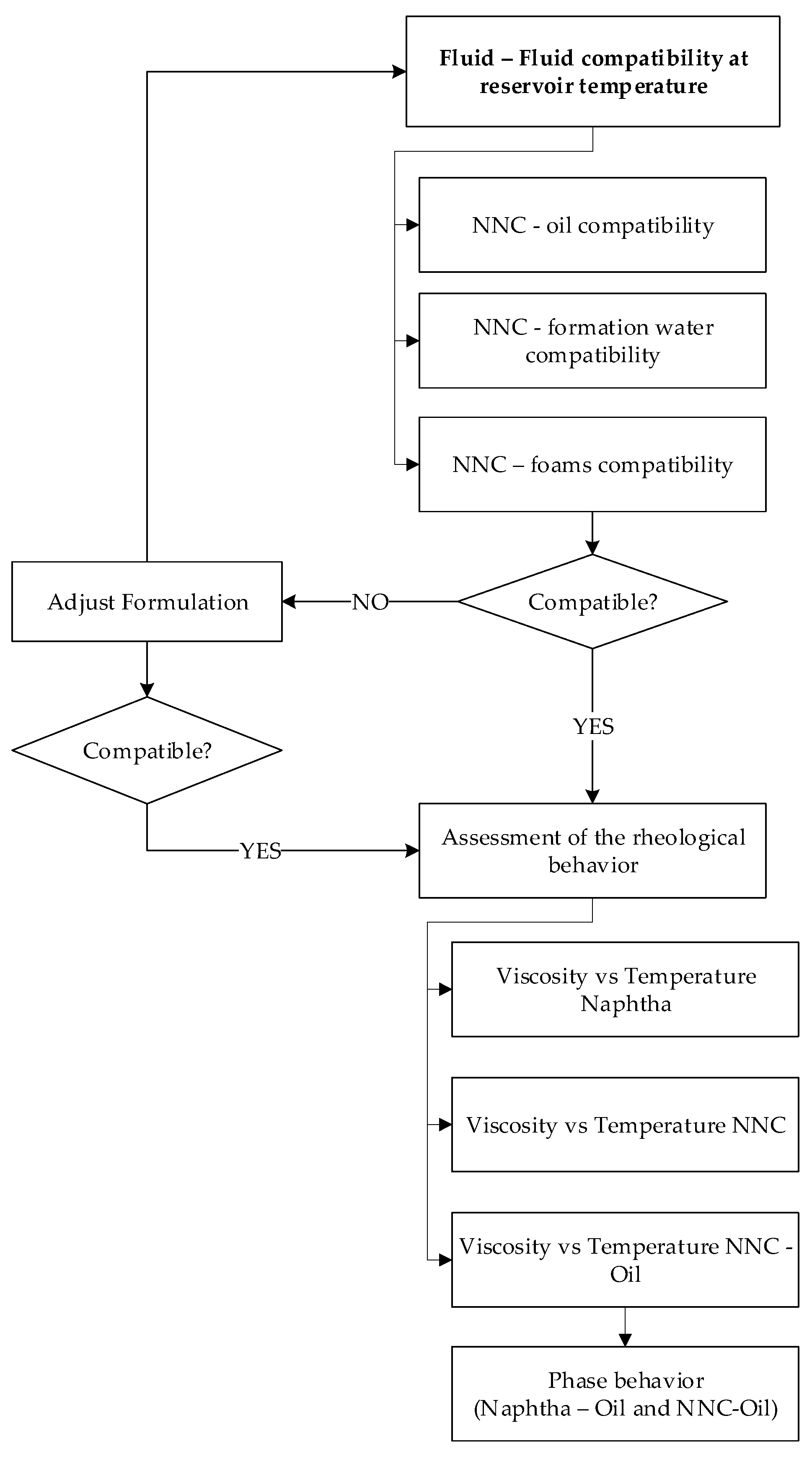
3.3. Fluid-Fluid Interaction
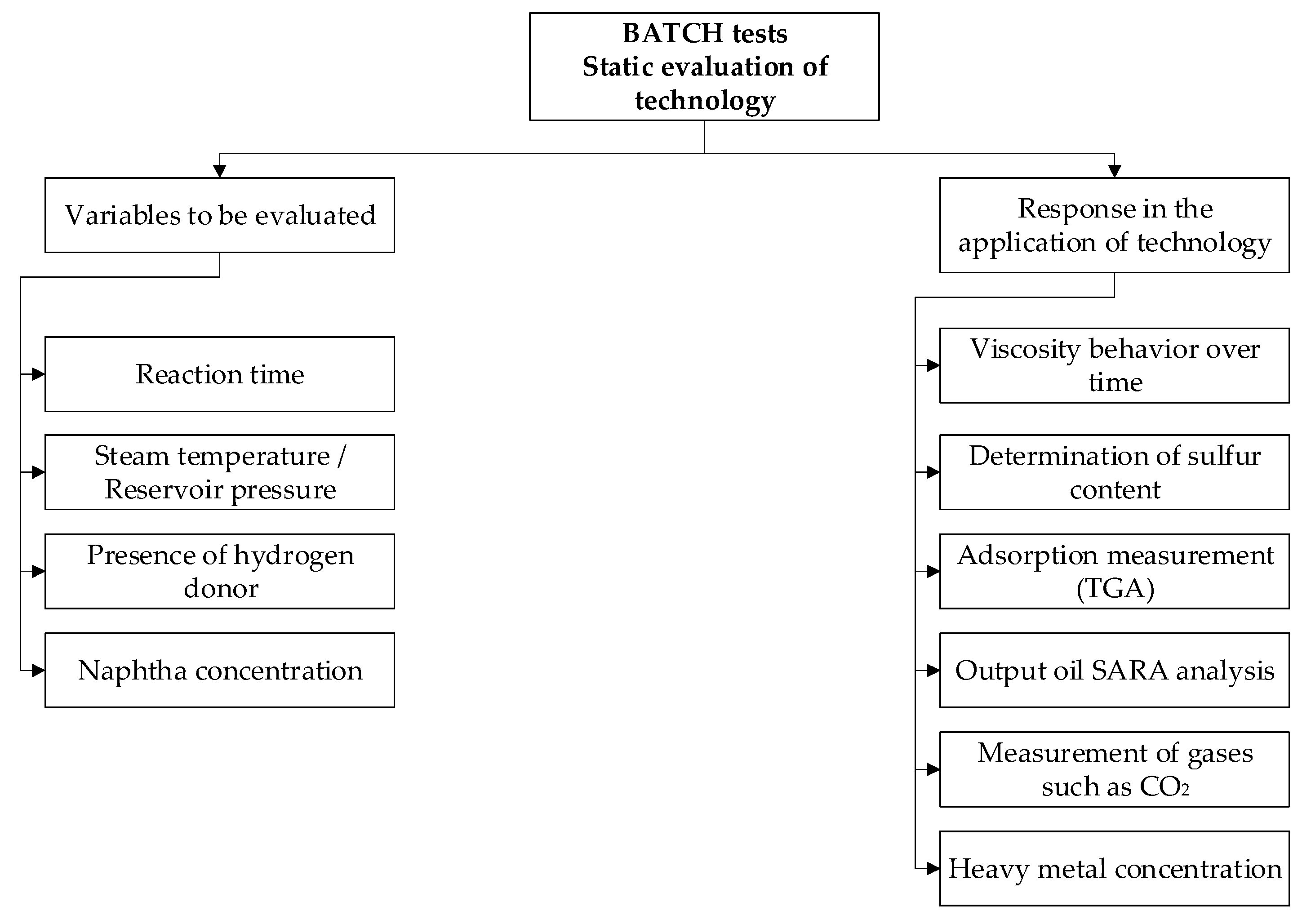
3.4. Experimental Test at Static Conditions
3.5. Rock-Fluid Experimental Test
- Steam temperature: can decrease with increasing depth, obtaining less energy than required for the reaction;
- Residence time: can affect the interaction between nanoparticles and heavy oil;
- Rock adsorption of the nanocatalyst: is a benefit that allows increasing the catalyst time over the cycles.
4. Conclusions and Recommendations
- The percentage of viscosity reduction in laboratory experiments in static tests is high compared to dynamic in situ experiments;
- Adequate dispersion of the nanocatalyst;
- Short time for nanoparticles to interact with heavy oil;
- The nanocatalyst must withstand the temperature gradient as the steam moves away from the injector well (temperature losses) to avoid losing the heat required for the reaction;
- Nanoparticle aggregation is a problem mainly due to its large surface area and destabilizing conditions such as temperature, pressure, salinity, oil, or other chemical species in the reservoir [80].
5. Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bera, A.; Babadagli, T. Status of electromagnetic heating for enhanced heavy oil/bitumen recovery and future prospects: A review. Appl. Energy 2015, 151, 206–226. [Google Scholar] [CrossRef]
- Franco, C.; Flórez, A.; Ochoa, M. Análisis de la cadena de suministros de biocombustibles en Colombia. Rev. Dinám. Sist. 2008, 4, 109–133. [Google Scholar]
- Montoya, T.; Argel, B.L.; Nassar, N.N.; Franco, C.A.; Cortés, F.B. Kinetics and mechanisms of the catalytic thermal cracking of asphaltenes adsorbed on supported nanoparticles. Pet. Sci. 2016, 13, 561–571. [Google Scholar] [CrossRef]
- Husein, M.M.; Alkhaldi, S.J. In situ preparation of alumina nanoparticles in heavy oil and their thermal cracking performance. Energy Fuels 2014, 28, 6563–6569. [Google Scholar] [CrossRef]
- Yi, S.; Babadagli, T.; Andy Li, H. Use of nickel nanoparticles for promoting aquathermolysis reaction during cyclic steam stimulation. SPE J. 2017, 23, 145–156. [Google Scholar] [CrossRef]
- Hou, J.; Li, C.; Gao, H.; Chen, M.; Huang, W.; Chen, Y.; Zhou, C. Recyclable oleic acid modified magnetic NiFe2O4 nanoparticles for catalytic aquathermolysis of Liaohe heavy oil. Fuel 2017, 200, 193–198. [Google Scholar] [CrossRef]
- Kaminski, T.; Anis, S.F.; Husein, M.M.; Hashaikeh, R. Hydrocracking of Athabasca VR Using NiO-WO3 Zeolite-Based Catalysts. Energy Fuels 2018, 32, 2224–2233. [Google Scholar] [CrossRef]
- Franco, C.A.; Martínez, M.; Benjumea, P.; Patiño, E.; Cortés, F.B. Influence of asphaltene aggregation on the adsorption and catalytic behavior of nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Tang, X.-D.; Liang, G.-J.; Li, J.-J.; Wei, Y.-T.; Dang, T. Catalytic effect of in-situ preparation of copper oxide nanoparticles on the heavy oil low-temperature oxidation process in air injection recovery. Pet. Sci. Technol. 2017, 35, 1321–1326. [Google Scholar] [CrossRef]
- Biyouki, A.A.; Hosseinpour, N.; Nassar, N.N. Pyrolysis and Oxidation of Asphaltene-Born Coke-like Residue Formed onto in Situ Prepared NiO Nanoparticles toward Advanced in Situ Combustion Enhanced Oil Recovery Processes. Energy Fuels 2018, 32, 5033–5044. [Google Scholar] [CrossRef]
- Biyouki, A.A.; Hosseinpour, N.; Bahramian, A.; Vatani, A. In-situ upgrading of reservoir oils by in-situ preparation of NiO nanoparticles in thermal enhanced oil recovery processes. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 289–300. [Google Scholar] [CrossRef]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni-Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Luna, G.; Pereira-Almao, P. Kinetics of the catalytic thermo-oxidation of asphaltenes at isothermal conditions on different metal oxide nanoparticle surfaces. Catal. Today 2013, 207, 127–132. [Google Scholar] [CrossRef]
- Desouky, S.; Al Sabagh, A.; Betiha, M.; Badawi, A.; Ghanem, A.; Khalil, S. Catalytic aquathermolysis of Egyptian heavy crude oil. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2013, 7, 638–643. [Google Scholar]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Farouq, S.M.; Abad, B. Bitumen Recovery from Oil Sands, Using Solvents in Conjunction with Steam. J. Can. Pet. Technol. 1976, 15, 80–90. [Google Scholar]
- Shu, W.R.; Hartman, K.J. Effect of Solvent on Steam Recovery of Heavy Oil. SPE Reserv. Eng. 1988, 3, 457–465. [Google Scholar] [CrossRef]
- Castro, Y.E.; Veliz, A.M.; Sanchez, D.A.; Rodriguez, M.M.; Rondon, N.G.; Rivero, S.; Cortez, M.L. Cyclic Steam Injection with Solvents as Method of Thermal Recovery for Heavy and Extra-Heavy Oils: Laboratory Tests. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar] [CrossRef]
- Ali, S.M.F.; Snyder, S.G. Miscible Thermal Methods Applied to a Two-Dimensional, Vertical Tar Sand Pack, With Restricted Fluid Entry. J. Can. Pet. Technol. 1973, 12, 20–26. [Google Scholar] [CrossRef]
- Gupta, S.C.; Gittins, S.D. Christina lake solvent aided process pilot. J. Can. Pet. Technol. 2006, 45, 15–18. [Google Scholar] [CrossRef]
- Ayodele, O.R.; Nasr, T.N.; Ivory, J.; Beaulieu, G.; Heck, G. Testing and history matching ES-SAGD (using hexane). In Proceedings of the SPE Western Regional Meeting, Anaheim, CA, USA, 27–29 May 2010; pp. 1108–1122. [Google Scholar] [CrossRef]
- Andarcia, L.; Bermudez, J.; Reyes, Y.; Caycedo, H.; Suarez, A. Potential of steam solvent hybrid processes in Llanos Basin, Colombia. In Proceedings of the SPE Heavy and Extra Heavy Oil Conference: Latin America, Medellín, Colombia, 24–26 September 2014; pp. 737–752. [Google Scholar] [CrossRef]
- Alikhan, A.A.; Ali, S.M.F. Heavy Oil Recovery by Steam-Driven Hydrocarbon Slugs from Linear Porous Media. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Houston, TX, USA, 6–9 October 1974. [Google Scholar] [CrossRef]
- Doscher, T.M.; Ershaghi, I.; Herzberg, D.E.; Gourene, Z.S. An Economic Evaluation of Solvent/Steam Stimulation. J. Pet. Technol. 1979, 31, 951–954. [Google Scholar] [CrossRef]
- Taborda, E.A.; Alvarado, V.; Cortés, F.B. Effect of SiO2-based nanofluids in the reduction of naphtha consumption for heavy and extra-heavy oils transport: Economic impacts on the Colombian market. Energy Convers. Manag. 2017, 148, 30–42. [Google Scholar] [CrossRef]
- Iskandar, F.; Dwinanto, E.; Abdullah, M.; Khairurrijal; Muraza, O. Viscosity reduction of heavy oil using nanocatalyst in aquathermolysis reaction. KONA Powder Part. J. 2016, 33, 3–16. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P. Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: A study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 2013, 27, 2194–2201. [Google Scholar] [CrossRef]
- Raffa, D.; Picchioni, F. Plenty of room at the bottom: Nanotechnology as solution to an old issue in enhanced oil recovery. Appl. Sci. 2018, 8, 2596. [Google Scholar]
- Callaghan, C.A. Kinetics and Catalysis of the Water-Gas-Shift Reaction: A Microkinetic and Graph Theoretic Approach; Naval Undersea Warfare Center: Worcester, MA, USA, 2006. [Google Scholar]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Heavy oil upgrading and enhanced recovery in a steam injection process assisted by NiO- and PdO-functionalized SiO2 nanoparticulated catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; He, J.; Li, P.; Yang, C. Mechanism of catalytic aquathermolysis: Influences on heavy oil by two types of efficient catalytic ions: Fe3+ and Mo6+. Energy Fuels 2010, 24, 1502–1510. [Google Scholar] [CrossRef]
- Hamedi, S.Y.; Babadagli, T. Effects of nano-sized metals on viscosity reduction of heavy oil/bitumen during thermal applications. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar] [CrossRef]
- Junaid, A.S.M.; Rahman, M.M.; Rocha, G.; Wang, W.; Kuznicki, T.; McCaffrey, W.C.; Kuznicki, S.M. On the role of water in natural-Zeolite-Catalyzed cracking of Athabasca oilsands bitumen. Energy Fuels 2014, 28, 3367–3376. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Hyne, J.B. Aquathermolysis: A Synopsis of Work on the Chemical Reaction between Water (Steam) and Heavy Oil Sands during Simulated Steam Stimulation; Alberta Oil Sands Technology and Research Authority: Edmonton, AB, Canada, 1986. [Google Scholar]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Bell, A. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef]
- Tajmiri, M.; Ehsani, M.R. The Potential of ZnO nanoparticles to reduce water consuming in Iranian Heavy Oil reservoir. Nanotechnol. J. Water Environ. Nanotechnol. 2016, 1, 84–90. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Transportation and interaction of nano and micro size metal particles injected to improve thermal recovery of heavy-oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Li, W.; Zhu, J.h.; Qi, J.h. Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J. Fuel Chem. Technol. 2007, 35, 176–180. [Google Scholar] [CrossRef]
- Wu, C.; Su, J.; Zhang, R.; Lei, G.; Cao, Y. The use of a nano-nickel catalyst for upgrading extra-heavy oil by an aquathermolysis treatment under steam injection conditions. Pet. Sci. Technol. 2013, 21, 2211–2218. [Google Scholar] [CrossRef]
- Noorlaily, M.I.; Khairurrijala, N.; Abdullah, M.; Iskandar, F. Ethylene glycol route synthesis of nickel oxide nanoparticles as a catalyst in aquathermolysis. Mater. Sci. Forum 2013, 737, 93–97. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Zhong, Y.T.; Tang, X.D.; Li, J.J.; da Zhou, T.; Deng, C.L. Thermocatalytic upgrading and viscosity reduction of heavy oil using copper oxide nanoparticles. Pet. Sci. Technol. 2020, 38, 891–903. [Google Scholar] [CrossRef]
- Afzal, S.; Ehsani, M.R.; Nikookar, M.; Roayaei, E. Effect of Fe2O3 and WO3 nanoparticle on steam injection recovery. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 251–258. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wu, C.; Xia, F. Laboratory experiments and field tests of an amphiphilic metallic chelate for catalytic aquathermolysis of heavy oil. Energy Fuels 2008, 22, 1502–1508. [Google Scholar] [CrossRef]
- Nugraha, M.I.; Noorlaily, M.; Khairurrijal, A.; Iskandara, F. Synthesis of NixFe3−xO4 nanoparticles by microwave-assisted coprecipitation and their application on viscosity reduction of heavy oil. Mater. Sci. Forum 2013, 737, 204–208. [Google Scholar] [CrossRef]
- Nurhayati, T.; Iskandar, F.; Abdullah, M.; Khairurrijal, A. Syntheses of hematite (α-Fe2O3) nanoparticles using microwave-assisted calcination method. Mater. Sci. Forum 2013, 737, 197–203. [Google Scholar] [CrossRef]
- Iskandar, F.; Fitriani; Merissa, S.; Mukti, R.R.; Khairurrijal; Abdullah, M. Fe3O4/Zeolite nanocomposites synthesized by microwave assisted coprecipitation and its performance in reducing viscosity of heavy oil. AIP Conf. Proc. 2014, 1586, 132–135. [Google Scholar] [CrossRef]
- Gao, Y.; Ghorbanian, B.; Gargari, H.N.; Gao, W. Steam reforming of gaseous by-products from bitumen oil using various supported Ni-based catalysts. Pet. Sci. Technol. 2018, 36, 34–39. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Cortés, F.B. NiO and PdO Supported on Fumed Silica Nanoparticles for Adsorption and Catalytic Steam Gasification of Colombian n-C7 Asphaltenes. In Handbook on Oil Production Research; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 101–145. [Google Scholar]
- Rojas, L.C. Efecto de Nanopartículas en Procesos con Inyección de Vapor a diferentes calidades; Universidad Nacional de Colombia: Bogotá, Colombia, 2017. [Google Scholar]
- Nassar, N.N.; Hassan, A.; Vitale, G. Comparing kinetics and mechanism of adsorption and thermo-oxidative decomposition of Athabasca asphaltenes onto TiO2, ZrO2, and CeO2 nanoparticles. Appl. Catal. A Gen. 2014, 484, 161–171. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the load of transition metal oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 nanoparticles in catalytic steam decomposition of n-C7 asphaltenes at low temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Diez, R.; Cortés, F.B.; Franco Ariza, C.A.; Giraldo, L.J.; Arias Madrid, D.; Gallego Marin, J. Síntesis y Caracterización de Nanopartículas Janus de Sílice/Níquel para Procesos EOR por Inyección de Vapor. In Proceedings of the Congreso Mexicano Del Petróleo, Mexico City, Mexico, 26–29 September 2018. [Google Scholar]
- Betancur, S.; Franco, C.A.; Cortés, F.B. Magnetite-silica nanoparticles with a core-shell structure for inhibiting the formation damage caused by the precipitation/deposition of asphaltene. J. Magnetohydrodyn. Plasma Res. 2016, 21, 289–322. [Google Scholar]
- Shokrlu, Y.H.; Babadagli, T. Viscosity reduction of heavy oil/bitumen using micro- and nano-metal particles during aqueous and non-aqueous thermal applications. J. Pet. Sci. Eng. 2014, 119, 210–220. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Zhong, L.G.; Liu, Y.J.; Fan, H.F.; Jiang, S.J. Liaohe extra-heavy crude oil underground aquathermolytic treatments using catalyst and hydrogen donors under steam injection conditions. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 20–21 October 2003. [Google Scholar]
- Shokrlu, Y.H. Enhancement of Heavy Oil/Bitumen Thermal Recovery Using Nano Metal Particles; Universidad de Alberta: Edmonton, AB, Canada, 2013. [Google Scholar]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. ASME Publ. Fed. 1995, 231, 99–106. [Google Scholar]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Feng, Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: A review. Nanoscale Res. Lett. 2011, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Hascakir, B.; Babadagli, T.; Akin, S. Experimental and numerical simulation of oil recovery from oil shales by electrical heating. Energy Fuels 2008, 22, 3976–3985. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.; Lin, Y. The catalytic effects of minerals on aquathermolysis of heavy oils. Fuel 2004, 83, 2035–2039. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquin, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Belgrave, J.; Moore, R.; Ursenbach, M. Comprehensive kinetic models for the aquathermolysis of heavy oils. J. Can. Pet. Technol. 1997, 36, 38–44. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Ruiz, M.A.; Pereira-Almao, P.; Cortés, F.B. Nanoparticles for inhibition of asphaltenes damage: Adsorption study and displacement test on porous media. Energy Fuels 2013, 27, 2899–2907. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. Pore-scale monitoring of wettability alteration by silica nanoparticles during polymer flooding to heavy oil in a five-spot glass micromodel. TransPorous Media 2011, 87, 653–664. [Google Scholar] [CrossRef]
- Galarraga, C.E.; Pereira-Almao, P. Hydrocracking of Athabasca bitumen using submicronic multimetallic catalysts at near in-reservoir conditions. Energy Fuels 2010, 24, 2383–2389. [Google Scholar] [CrossRef]
- Aladag, B.; Halelfadl, S.; Doner, N.; Maré, T.; Duret, S.; Estellé, P. Experimental investigations of the viscosity of nanofluids at low temperatures. Appl. Energy 2012, 97, 876–880. [Google Scholar] [CrossRef]
- Farzaneh, H.; Behzadmehr, A.; Yaghoubi, M.; Samimi, A.; Sarvari, S. Stability of nanofluids: Molecular dynamic approach and experimental study. Energy Convers. Manag. 2016, 111, 1–14. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Narahari, M.; Susin, L. Stability, rheology and thermal analysis of functionalized alumina-thermal oil-based nanofluids for advanced cooling systems. Energy Convers. Manag. 2017, 142, 215–229. [Google Scholar] [CrossRef]
- Islam, M.R.; Shabani, B.; Rosengarten, G. Nanofluids to improve the performance of PEM fuel cell cooling systems: A theoretical approach. Appl. Energy 2016, 178, 660–671. [Google Scholar] [CrossRef]
- Kibria, M.; Anisur, M.; Mahfuz, M.; Saidur, R.; Metselaar, I. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef]
- Mortazavi-Manesh, S.; Shaw, J. Effect of diluents on the rheological properties of Maya crude oil. Energy Fuels 2016, 30, 766–772. [Google Scholar] [CrossRef]
- Kaur, I.; Ellis, L.-J.; Römer, I.; Tantra, R.; Carriere, M.; Allard, S.; Hermite, M.M.-L.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of nanomaterials in aqueous media: Towards protocol optimization. J. Vis. Exp. 2017, 130, e56074. [Google Scholar] [CrossRef] [PubMed]
- Hendraningrat, L.; Torsæter, O. Effects of the initial rock wettability on silica-based nanofluid-enhanced oil recovery processes at reservoir temperatures. Energy Fuels 2014, 28, 6228–6241. [Google Scholar] [CrossRef]
- De la Cruz Flores, V.G. Actividad y Desactivación de Ni en HMS para el Reformado de Metano con CO2; Universidad Autónoma de Nuevo León: San Nicolás de los Garza, Mexico, 2015. [Google Scholar]
- Cortés, F.B.; Mejía, J.M.; Ruiz, M.A.; Benjumea, P.; Riffel, D.B. Sorption of asphaltenes onto nanoparticles of nickel oxide supported on nanoparticulated silica gel. Energy Fuels 2012, 26, 1725–1730. [Google Scholar] [CrossRef]
- Pérez, R.A.; Rodréguez, H.A.; Rendón, G.J.; Plata, B.G.; Salinas, L.M.; Barbosa, C.; García, L.E.; Rojas, F.A.; Orrego, J.A.; León, L.J.; et al. Optimizing Production Performance, Energy Efficiency and Carbon Intensity with Preformed Foams in Cyclic Steam Stimulation in a Mature Heavy Oil Field: Pilot Results and Development Plans. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 25–29 April 2022. [Google Scholar] [CrossRef]




| Reference | Observed Catalytic Performance | Remarks |
|---|---|---|
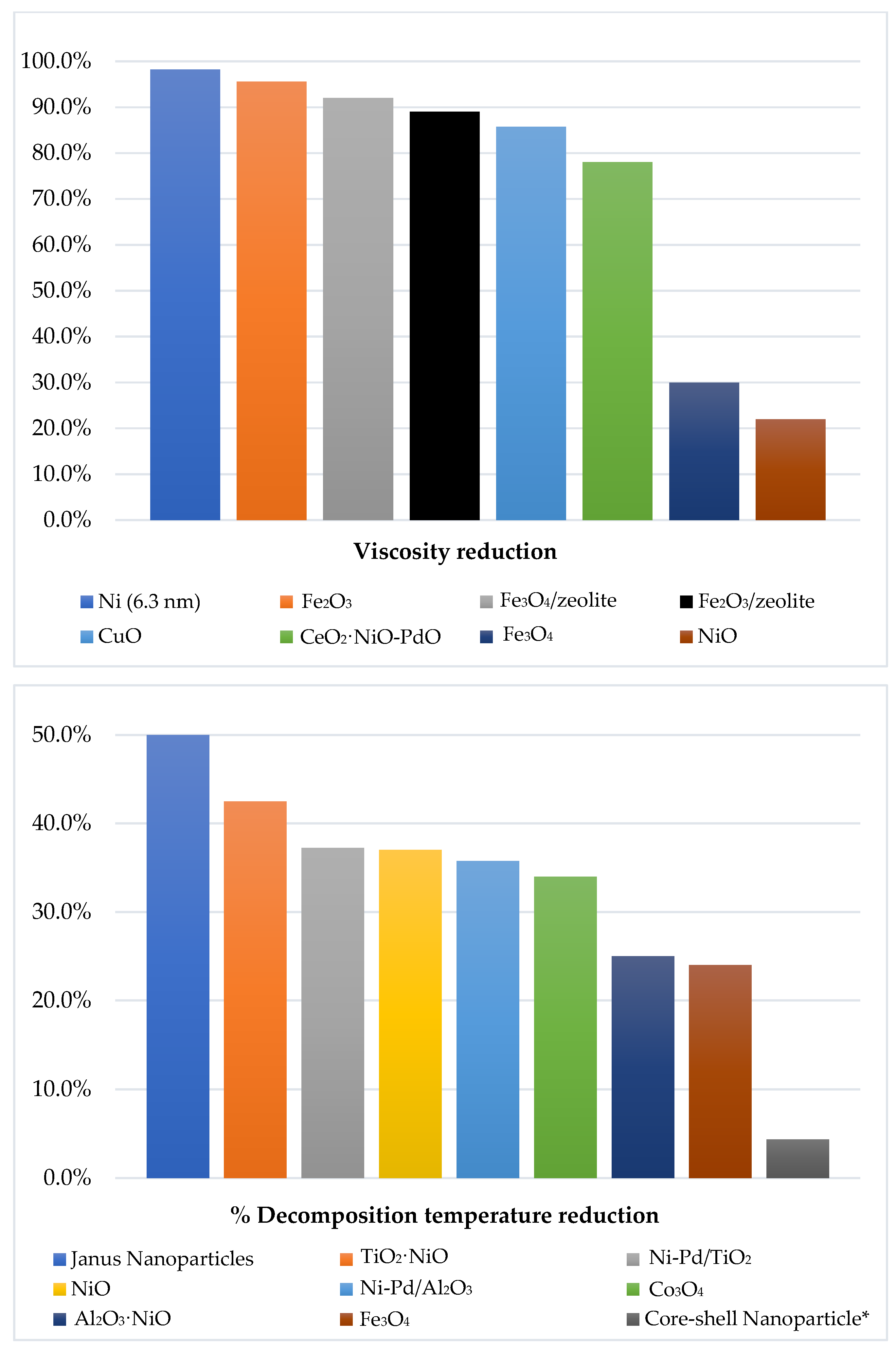
| Y.H. Shokrlu & Babadagli (2011) [39] | Oil viscosity reduction: 29.63% C-S chain breaking, but with time this effect is reduced [5] | Sandstone core in CSS. Concentration used: 500 ppm. |
| Li, Zhu, & Qi (2007) [40] | Oil viscosity reduction: 98.2% H/C: 1.46 | Particle size: 6.3 nm |
| Wu, Su, Zhang, Lei, & Cao (2013) [41] | Oil viscosity reduction: 90.36% H/C: 2.09 | Particle size: 4.2 nm Asphaltenes molecular weight reduction: 28.06% |
| Nanoparticles | Observed Catalytic Performance | Remarks | Reference |
|---|---|---|---|
| NiO | Oil viscosity reduction: 22% Temperature decomposition reduction (TD): 37% in presence of steam | Nanoparticle size: 60–70 nm. Athabasca asphaltenes 12 nm (crystal size) Asphaltenes conversion: 37% | Noorlaily, Nugrah, Khairurrijala, Abdullah, & Iskandar (2013) [42] Nashaat Nassar, Hassan, & Pereira-Almao (2011) [43] |
| ZnO | Incremental sweep efficiency: 35.5% compared to conventional SAGD | Tajmiri & Ehsani (2016) [38] | |
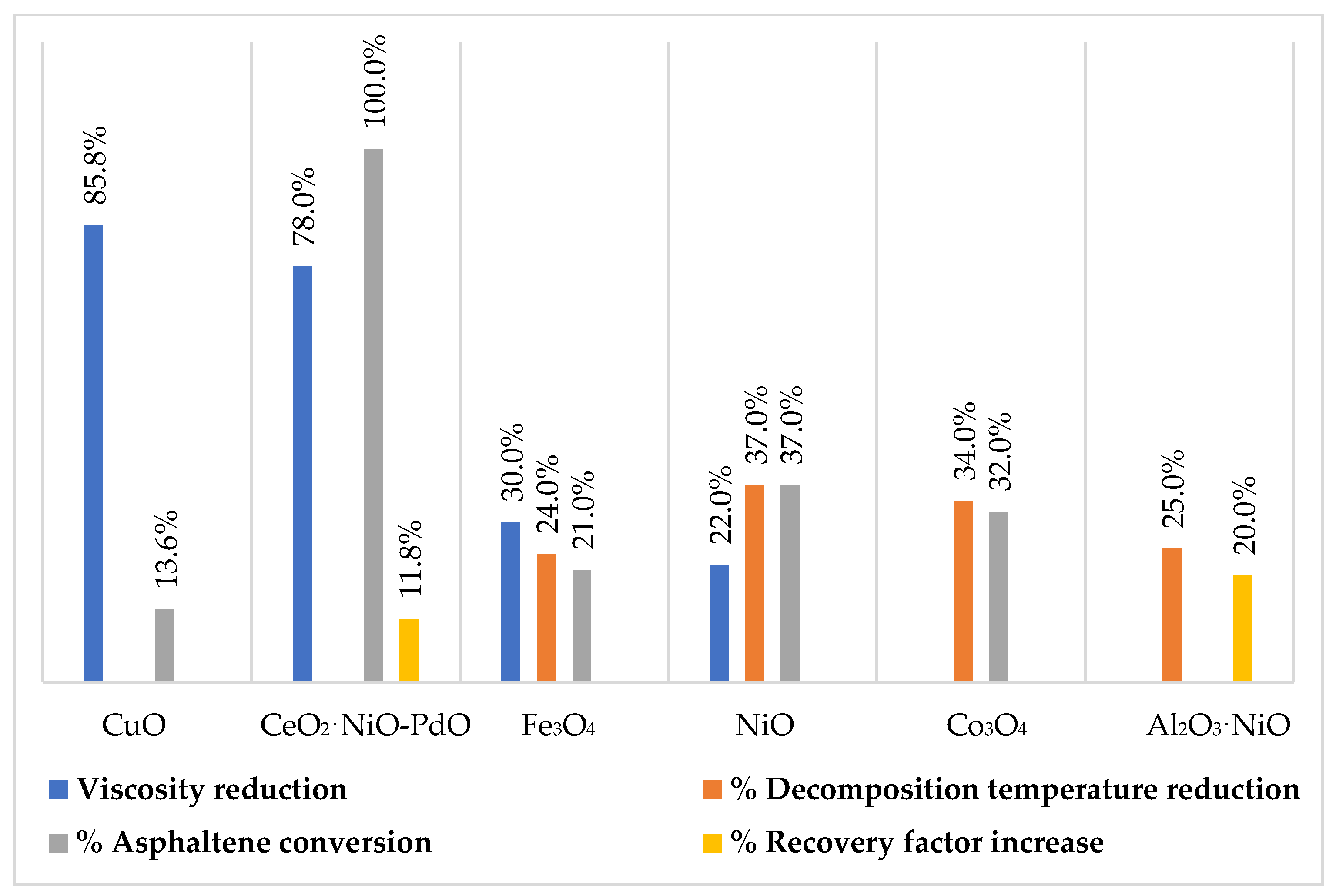
| CuO | Oil viscosity reduction: 85.75% at 350 °C for 40 min | Used concentration: 0.2% p/p % Asphaltenes reduction: 13.62% | Zhong, Tang, Zhou, & Deng (2020) [44] |
| Fe2O3 | Oil viscosity reduction: less than 40% | Concentration 0.2% p/p | Afzal, Ehsani, Nikookar, & Roayaei (2018) [45] |
| α-Fe2O3 | Oil viscosity reduction: 93.3% Oil viscosity reduction: 95.6% | Resins and asphaltenes reduction. Aromatics and saturated increase. | Chen, Wang, Wu, & Xia (2008) [46] Wang et al. (2010) [31] |
| Fe3O4 | TD reduction: 24% Less catalytic activity compared to NiO and Co3O4. Oil viscosity reduction: 30% | Concentration: 0.2% p/p Crystal size: 22 nm Asphaltenes conversion: 21% Crystal size: 43 nm | Nashaat Nassar et al. (2011) [43] Nugraha, Noorlaily, Abdullah, Khairurrijal, & Iskandara (2013) [47] |
| Co3O4 | TD reduction: 34% | Crystal size: 22 nm Asphaltenes conversion: 32% | Nashaat Nassar et al. (2011) [43] |
| Nanoparticles | Observed Catalytic Performance | Remarks | Reference |
|---|---|---|---|
| α-Fe2O3/zeolite | Oil viscosity reduction: 89% | Particle size: 135 nm Composition α-Fe2O3: zeolite of 1:3 | Nurhayati, Iskandar, Abdullah, & Khairurrijal (2013) [48] |
| Fe3O4/zeolite | Oil viscosity reduction: 92% | Particle size: 96 nm Composition Fe3O4:zeolite of 1:4 | Iskandar et al. (2014) [49] |
| Functionalized Ni | Tar reduction: Ni/Al2O3: 99% Ni/Olivina: 93.1% Ni/Fe2O3: 83.6% | Gao, Ghorbanian, Gargari, & Gao (2018) [50] | |
| Functionalized Ni-Pd | Asphaltenes TD reduction: Ni-Pd/TiO2: 37.25% Ni-Pd/Al2O3: 35.75% | Ni-Pd/CeO2: 93% n-C7 asphaltenes conversion in presence of steam in less than 90 min. | Nashaat N. Nassar et al. (2015) [12] |
| Functionalized SiO2 with 1% NiO and 1% PdO | API increase: 40.5% Better asphaltenes thermal cracking compared to SiO2 nanoparticles by itself. | CH4 production increase Sweep efficiency increase: 56% compared to steam injection | Franco, Montoya, Nassar, & Cortés (2014) [51] |
| Functionalized Al2O3 | Functionalized Al2O3 with 2%NiO: TD reduction of approx. 25%. API increasing of 5°. | Functionalized Al2O3 with 2%NiO: 20% increase in sweep efficiency Promote gas reduction like CH4 y CO over others like CO2, with a coke better performance of aprox 0.13% | Cardona Rojas (2017) [52] |
| Functionalized TiO2 with 2% NiO | Reduce TD approx. 170 °C: 42.5% | Residual coke is higher with a mass fraction of 0.17%. | Nashaat Nassar et al. (2015) [12]; Nashaat Nassar, Hassan, & Vitale (2014) [53] |
| Functionalized CeO2 with NiO and PdO | API increase: 50% Oil viscosity reduction: 78% | 0.89% of PdO and 1.1% of NiO over CeO2 Asphaltenes conversion: 100% in less than 80 min. Asphaltenes reduction: 15.8% Sweep efficiency improvement: 11.8% | Medina, Gallego, Arias-Madrid, Cortés, & Franco (2019) [54] |
| Janus nanoparticles | TD asphaltenes reduction at 200 °C: 50% | Interfacial tension decreased | Diez et al. (2018) [55] |
| NPs core (magnetite)—Shell (silica) | TD starts at 200 °C and max at 440 °C, 20 °C less than base case: 4.35% | Promote CH4 and light HCs formation during heavy fractions decomposition. | Betancur, Franco, & Cortés (2016) [56] |
| Ni/W/Mo | Promote a decrease in sulfur and nitrogen-based gases. | Hashemi et al. (2013) [27] |
| Lab Technique | Technique Description |
|---|---|
| SEM-EDS (Scanning electron microscopy) or TEM | Particle morphology and size |
| Dynamic Light Scattering (DLS) | Particle size (ideal for monodispersed samples) |
| ζ-Potential | Z potential determination |
| Infrared spectroscopy through Fourier transform (FTIR-ATR) | Structural analysis of the nanoparticles |
| Thermal Property Analyzer | Thermal conductivity determination |
| BET (Brunauer, Emmett and Teller) | Superficial area determination |
| Property | Technique Description |
|---|---|
| API Gravity | At 60 °F, a specific gravity function |
| Density | Digital densimeter: through frequency measurement |
| Viscosity | Capillary viscosimeter: measure through Hagen–Poiseuille equation |
| Paraffins, Isoparaffins, Olefins, Naphthene, Aromatics determination (PIANO) | Polar column separates paraffins and naphthene from aromatics, while heavy aromatics and alcohols are kept in the pre-column. |
| Property | Technique Description |
|---|---|
| API Gravity | At 60 °F, a specific gravity function |
| Density | Digital densimeter: through frequency measurement |
| Viscosity | Flow through two parallel plates, the superior plate turns creating a shear. |
| Total Acid Number | Titration (neutralization) with potassium hydroxide (KOH) |
| SARA Analysis | Separation using solvents (heptane-toluene) |
| Compositional analysis | CHNO analysis based on sample combustion. |
| Heavy metals | ICP: an atomized liquid sample is injected into an argon plasma. The sample ionize into the plasma and the ions emit different wavelengths. |
| Simulated distillation | Samples are analyzed in a nonpolar chromatographic capillary column that separates hydrocarbons according to their boiling point. It allows identification of the type of crude: aromatic, paraffinic, or naphthenic. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Duarte, H.A.; Ruiz-Cañas, M.C.; Pérez-Romero, R.A. Innovative Experimental Design for the Evaluation of Nanofluid-Based Solvent as a Hybrid Technology for Optimizing Cyclic Steam Stimulation Applications. Energies 2023, 16, 373. https://doi.org/10.3390/en16010373
García-Duarte HA, Ruiz-Cañas MC, Pérez-Romero RA. Innovative Experimental Design for the Evaluation of Nanofluid-Based Solvent as a Hybrid Technology for Optimizing Cyclic Steam Stimulation Applications. Energies. 2023; 16(1):373. https://doi.org/10.3390/en16010373
Chicago/Turabian StyleGarcía-Duarte, Hugo Alejandro, María Carolina Ruiz-Cañas, and Romel Antonio Pérez-Romero. 2023. "Innovative Experimental Design for the Evaluation of Nanofluid-Based Solvent as a Hybrid Technology for Optimizing Cyclic Steam Stimulation Applications" Energies 16, no. 1: 373. https://doi.org/10.3390/en16010373
APA StyleGarcía-Duarte, H. A., Ruiz-Cañas, M. C., & Pérez-Romero, R. A. (2023). Innovative Experimental Design for the Evaluation of Nanofluid-Based Solvent as a Hybrid Technology for Optimizing Cyclic Steam Stimulation Applications. Energies, 16(1), 373. https://doi.org/10.3390/en16010373







