Cooling Optimization Strategy for a 6s4p Lithium-Ion Battery Pack Based on Triple-Step Nonlinear Method
Abstract
:1. Introduction
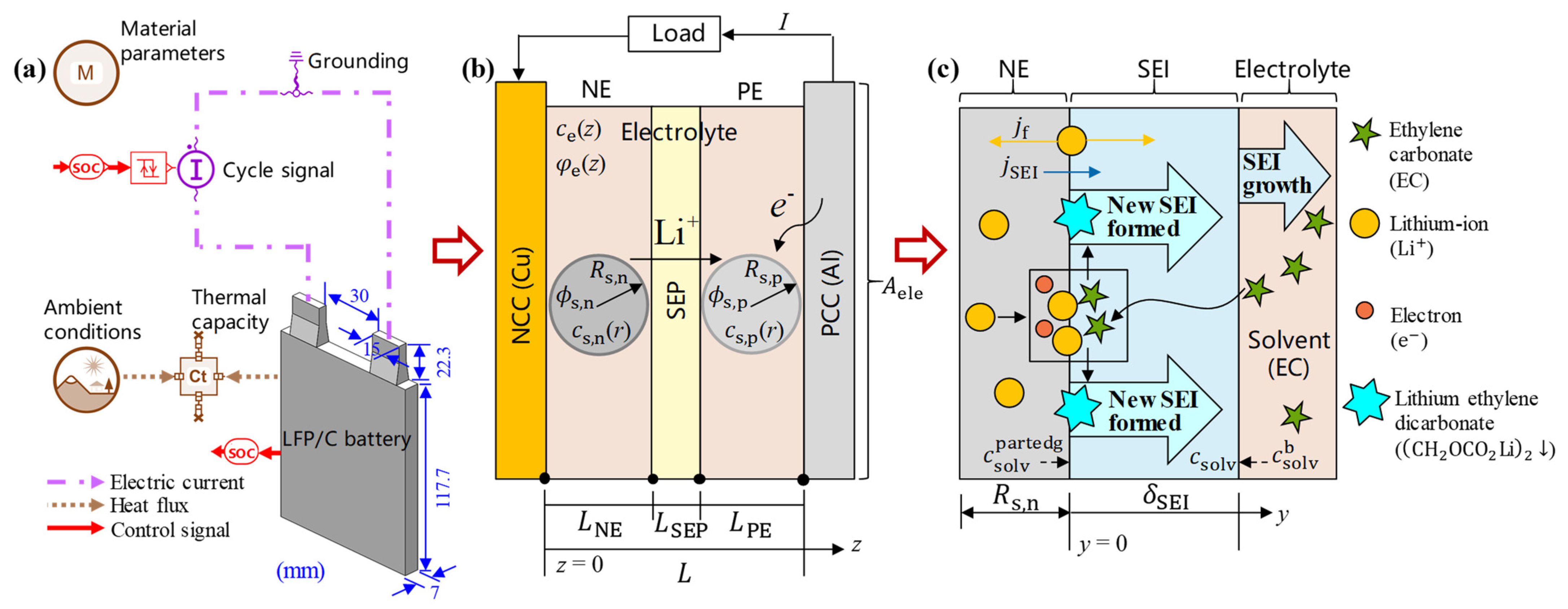
2. Materials and Methods
2.1. Charge–Discharge and Nominal Electrochemical Model of the Battery Cell
- (1)
- The passive sign convention is used: during charge and during discharge;
- (2)
- The particles in both electrodes can be represented as two mean spherical particles;
- (3)
- The active surface is assumed to be same in all areas: .
2.2. Aging Model of the Battery Cell
2.3. Thermal Models of the Battery Cell
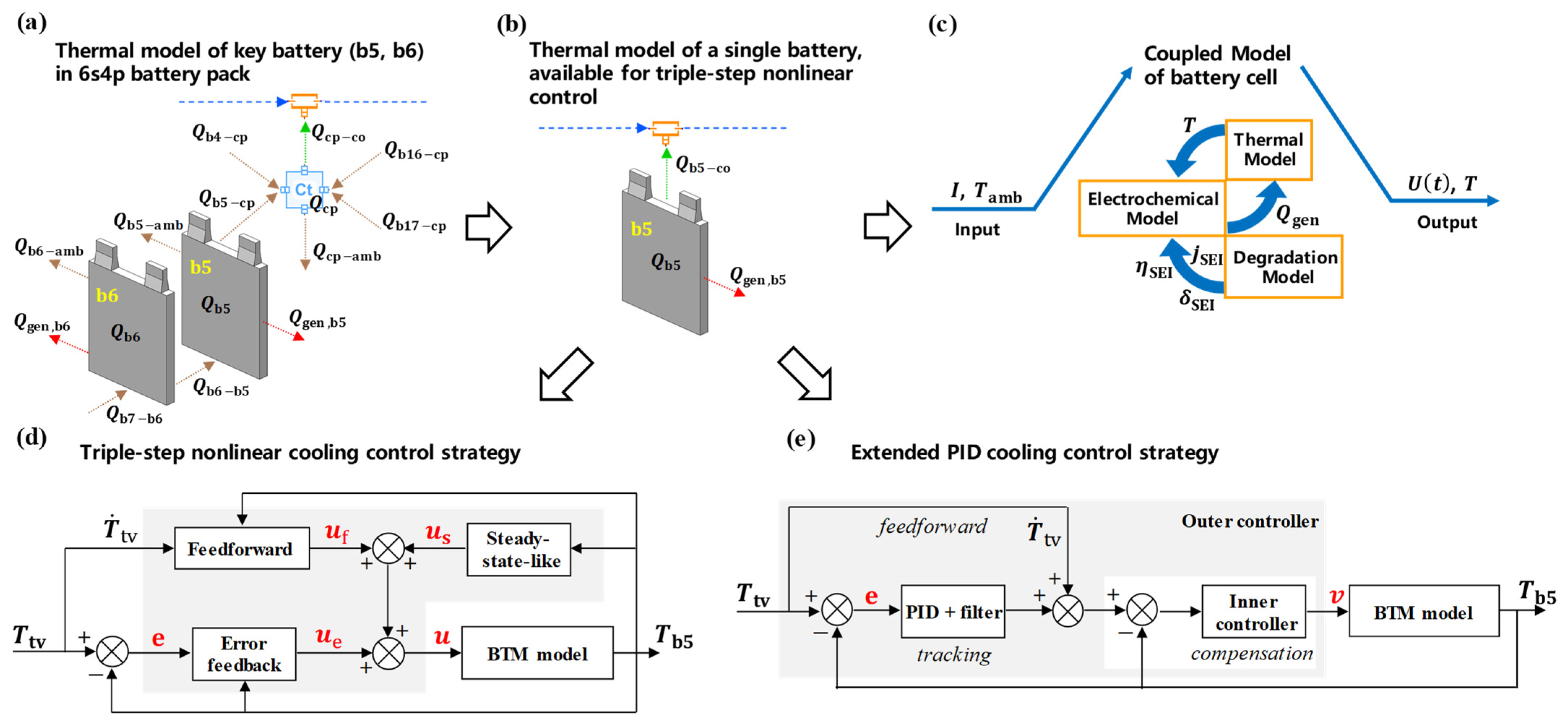
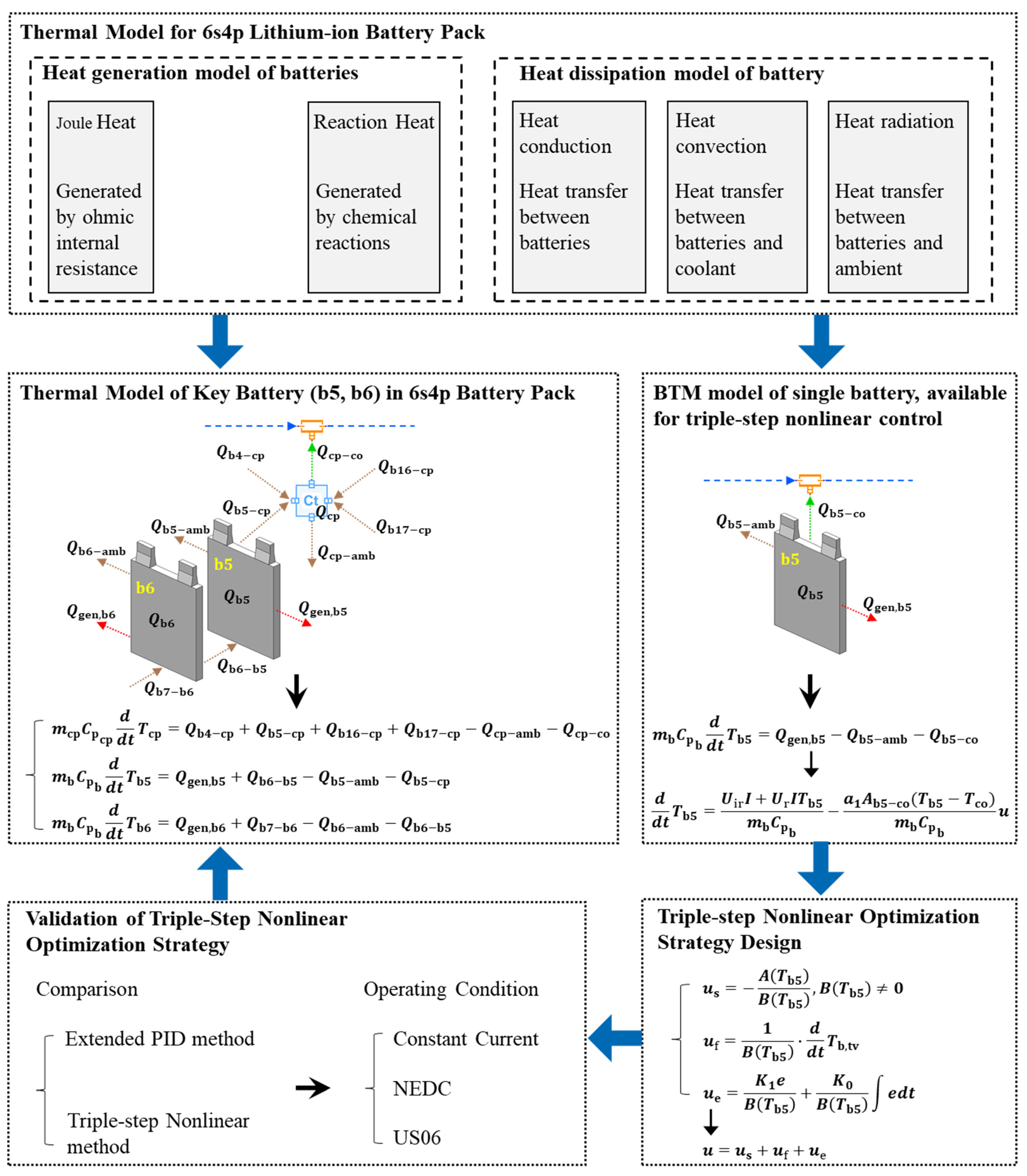
2.4. Thermal Management Model of the 6s4p Battery Pack
2.5. Triple-Step Nonlinear Cooling Control Strategy
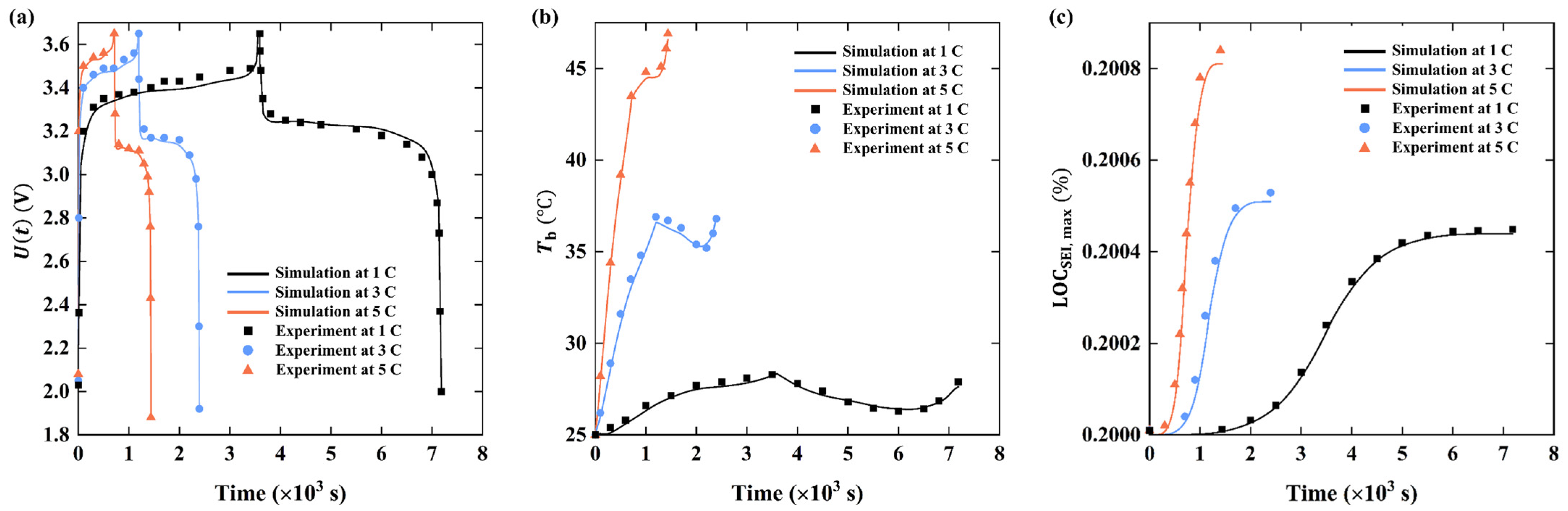
3. Model Validation
4. Results and Discussion
4.1. Performance of Battery Cell and 6s4p Battery Pack under Natural Cooling
4.2. Performance of 6s4p Battery Pack under Two Modes Cooling System
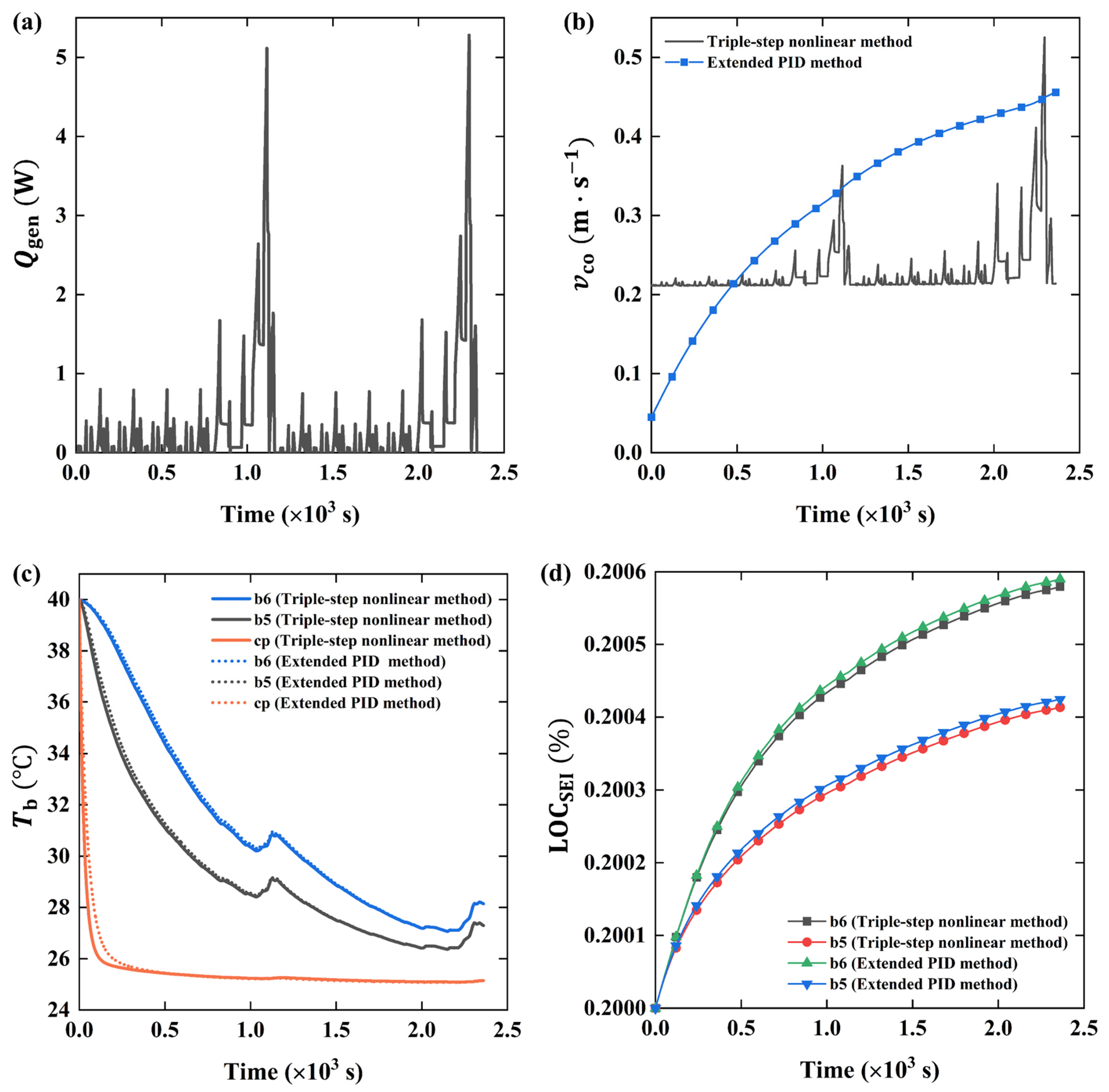
4.3. Cooling Optimization of 6s4p Battery Pack under Different Cycle Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Heat transferred from the battery to the ambient (W) | Error feedback control law | ||
| hconv | Convective heat transfer coefficient () | Input error | |
| Contact area between the battery and the ambient air () | Greek symbols | ||
| Battery temperature (K) | Surface emissivity | ||
| Ambient temperature (K) | Stefan-Boltzmann constant | ||
| Active area of positive electrode () | Density of battery () | ||
| Active area of separator () | Overpotential at z = L | ||
| Active area of negative electrode () | Overpotential at z = 0 | ||
| Active area of electrode () | Electrolyte potential at z = L | ||
| Electric current (A) | Electrolyte potential at z = 0 | ||
| Thermal conductivity of battery () | Hysteresis factor | ||
| Specific thermal capacity of battery () | Volume fraction of electrolyte | ||
| Thermodynamic potential of the positive electrode (V) | Effective solid phase conductivity () | ||
| Thermodynamic potential of the negative electrode (V) | Potential of solid phase (V) | ||
| z | Battery thickness direction | Electrolyte effective ionic conductivity () | |
| Bulk Li concentration in the solid phase for positive electrode () | Electrolyte effective ionic conductivity () | ||
| Bulk Li concentration in the solid phase for negative electrode () | Charge transfer coefficient of the anodic reaction | ||
| Maximum Li concentration in the positive electrode () | Charge transfer coefficient of the reduction reaction | ||
| Maximum Li concentration in the negative electrode () | Overpotential (V) | ||
| Reference potential of the positive electrode (V) | Electrolyte Bruggeman exponent | ||
| Reference potential of the negative electrode (V) | Solid phase conductivity | ||
| Transition factor | Volume fraction of the active material | ||
| x | Insertion rate | Solid phase overpotential of positive electrode (V) | |
| Charge reference potentials of the positive electrode (V) | Solid phase overpotential of negative electrode (V) | ||
| Discharge reference potentials of the positive electrode (V) | Solid ohmic overpotential of positive electrode (V) | ||
| Charge reference potentials of the negative electrode (V) | Solid ohmic overpotential of negative electrode (V) | ||
| Discharge reference potentials of the negative electrode (V) | Electrolyte diffusion overpotential (V) | ||
| Reference temperature | Electrolyte ohmic overpotential (V) | ||
| Li concentration in the active material particles () | Reaction charge transfer coefficient of the SEI layer formation | ||
| Radial coordinate inside a spherical particle (m) | SEI layer thickness (m) | ||
| Solid phase diffusion coefficient () | SEI layer conductivity () | ||
| Current per volume unit () | SEI layer porosity | ||
| Specific interfacial surface area () | Instantaneous formed SEI layer porosity | ||
| Faraday constant () | SEI layer density () | ||
| Concentration of the electrolyte () | SEI ohmic overvoltage (V) | ||
| Effective electrolyte phase diffusion coefficient () | Index of the SEI layer electronic conductivity data () | ||
| Lithium transference number | SEI Bruggeman exponent | ||
| Thickness of the positive electrode | Porosity of the negative electrode | ||
| Thickness of the negative electrode | Density of the coolant () | ||
| Exchange current density (A) | Dynamic viscosity of the coolant () | ||
| Ideal gas constant | Dynamic viscosity near the wall in the pipe () | ||
| Temperature | |||
| Electrolyte phase diffusion coefficient () | Subscripts and superscripts | ||
| Reaction rate constant () | conv | Convection | |
| Electrolyte activity coefficient | b | Battery | |
| Radius of spherical particle (m) | s | Solid phase | |
| Li concentration at the positive electrode-electrolyte interface phase () | p | Positive electrode | |
| Li concentration at the negative electrode-electrolyte interface phase () | n | Negative electrode | |
| Faraday constant | max | Maximum | |
| Parasitic current density for SEI layer formation () | e | Electrolyte | |
| SEI formation reaction constant () | eff | Effective | |
| Concentration of the solvent at the particle edge () | diff | Difference | |
| Solvent reduction potential of the SEI layer (V) | int | Initial | |
| Concentration of the solvent () | ir | Irreversible | |
| SEI layer solvent diffusivity () | r | Reversible | |
| Bulk concentration of the solvent () | co | Coolant | |
| Cycling lithium loss due to SEI formation () | tv | Target value | |
| Loss of capacity due to SEI formation | b5 | Battery 5 | |
| Total capacity () | |||
| SEI molar mass () | Acronyms | ||
| Negative insertion rate when the state of charge is zero | EVs | Electric vehicles | |
| Nominal capacity () | LIBs | Lithium-ion batteries | |
| Mass of the battery (kg) | BTM | Battery thermal management | |
| Total current intensity () | NE | Negative electrode | |
| Generated heat of battery (W) | PE | Positive electrode | |
| Heat transferred from cell 5 to coolant (W) | SEI | solid electrolyte interface | |
| Heat transfer coefficient between cell 5 and coolant () | SP | Single particle | |
| Contact area between cell 5 and coolant () | LFP | Lithium iron phosphate | |
| Nusselt number of the coolant | SOC | state of charge | |
| Reynolds number of the coolant | AMESim | Advanced Modeling Environment for performing Simulation of engineering systems | |
| Prandtl number of the coolant | NCC | Negative current collector | |
| Flow velocity of the coolant () | SEP | Separator | |
| Steady-state-like control law | PCC | Positive current collector | |
| Reference variable feed-forward control law | LOC | Loss of capacity | |
References
- Al-Zareer, M.; Dincer, I.; Rosen, M.A. A Review of Novel Thermal Management Systems for Batteries. Int. J. Energy Res. 2018, 42, 3182–3205. [Google Scholar] [CrossRef]
- Patil, M.S.; Seo, J.H.; Panchal, S.; Jee, S.W.; Lee, M.Y. Investigation on Thermal Performance of Water-Cooled Li-Ion Pouch Cell and Pack at High Discharge Rate with U-Turn Type Microchannel Cold Plate. Int. J. Heat Mass Transf. 2020, 155, 119728. [Google Scholar] [CrossRef]
- Panchal, S.; Gudlanarva, K.; Tran, M.K.; Fraser, R.; Fowler, M. High Reynold’s Number Turbulent Model for Micro-Channel Cold Plate Using Reverse Engineering Approach for Water-Cooled Battery in Electric Vehicles. Energies 2020, 13, 1638. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Guo, H.; Xiong, R.; Yu, Q. A Double-Scale and Adaptive Particle Filter-Based Online Parameter and State of Charge Estimation Method for Lithium-Ion Batteries. Energy 2018, 144, 789–799. [Google Scholar] [CrossRef]
- Tran, M.K.; Panchal, S.; Chauhan, V.; Brahmbhatt, N.; Mevawalla, A.; Fraser, R.; Fowler, M. Python-Based Scikit-Learn Machine Learning Models for Thermal and Electrical Performance Prediction of High-Capacity Lithium-Ion Battery. Int. J. Energy Res. 2022, 46, 786–794. [Google Scholar] [CrossRef]
- Hekmat, S.; Molaeimanesh, G.R. Hybrid Thermal Management of a Li-Ion Battery Module with Phase Change Material and Cooling Water Pipes: An Experimental Investigation. Appl. Therm. Eng. 2020, 166, 114759. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Yuan, Q.; Wang, T.; Ji, H.; Papović, S.; Raleva, K.; Pan, F.; Yang, T.; Li, J. Effects of Minor Mechanical Deformation on the Lifetime and Performance of Commercial 21700 Lithium-Ion Battery. J. Electrochem. Soc. 2022, 169, 060544. [Google Scholar] [CrossRef]
- Latini, D.; Vaccari, M.; Lagnoni, M.; Orefice, M.; Mathieux, F.; Huisman, J.; Tognotti, L.; Bertei, A. A Comprehensive Review and Classification of Unit Operations with Assessment of Outputs Quality in Lithium-Ion Battery Recycling. J. Power Sources 2022, 546, 231979. [Google Scholar] [CrossRef]
- Ghaeminezhad, N.; Wang, Z.; Ouyang, Q. A Review on Lithium-Ion Battery Thermal Management System Techniques: A Control-Oriented Analysis. Appl. Therm. Eng. 2023, 219, 119497. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A Critical Review of Battery Thermal Performance and Liquid Based Battery Thermal Management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Lee, H. Review on Battery Thermal Management System for Electric Vehicles. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Aswin Karthik, C.; Kalita, P.; Cui, X.; Peng, X. Thermal Management for Prevention of Failures of Lithium Ion Battery Packs in Electric Vehicles: A Review and Critical Future Aspects. Energy Storage 2020, 2, e137. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Liu, X.; Li, S.; Zhang, C.; Yang, S. A Review on Recent Progress, Challenges and Perspective of Battery Thermal Management System. Int. J. Heat Mass Transf. 2021, 167, 120834. [Google Scholar] [CrossRef]
- Mohammadian, S.K.; Zhang, Y. Thermal Management Optimization of an Air-Cooled Li-Ion Battery Module Using Pin-Fin Heat Sinks for Hybrid Electric Vehicles. J. Power Sources 2015, 273, 431–439. [Google Scholar] [CrossRef]
- Mohammadian, S.K.; Rassoulinejad-Mousavi, S.M.; Zhang, Y. Thermal Management Improvement of an Air-Cooled High-Power Lithium-Ion Battery by Embedding Metal Foam. J. Power Sources 2015, 296, 305–313. [Google Scholar] [CrossRef]
- An, Z.; Shah, K.; Jia, L.; Ma, Y. A Parametric Study for Optimization of Minichannel Based Battery Thermal Management System. Appl. Therm. Eng. 2019, 154, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, H.; Li, W.; Shi, J.; Wang, H.; Chen, J. Thermal Characteristics of Power Battery Pack with Liquid-Based Thermal Management. Appl. Therm. Eng. 2020, 164, 114421. [Google Scholar] [CrossRef]
- Mortazavi, B.; Yang, H.; Mohebbi, F.; Cuniberti, G.; Rabczuk, T. Graphene or H-BN Paraffin Composite Structures for the Thermal Management of Li-Ion Batteries: A Multiscale Investigation. Appl. Energy 2017, 202, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ling, G.; Zhuang, L.; Liang, Z. The Effect of Reducing the Thermal Contact Resistance on the Performance of Battery Thermal Management System. Int. J. Energy Res. 2021, 45, 9970–9982. [Google Scholar] [CrossRef]
- Liu, F.F.; Lan, F.C.; Chen, J.Q.; Li, Y.G. Experimental Investigation on Cooling/Heating Characteristics of Ultra-Thin Micro Heat Pipe for Electric Vehicle Battery Thermal Management. Chinese J. Mech. Eng. 2018, 31, 53. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, K. Experimental and Numerical Study of a Passive Thermal Management System Using Flat Heat Pipes for Lithium-Ion Batteries. Appl. Therm. Eng. 2020, 166, 114660. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Zhang, G.; Huang, Q.; Zhou, D. Experiment and Simulation for Pouch Battery with Silica Cooling Plates and Copper Mesh Based Air Cooling Thermal Management System. Appl. Therm. Eng. 2019, 146, 866–880. [Google Scholar] [CrossRef]
- Dan, D.; Yao, C.; Zhang, Y.; Zhang, H.; Zeng, Z.; Xu, X. Dynamic Thermal Behavior of Micro Heat Pipe Array-Air Cooling Battery Thermal Management System Based on Thermal Network Model. Appl. Therm. Eng. 2019, 162, 114183. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, Q.; Hu, S.; Xu, X. Research on Performance of Thermal Management System Integrated with Multiple Heat Exchange Methods. Ionics 2022, 28, 789–799. [Google Scholar] [CrossRef]
- Kabir, M.M.; Demirocak, D.E. Degradation Mechanisms in Li-Ion Batteries: A State-of-the-Art Review. Int. J. Energy Res. 2017, 41, 1963–1986. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-Ion Battery Aging Mechanisms and Diagnosis Method for Automotive Applications: Recent Advances and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Kamyab, N.; Weidner, J.W.; White, R.E. Mixed Mode Growth Model for the Solid Electrolyte Interface (SEI). J. Electrochem. Soc. 2019, 166, A334–A341. [Google Scholar] [CrossRef]
- Yang, S.-c.; Hua, Y.; Qiao, D.; Lian, Y.-b.; Pan, Y.-w.; He, Y.-l. A Coupled Electrochemical-Thermal-Mechanical Degradation Modelling Approach for Lifetime Assessment of Lithium-Ion Batteries. Electrochim. Acta 2019, 326, 134928. [Google Scholar] [CrossRef]
- Sun, S.; Guan, T.; Cheng, X.; Zuo, P.; Gao, Y.; Du, C.; Yin, G. Accelerated Aging and Degradation Mechanism of LiFePO4/Graphite Batteries Cycled at High Discharge Rates. RSC Adv. 2018, 8, 25695–25703. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gan, Y.; Yao, M.; Li, Y. Numerical Analysis of Capacity Fading for a LiFePO4 Battery under Different Current Rates and Ambient Temperatures. Int. J. Heat Mass Transf. 2021, 165, 120615. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Zhang, R.; Lin, Y.; Fang, H. Thermal Analysis of a 6s4p Lithium-Ion Battery Pack Cooled by Cold Plates Based on a Multi-Domain Modeling Framework. Appl. Therm. Eng. 2020, 173, 115216. [Google Scholar] [CrossRef]
- An, Z.; Jia, L.; Wei, L.; Yang, C. Numerical Modeling and Analysis of Thermal Behavior and Li+ Transport Characteristic in Lithium-Ion Battery. Int. J. Heat Mass Transf. 2018, 127, 1351–1366. [Google Scholar] [CrossRef]
- Zhao, P.; Li, M.; Kang, J.; Rizzoni, G. Analysis of Fading Characteristics of a Lithium Ion Battery Based on an Integration Model. Int. J. Heat Mass Transf. 2017, 104, 1317–1324. [Google Scholar] [CrossRef]
- Dong, T.; Peng, P.; Jiang, F. Numerical Modeling and Analysis of the Thermal Behavior of NCM Lithium-Ion Batteries Subjected to Very High C-Rate Discharge/Charge Operations. Int. J. Heat Mass Transf. 2018, 117, 261–272. [Google Scholar] [CrossRef]
- Haran, B.S.; Popov, B.N.; White, R.E. Determination of the Hydrogen Diffusion Coefficient in Metal Hydrides by Impedance Spectroscopy. J. Power Sources 1998, 75, 56–63. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and Optimization of the Dual Lithium Ion Insertion Cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rahimian, S.K.; Rayman, S.; White, R.E. Comparison of Single Particle and Equivalent Circuit Analog Models for a Lithium-Ion Cell. J. Power Sources 2011, 196, 8450–8462. [Google Scholar] [CrossRef]
- Li, J.; Adewuyi, K.; Lotfi, N.; Landers, R.G.; Park, J. A Single Particle Model with Chemical/Mechanical Degradation Physics for Lithium Ion Battery State of Health (SOH) Estimation. Appl. Energy 2018, 212, 1178–1190. [Google Scholar] [CrossRef]
- Richardson, G.; Korotkin, I.; Ranom, R.; Castle, M.; Foster, J.M. Generalised Single Particle Models for High-Rate Operation of Graded Lithium-Ion Electrodes: Systematic Derivation and Validation. Electrochim. Acta 2020, 339, 135862. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, G.; Kang, J.; Wang, J.V.; Luo, B.; Chen, C.; Xiang, K. An Algorithm for State of Charge Estimation Based on a Single-Particle Model. J. Energy Storage 2021, 39, 102644. [Google Scholar] [CrossRef]
- Ji, B.; Song, X.G.; Cao, W.P.; Pickert, V. Active Temperature Control of Li-Ion Batteries in Electric Vehicles. IET Conf. Publ. 2013, 2013, 3–7. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, F.; Zhang, H.; Mi, C.C. Robust Predictive Battery Thermal Management Strategy for Connected and Automated Hybrid Electric Vehicles Based on Thermoelectric Parameter Uncertainty. IEEE J. Emerg. Sel. Top. Power Electron. 2018, 6, 1796–1805. [Google Scholar] [CrossRef]
- Ma, Y.; Mou, H.; Zhao, H. Cooling Optimization Strategy for Lithium-Ion Batteries Based on Triple-Step Nonlinear Method. Energy 2020, 201, 117678. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, H.; Mou, H.; Gao, J. Battery Thermal Management Strategy for Electric Vehicles Based on Nonlinear Model Predictive Control. Meas. J. Int. Meas. Confed. 2021, 186, 110115. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Z.; Wang, X.; Jia, L.; Yang, L. Two-Dimensional Electrochemical-Thermal Coupled Modeling of Cylindrical LiFePO4 Batteries. J. Power Sources 2014, 256, 233–243. [Google Scholar] [CrossRef]
- Kim, G.H.; Pesaran, A.; Spotnitz, R. A Three-Dimensional Thermal Abuse Model for Lithium-Ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Zhang, R.; Lin, Y.; Fang, H. On the Characteristics Analysis and Tab Design of an 18650 Type Cylindrical LiFePO4 Battery. Appl. Therm. Eng. 2021, 182, 116144. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Guo, Q.; Ramadass, P.; White, R.E. Review of Models for Predicting the Cycling Performance of Lithium Ion Batteries. J. Power Sources 2006, 156, 620–628. [Google Scholar] [CrossRef]
- Prada, E.; Di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Simplified Electrochemical and Thermal Model of LiFePO4-Graphite Li-Ion Batteries for Fast Charge Applications. J. Electrochem. Soc. 2012, 159, A1508–A1519. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Chen, H. Analysis of Prismatic Lithium-Ion Battery Degradation Based on an Electrochemical-Thermal-Degradation Model. Int. J. Energy Res. 2022, 46, 23658–23681. [Google Scholar] [CrossRef]
- Prada, E.; Di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. A Simplified Electrochemical and Thermal Aging Model of LiFePO4-Graphite Li-Ion Batteries: Power and Capacity Fade Simulations. J. Electrochem. Soc. 2013, 160, A616–A628. [Google Scholar] [CrossRef] [Green Version]
- Ploehn, H.J.; Ramadass, P.; White, R.E. Solvent Diffusion Model for Aging of Lithium-Ion Battery Cells. J. Electrochem. Soc. 2004, 151, A456. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Vora, A.; Hoshing, V.; Saha, T.; Shaver, G.; García, R.E.; Wasynczuk, O.; Varigonda, S. Physically-Based Reduced-Order Capacity Loss Model for Graphite Anodes in Li-Ion Battery Cells. J. Power Sources 2017, 342, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.S.; Kang, D.M. Prediction of Thermal Behaviors of an Air-Cooled Lithium-Ion Battery System for Hybrid Electric Vehicles. J. Power Sources 2014, 270, 273–280. [Google Scholar] [CrossRef]
- Sahel, D.; Ameur, H.; Benzeguir, R.; Kamla, Y. Prediction of Heat Transfer Development in a Smooth Tube. J. Eng. Phys. Thermophys. 2018, 91, 682–687. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, B.; Ren, B.; Chen, H. Integrated Control of In-Wheel Motor Electric Vehicles Using a Triple-Step Nonlinear Method. J. Frankl. Inst. 2015, 352, 519–540. [Google Scholar] [CrossRef]
- Wang, F.; Hao, N.; Song, L.; Chen, H. Triple-Step Nonlinear Control Design for Road Vehicles after a Tire Blow-out on the Highway. In Proceedings of the 2016 12th World Congress on Intelligent Control and Automation (WCICA), Guilin, China, 12–15 June 2016; pp. 1414–1419. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Song, S. Effect of Delayed Impulses on Input-to-State Stability of Nonlinear Systems. Automatica 2017, 76, 378–382. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Zhao, P.; Wang, X. Fast Charging Optimization for Lithium-Ion Batteries Based on Dynamic Programming Algorithm and Electrochemical-Thermal-Capacity Fade Coupled Model. J. Power Sources 2019, 438, 227015. [Google Scholar] [CrossRef]










| Parameter | Value |
|---|---|
| Cell length | 140 mm |
| Cell width | 70 mm |
| Cell thickness | 7 mm |
| Nominal voltage | 3.2 V |
| Charge cut-off voltage | 3.65 V |
| Discharge cut-off voltage | 2 V |
| Nominal capacity | 5 |
| Energy density | 245 |
| Positive electrode material | LiFePO4 |
| Negative electrode material | Graphite-based carbon |
| Electrolyte material | Carbonate based |
| Thermal conductivity, () | 55.66 |
| Density, () | 2318.07 |
| Specific thermal capacity, () | 1056.08 |
| Physical and Chemical Mechanisms | Equations | Boundary Conditions |
|---|---|---|
| Solid phase: conservation of Li+ species | ||
| Electrolyte phase: conservation of Li+ species | ||
| Solid phase: charge conservation | ||
| Electrolyte phase: charge conservation | ||
| Electrochemical kinetics | ||
| Electrolyte ionic diffusivity | = | |
| Electrolyte ionic conductivity | ||
| Electrolyte ionic diffusional conductivity | ||
| Solid phase electronic conductivity | ||
| Specific interfacial surface area |
| Physical and Chemical Mechanisms | Equations | Boundary Conditions |
|---|---|---|
| SEI formation reaction kinetics | ||
| Solvent diffusion in the SEI | ||
| Loss of capacity variation | ||
| SEI porosity variation | ||
| SEI layer thickness |
| Parameters | Equations | Refs. |
|---|---|---|
| Active material in solid phase | ||
| Solid phase diffusivity | [50,52] | |
| [50,52] | ||
| Solid phase conductivity () | [50,52] | |
| [50,52] | ||
| Electrode reaction rate constant () | [50,52] | |
| [50,52] | ||
| Electrolyte | ||
| Electrolyte ionic diffusivity () | [50,52] | |
| Electrolyte ionic conductivity () | [50,52] | |
| SEI formation | ||
| SEI formation reaction rate constant () | [50,52] | |
| SEI solvent diffusivity () | [50,52] | |
| SEI conductivity () | [50,52] | |
| Parameters | Value | Ref. |
|---|---|---|
| (%) | 100 | Estimated |
| () | 0.395 | [51] |
| () | 5 | [51] |
| 0.5 | [51] | |
| 0.5 | [51] | |
| R () | 8.314 | |
| F () | 96,487 | |
| Positive electrode | ||
| () | 70 | [51] |
| at SOC 0% | 0.74 | [51] |
| at SOC 100% | 0.035 | [51] |
| 0.332 | [51] | |
| 0.42 | [51] | |
| () | 0.11 | [51] |
| 2.1 | [51] | |
| () | 22,806 | [51] |
| Negative electrode | ||
| () | 0.395 | [51] |
| () | 34 | [51] |
| at SOC 0% | 0.0132 | [51] |
| at SOC 100% | 0.811 | [51] |
| 0.33 | [51] | |
| 0.555 | [51] | |
| () | 5 | [51] |
| 2.3 | [51] | |
| () | 31,370 | [51] |
| Aging due to SEI layer formation | ||
| () | 0.01 | [52] |
| 0.01 | [52] | |
| (V) | 0.04 | [52] |
| () | 1690 | [52] |
| () | 4541 | [52] |
| 0.5 | [52] | |
| 1.5 | [52] | |
| () | 0.162 | [52] |
| [52] | ||
| Electrolyte | ||
| () | 1200 | [51] |
| 0.363 | [51] | |
| Separator | ||
| () | 25 | [51] |
| 0.54 | [51] | |
| 1.5 | [51] | |
| Parameter | Cold Plate | Coolant | Ambient Air |
|---|---|---|---|
| Initial temperature (K) | 298.15 | 298.15 | 298.15 |
| Thermal conductivity, k () | 237 | 0.4156 | - |
| Density, () | 2700 | 1069 | 1.1691 |
| Specific thermal capacity, () | 897 | 3310 | - |
| Convective heat transfer coefficient, () | - | - | 10 |
| Dynamic viscosity, () | - | 0.004563 |
| Cooling Type | Parallel | Series | |||
|---|---|---|---|---|---|
| Pump rotary speed (r·min−1) | 1000 | 3000 | 1000 | 3000 | |
| Mechanical power provided by the pump to the coolant (W) | 7.168 | 218.873 | 2.620 | 81.552 | |
| Volumetric flow rate (L·min−1) | 18.325 (4.581) | 62.3352 (15.584) | 6.65476 | 23.0198 | |
| Velocity (m·s−1) | 3.868 (0.967) | 13.153 (3.288) | 1.404 | 4.858 | |
| Mass flow rate (kg·s−1) | 0.325 (0.081) | 1.104 (0.276) | 0.118 | 0.408 | |
| C-rates | |||||
| 5 C | Case A | Case F | Case K | Case P | |
| 4 C | Case B | Case G | Case L | Case Q | |
| 3 C | Case C | Case H | Case M | Case R | |
| 2 C | Case D | Case I | Case N | Case S | |
| 1 C | Case E | Case J | Case O | Case T | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, H.; Fang, H. Cooling Optimization Strategy for a 6s4p Lithium-Ion Battery Pack Based on Triple-Step Nonlinear Method. Energies 2023, 16, 460. https://doi.org/10.3390/en16010460
Zhang H, Chen H, Fang H. Cooling Optimization Strategy for a 6s4p Lithium-Ion Battery Pack Based on Triple-Step Nonlinear Method. Energies. 2023; 16(1):460. https://doi.org/10.3390/en16010460
Chicago/Turabian StyleZhang, Hongya, Hao Chen, and Haisheng Fang. 2023. "Cooling Optimization Strategy for a 6s4p Lithium-Ion Battery Pack Based on Triple-Step Nonlinear Method" Energies 16, no. 1: 460. https://doi.org/10.3390/en16010460






