Microbial Granule Technology—Prospects for Wastewater Treatment and Energy Production
Abstract
:1. Introduction and Background
2. Microbial Granule Technology as an Alternative
3. Why Granular Microbial Consortia?
4. Barriers and Limitations
5. New Directions of Biotechnological Granulation
6. Energy Aspects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tušer, I.; Oulehlová, A. Risk Assessment and Sustainability of Wastewater Treatment Plant Operation. Sustainability 2021, 13, 5120. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Ngo, H.H.; Guo, W.; Nguyen, T.L.H.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Lei, Z.; Deng, L. Environmental Impacts and Greenhouse Gas Emissions Assessment for Energy Recovery and Material Recycle of the Wastewater Treatment Plant. Sci. Total Environ. 2021, 784, 147135. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhaskar, T.; Ghosh, D. A Biorefinery Approach for Sewage Sludge. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–421. [Google Scholar] [CrossRef]
- Marami, H.; He, L.; Rafiee, S.; Khoshnevisan, B.; Tsapekos, P.; Mobli, H.; Elyasi, S.N.; Liu, H.; Angelidaki, I. Bridging to Circular Bioeconomy through a Novel Biorefinery Platform on a Wastewater Treatment Plant. Renew. Sustain. Energy Rev. 2022, 154, 111895. [Google Scholar] [CrossRef]
- Ratna, S.; Rastogi, S.; Kumar, R. Current Trends for Distillery Wastewater Management and Its Emerging Applications for Sustainable Environment. J. Environ. Manag. 2021, 290, 112544. [Google Scholar] [CrossRef]
- Gupta, S.; Nayak, A.; Roy, C.; Yadav, A.K. An Algal Assisted Constructed Wetland-Microbial Fuel Cell Integrated with Sand Filter for Efficient Wastewater Treatment and Electricity Production. Chemosphere 2021, 263, 128132. [Google Scholar] [CrossRef] [PubMed]
- Mirghorayshi, M.; Zinatizadeh, A.A.; van Loosdrecht, M. Simultaneous Biodegradability Enhancement and High-Efficient Nitrogen Removal in an Innovative Single Stage Anaerobic/Anoxic/Aerobic Hybrid Airlift Bioreactor (HALBR) for Composting Leachate Treatment: Process Modeling and Optimization. Chem. Eng. J. 2021, 407, 127019. [Google Scholar] [CrossRef]
- Rout, P.R.; Shahid, M.K.; Dash, R.R.; Bhunia, P.; Liu, D.; Varjani, S.; Zhang, T.C.; Surampalli, R.Y. Nutrient Removal from Domestic Wastewater: A Comprehensive Review on Conventional and Advanced Technologies. J. Environ. Manag. 2021, 296, 113246. [Google Scholar] [CrossRef]
- Elmansour, T.E.; Mandi, L.; Hejjaj, A.; Ouazzani, N. Nutrients’ Behavior and Removal in an Activated Sludge System Receiving Olive Mill Wastewater. J. Environ. Manag. 2022, 305, 114254. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Bakare, B.F.; Tetteh, E.K.; Rathilal, S. Sequencing Batch Reactor Performance Evaluation on Orthophosphates and COD Removal from Brewery Wastewater. Fermentation 2022, 8, 296. [Google Scholar] [CrossRef]
- Czerwionka, K.; Wilinska, A.; Tuszynska, A. The Use of Organic Coagulants in the Primary Precipitation Process at Wastewater Treatment Plants. Water 2020, 12, 1650. [Google Scholar] [CrossRef]
- Cardoso, B.J.; Rodrigues, E.; Gaspar, A.R.; Gomes, Á. Energy Performance Factors in Wastewater Treatment Plants: A Review. J. Clean. Prod. 2021, 322, 129107. [Google Scholar] [CrossRef]
- Nierychlo, M.; Andersen, K.S.; Xu, Y.; Green, N.; Jiang, C.; Albertsen, M.; Dueholm, M.S.; Nielsen, P.H. MiDAS 3: An Ecosystem-Specific Reference Database, Taxonomy and Knowledge Platform for Activated Sludge and Anaerobic Digesters Reveals Species-Level Microbiome Composition of Activated Sludge. Water Res. 2020, 182, 115955. [Google Scholar] [CrossRef] [PubMed]
- Debowski, M.; Zielinski, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Kanafina, D.; Malamis, S.; Katsou, E.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment: A Literature Review. Membranes 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Simioni, T.; Agustini, C.B.; Dettmer, A.; Gutterres, M. Performance Enhancement Strategies of Anaerobic Digestion Technology: A Critical Assessment. In Anaerobic Biodigesters for Human Waste Treatment; Springer: Singapore, 2022; pp. 167–189. [Google Scholar] [CrossRef]
- Ghimire, U.; Sarpong, G.; Gude, V.G. Transitioning Wastewater Treatment Plants toward Circular Economy and Energy Sustainability. ACS Omega 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J. Anaerobic Reactor Filling for Phosphorus Removal by Metal Dissolution Method. Materials 2022, 15, 2263. [Google Scholar] [CrossRef]
- Podder, A.; Reinhart, D.; Goel, R. Integrated Leachate Management Approach Incorporating Nutrient Recovery and Removal. Waste Manag. 2020, 102, 420–431. [Google Scholar] [CrossRef]
- Gómez-Camacho, C.E.; Pirone, R.; Ruggeri, B. Is the Anaerobic Digestion (AD) Sustainable from the Energy Point of View? Energy Convers. Manag. 2021, 231, 113857. [Google Scholar] [CrossRef]
- Fernández del Castillo, A.; Garibay, M.V.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; de Anda, J.; Gradilla-Hernández, M.S. A Review of the Sustainability of Anaerobic Reactors Combined with Constructed Wetlands for Decentralized Wastewater Treatment. J. Clean. Prod. 2022, 371, 133428. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Grządziel, J.; Gałązka, A.; Paluch, E.; Borowiak, K.; Pilarski, K. Quantitative and Qualitative Changes in the Genetic Diversity of Bacterial Communities in Anaerobic Bioreactors with the Diatomaceous Earth/Peat Cell Carrier. Cells 2022, 11, 2571. [Google Scholar] [CrossRef]
- Khattabi Rifi, S.; Aguelmous, A.; El Fels, L.; Hafidi, M.; Souabi, S. Effectiveness Assessment of Olive Mill Wastewater Treatment by Combined Process: Natural Flotation and Anaerobic-Aerobic Biodegradation. Water Environ. J. 2021, 35, 986–997. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.V.N.; Mahlia, T.M.I. Strategies to Improve Membrane Performance in Wastewater Treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef]
- Akhbari, A.; Kutty, P.K.; Chuen, O.C.; Ibrahim, S. A Study of Palm Oil Mill Processing and Environmental Assessment of Palm Oil Mill Effluent Treatment. Environ. Eng. Res. 2020, 25, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Yao, J.C.; Liu, Y.D.; Li, W. Performance, Sludge Characteristics and Microbial Community in a Salt-Tolerant Aerobic Granular SBR by Seeding Anaerobic Granular Sludge. Int. Biodeterior. Biodegradation 2021, 163, 105258. [Google Scholar] [CrossRef]
- Ran, X.; Zhou, M.; Wang, T.; Wang, W.; Kumari, S.; Wang, Y. Multidisciplinary Characterization of Nitrogen-Removal Granular Sludge: A Review of Advances and Technologies. Water Res. 2022, 214, 118214. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Liu, Y. Assessment of Microalgal-Bacterial Granular Sludge Process for Environmentally Sustainable Municipal Wastewater Treatment. ACS ES&T Water 2021, 1, 2459–2469. [Google Scholar] [CrossRef]
- Ji, B.; Shi, Y.; Yılmaz, M. Microalgal-Bacterial Granular Sludge Process for Sustainable Municipal Wastewater Treatment: Simple Organics versus Complex Organics. J. Water Process Eng. 2022, 46, 102613. [Google Scholar] [CrossRef]
- Fan, S.; Zhu, L.; Ji, B. Deciphering the Effect of Light Intensity on Microalgal-Bacterial Granular Sludge Process for Non-Aerated Municipal Wastewater Treatment. Algal Res. 2021, 58, 102437. [Google Scholar] [CrossRef]
- Wang, H.; Deng, L.; Qi, Z.; Wang, W. Constructed Microalgal-Bacterial Symbiotic (MBS) System: Classification, Performance, Partnerships and Perspectives. Sci. Total Environ. 2022, 803, 150082. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Gupta, D.K. Constructed Wetland, an Eco-Technology for Wastewater Treatment: A Review on Various Aspects of Microbial Fuel Cell Integration, Low Temperature Strategies and Life Cycle Impact of the Technology. Renew. Sustain. Energy Rev. 2021, 148, 111261. [Google Scholar] [CrossRef]
- Sun, Y.; Bi, K.; Yin, S. Measuring and Integrating Risk Management into Green Innovation Practices for Green Manufacturing under the Global Value Chain. Sustainability 2020, 12, 545. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of Techno-Economic Analysis and Life Cycle Assessment for Sustainable Process Design—A Review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Erans, M.; Sanz-Pérez, E.S.; Hanak, D.P.; Clulow, Z.; Reiner, D.M.; Mutch, G.A. Direct Air Capture: Process Technology, Techno-Economic and Socio-Political Challenges. Energy Environ. Sci. 2022, 15, 1360–1405. [Google Scholar] [CrossRef]
- Narayanamoorthy, S.; Brainy, J.V.; Sulaiman, R.; Ferrara, M.; Ahmadian, A.; Kang, D. An Integrated Decision Making Approach for Selecting a Sustainable Waste Water Treatment Technology. Chemosphere 2022, 301, 134568. [Google Scholar] [CrossRef]
- Trianni, A.; Negri, M.; Cagno, E. What Factors Affect the Selection of Industrial Wastewater Treatment Configuration? J. Environ. Manag. 2021, 285, 112099. [Google Scholar] [CrossRef]
- Ullah, A.; Hussain, S.; Wasim, A.; Jahanzaib, M. Development of a Decision Support System for the Selection of Wastewater Treatment Technologies. Sci. Total Environ. 2020, 731, 139158. [Google Scholar] [CrossRef]
- Bhambore, N.; Suresh Kumar, M. Municipal Solid Waste Generation, Management Scenarios, and Leachate Treatment Using Sequencing Batch Biofilter Granular Reactor. Process. Saf. Environ. Prot. 2022, 167, 454–468. [Google Scholar] [CrossRef]
- Derlon, N.; Garcia Villodres, M.; Kovács, R.; Brison, A.; Layer, M.; Takács, I.; Morgenroth, E. Modelling of Aerobic Granular Sludge Reactors: The Importance of Hydrodynamic Regimes, Selective Sludge Removal and Gradients. Water Sci. Technol. 2022, 86, 410–431. [Google Scholar] [CrossRef]
- Xu, L.; Pang, Y.; Liu, W.; Chen, H.; Huang, S.; Zhu, L. Hypersaline Wastewater Produced from Pickled Mustard Tuber (Chinese Zhacai): Current Treatment Status and Prospects. Water 2022, 14, 1508. [Google Scholar] [CrossRef]
- Kim, S.M.; Sim, Y.-B.; Baik, J.-H.; Yang, J.; Pandey, A.K.; Joo, H.-H.; Jung, J.-H.; Kim, S.-H. Formation and Characterization of H2-Producing Granule in a Pilot-Scale Dynamic Membrane Bioreactor. Chem. Eng. J. 2023, 452, 139384. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M. Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustainability 2022, 14, 10904. [Google Scholar] [CrossRef]
- Krzemieniewski, M.; Debowski, M.; Dobrzynska, A.; Zielinski, M. Chemical Oxygen Demand Reduction of Various Wastewater Types Using Magnetic Field-Assisted Fenton Reaction. Water Environ. Res. 2004, 76, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Kumar Padhi, S.; Kumar Dikshit, P.; Pattanaik, L. Recent Developments in Landfill Leachate Treatment: Aerobic Granular Reactor and Its Future Prospects. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100689. [Google Scholar] [CrossRef]
- Tsai, S.W.; Schwinghammer, L.; Lee, C.H.; Lin, C.F.; Hou, C.H. Shifts of Microbial Community Structure along Substrate Concentration Gradients in Immobilized Biomass for Nitrogen Removal. NPJ Clean Water 2022, 5, 3893. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy Mechanism, Microbial Population, and Process Optimization for Volatile Fatty Acids Metabolism in Anaerobic Digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Franca, R.D.G.; Pinheiro, H.M.; Lourenço, N.D. Recent Developments in Textile Wastewater Biotreatment: Dye Metabolite Fate, Aerobic Granular Sludge Systems and Engineered Nanoparticles. Rev. Environ. Sci. Bio/Technol. 2020, 19, 149–190. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, H.; Song, Y.; Lu, C.; Han, Y.; Hou, Y. Granulation and Response of Anaerobic Granular Sludge to Allicin Stress While Treating Allicin-Containing Wastewater. Biochem. Eng. J. 2021, 169, 107971. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Sun, J.; Xu, Q.; Ni, B.J. Long-Term Effects of Polyvinyl Chloride Microplastics on Anaerobic Granular Sludge for Recovering Methane from Wastewater. Environ. Sci. Technol. 2020, 54, 9662–9671. [Google Scholar] [CrossRef]
- Ma, H.; Wu, M.; Liu, H.; Wang, Z.; Guo, C.; Wang, S. Study on Enhancing Sludge Methanogenesis by Adding Acetylene Black and Effect on the Characteristics & Microbial Community of Anaerobic Granular Sludge. RSC Adv. 2019, 9, 23086–23095. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, H.; Lee, C. Formation and Characterization of Conductive Magnetite-Embedded Granules in Upflow Anaerobic Sludge Blanket Reactor Treating Dairy Wastewater. Bioresour. Technol. 2022, 345, 126492. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiol. Russian Fed. 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Zheng, Y.; Quan, X.; Zhuo, M.; Zhang, X.; Quan, Y. In-Situ Formation and Self-Immobilization of Biogenic Fe Oxides in Anaerobic Granular Sludge for Enhanced Performance of Acidogenesis and Methanogenesis. Sci. Total Environ. 2021, 787, 147400. [Google Scholar] [CrossRef]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.A.; Tyagi, V.K. Conductive Material Engineered Direct Interspecies Electron Transfer (DIET) in Anaerobic Digestion: Mechanism and Application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Koch, K.; Mata-Alvarez, J.; Dosta, J. Co-Digestion of Sewage Sludge and Food Waste in a Wastewater Treatment Plant Based on Mainstream Anaerobic Membrane Bioreactor Technology: A Techno-Economic Evaluation. Bioresour. Technol. 2021, 330, 124978. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.P.; Peng, D.C.; Zhang, A.L.; Wang, X.B.; Pei, L.Y. Sludge Granulation in ASBR: Reactor Performance, Sludge Physiochemical Properties Evolution and the Unique Clustered Structure of the Granular Sludge. J. Water Process. Eng. 2022, 49, 102948. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Zhang, H.; Peng, Y. Formation of Partial-Denitrification (PD) Granular Sludge from Low-Strength Nitrate Wastewater: The Influence of Loading Rates. J. Hazard. Mater. 2020, 384, 121273. [Google Scholar] [CrossRef]
- Muñoz-Palazón, B.; Hurtado-Martinez, M.; Gonzalez-Lopez, J. Simultaneous Nitrification and Denitrification Processes in Granular Sludge Technology. In Nitrogen Cycle; CRC Press: Boca Raton, FL, USA, 2021; pp. 222–244. [Google Scholar] [CrossRef]
- Wang, H.; Lyu, W.; Song, Q.; Zhou, D.; Hu, X.; Wang, B.; Chen, R. Role of Weak Magnetic Strength in the Operation of Aerobic Granular Reactor for Wastewater Treatment Containing Ammonia Nitrogen Concentration Gradient. Bioresour. Technol. 2021, 322, 124570. [Google Scholar] [CrossRef]
- Campo, R.; Lubello, C.; Lotti, T.; Di Bella, G. Aerobic Granular Sludge–Membrane BioReactor (AGS–MBR) as a Novel Configuration for Wastewater Treatment and Fouling Mitigation: A Mini-Review. Membranes 2021, 11, 261. [Google Scholar] [CrossRef]
- Di Biase, A.; Corsino, F.S.; Devlin, T.R.; Torregrossa, M.; Munz, G.; Oleszkiewicz, J.A. Aerobic Granular Sludge Treating Anaerobically Pretreated Brewery Wastewater at Different Loading Rates. Water Sci. Technol. 2020, 82, 1523–1534. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Lawal, I.M.; Abubakar, S.; Hassan, I.; Zubairu, I.; Umaru, I.; Abdurrasheed, A.S.; Adam, A.A.; Ghaleb, A.A.S.; et al. Sequencing Batch Reactor Technology for Landfill Leachate Treatment: A State-of-the-Art Review. J. Environ. Manag. 2021, 282, 111946. [Google Scholar] [CrossRef]
- Liu, S.; Daigger, G.T.; Liu, B.; Zhao, W.; Liu, J. Enhanced Performance of Simultaneous Carbon, Nitrogen and Phosphorus Removal from Municipal Wastewater in an Anaerobic-Aerobic-Anoxic Sequencing Batch Reactor (AOA-SBR) System by Alternating the Cycle Times. Bioresour. Technol. 2020, 301, 122750. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, R.; Paul, A.; Lewandowski, M. Improving SBR Performance Alongside with Cost Reduction through Optimizing Biological Processes and Dissolved Oxygen Concentration Trajectory. Appl. Sci. 2019, 9, 2268. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, E.J.H.; Pronk, M.; van Loosdrecht, M.C.M. A Settling Model for Full-Scale Aerobic Granular Sludge. Water Res. 2020, 186, 116135. [Google Scholar] [CrossRef]
- Mariraj Mohan, S.; Swathi, T. A Review on Upflow Anaerobic Sludge Blanket Reactor: Factors Affecting Performance, Modification of Configuration and Its Derivatives. Water Environ. Res. 2022, 94, e1665. [Google Scholar] [CrossRef]
- Show, K.Y.; Yan, Y.; Yao, H.; Guo, H.; Li, T.; Show, D.Y.; Chang, J.S.; Lee, D.J. Anaerobic Granulation: A Review of Granulation Hypotheses, Bioreactor Designs and Emerging Green Applications. Bioresour. Technol. 2020, 300, 122751. [Google Scholar] [CrossRef] [PubMed]
- Pronk, M.; van Dijk, E.J.; Mark, C.V. Aerobic Granular Sludge. In Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2020; pp. 497–522. [Google Scholar] [CrossRef]
- Janiak, K.; Zięba, B.; Szetela, R.; Muszyński-Huhajło, M.; Miodoński, S.; Jurga, A.; Trusz, A. Approach to Modeling Protozoa Grazing on the Basis of the Current State of Knowledge. Ecol. Modell. 2021, 447, 109503. [Google Scholar] [CrossRef]
- Imachi, H.; Nobu, M.K.; Miyazaki, M.; Tasumi, E.; Saito, Y.; Sakai, S.; Ogawara, M.; Ohashi, A.; Takai, K. Cultivation of Previously Uncultured Microorganisms with a Continuous-Flow down-Flow Hanging Sponge (DHS) Bioreactor, Using a Syntrophic Archaeon Culture Obtained from Deep Marine Sediment as a Case Study. Nat. Protoc. 2022, 17, 2784–2814. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; Wang, Y.; Du, G.; Tay, J.H.; Li, J. Piggery Wastewater Treatment by Aerobic Granular Sludge: Granulation Process and Antibiotics and Antibiotic-Resistant Bacteria Removal and Transport. Bioresour. Technol. 2019, 273, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Extensive Studies on the Treatment of Pulp Mill Wastewater Using Aerobic Granular Sludge (AGS) Technology. Chem. Eng. J. 2019, 359, 1175–1194. [Google Scholar] [CrossRef]
- Kent, J.; Tay, J.H. Treatment of 17α-ethinylestradiol, 4-nonylphenol, and Carbamazepine in Wastewater Using an Aerobic Granular Sludge Sequencing Batch Reactor. Sci. Total Environ. 2019, 652, 1270–1278. [Google Scholar] [CrossRef]
- Cai, W.; Huang, W.; Lei, Z.; Zhang, Z.; Lee, D.J.; Adachi, Y. Granulation of Activated Sludge Using Butyrate and Valerate as Additional Carbon Source and Granular Phosphorus Removal Capacity during Wastewater Treatment. Bioresour. Technol. 2019, 282, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sguanci, S.; Lubello, C.; Caffaz, S.; Lotti, T. Long-Term Stability of Aerobic Granular Sludge for the Treatment of Very Low-Strength Real Domestic Wastewater. J. Clean. Prod. 2019, 222, 882–890. [Google Scholar] [CrossRef]
- Zakoura, M.; Kopsahelis, A.; Tsigkou, K.; Ntougias, S.; Ali, S.S.; Kornaros, M. Performance Evaluation of Three Mesophilic Upflow Anaerobic Sludge Blanket Bioreactors Treating Olive Mill Wastewater: Flocculent and Granular Inocula Tests, Organic Loading Rate Effect and Anaerobic Consortia Structure. Fuel 2022, 313, 122951. [Google Scholar] [CrossRef]
- De Barros, V.G.; Duda, R.M.; de Oliveira, R.A. Biomethane Production from Vinasse in Upflow Anaerobic Sludge Blanket Reactors Inoculated with Granular Sludge. Brazilian J. Microbiol. 2016, 47, 628–639. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular Biopolymers Recovered as Raw Biomaterials from Waste Granular Sludge and Potential Applications: A Critical Review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef]
- Tanavarotai, K.; Kamyab, H.; Nor Anuar, A.; Khademi, T.; Yuzir, A.; Ashokkumar, V.; Rezania, S. Storage and Reactivation of Aerobic Granular Sludge: A Review. Fuel 2022, 330, 125536. [Google Scholar] [CrossRef]
- Zhang, L.; Long, B.; Cheng, Y.; Wu, J.; Zhang, B.; Zeng, Y.; Huang, S.; Zeng, M. Rapid Cultivation and Stability of Autotrophic Nitrifying Granular Sludge. Water Sci. Technol. 2020, 81, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Yenji, S.S.; Munavalli, G.R.; Koli, M.M. Field-Scale Anaerobic Baffled Reactor for Domestic Wastewater Treatment: Effect of Dynamic Operating Conditions. Water Pract. Technol. 2021, 16, 42–58. [Google Scholar] [CrossRef]
- Tavares Ferreira, T.J.; Luiz de Sousa Rollemberg, S.; Nascimento de Barros, A.; Machado de Lima, J.P.; Bezerra dos Santos, A. Integrated Review of Resource Recovery on Aerobic Granular Sludge Systems: Possibilities and Challenges for the Application of the Biorefinery Concept. J. Environ. Manag. 2021, 291, 112718. [Google Scholar] [CrossRef]
- Mdladla, C.T.; Dyosile, P.A.; Njoya, M.; Basitere, M.; Ntwampe, S.K.O.; Kaskote, E. Poultry Slaughterhouse Wastewater Remediation Using a Bio-Delipidation Pre-Treatment Unit Coupled with an Expanded Granular Sludge Bed Reactor. Processes 2021, 9, 1938. [Google Scholar] [CrossRef]
- Liu, W.; Lian, J.; Guo, J.; Guo, Y.; Yue, L.; Niu, Y.; Duan, L. Perchlorate Bioreduction by Anaerobic Granular Sludge Immobilised with Fe-HA Complex: Performance, Extracellular Polymeric Substances and Microbial Community Structure. J. Hazard. Mater. 2020, 398, 122898. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Amorim, C.L.; Mesquita, D.P.; Ferreira, E.C.; van Loosdrecht, M.; Castro, P.M.L. Increased Extracellular Polymeric Substances Production Contributes for the Robustness of Aerobic Granular Sludge during Long-Term Intermittent Exposure to 2-Fluorophenol in Saline Wastewater. J. Water Process. Eng. 2021, 40, 101977. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Cheng, M.; Zhang, H.; Yang, Y.; Liu, N.; Liao, J. Performance and Mechanism of Anaerobic Granular Sludge Enhancing Uranium Immobilization via Extracellular Polymeric Substances in Column Reactors and Batch Experiments. J. Clean. Prod. 2022, 363, 132517. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.; Morgan, G.; Iorhemen, O.T. A Review of the State of Development of Aerobic Granular Sludge Technology over the Last 20 Years: Full-Scale Applications and Resource Recovery. Case Stud. Chem. Environ. Eng. 2022, 5, 100173. [Google Scholar] [CrossRef]
- Lin, H.; Ma, R.; Hu, Y.; Lin, J.; Sun, S.; Jiang, J.; Li, T.; Liao, Q.; Luo, J. Reviewing bottlenecks in aerobic granular sludge technology: Slow granulation and low granular stability. Environ. Pollut. 2020, 263, 114638. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic Granular Sludge Process: A Fast Growing Biological Treatment for Sustainable Wastewater Treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Wang, S.; Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. Tetracycline-Induced Decoupling of Symbiosis in Microalgal-Bacterial Granular Sludge. Environ. Res. 2021, 197, 111095. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, J.A.; Muñoz, R.; Donoso-Bravo, A.; Bernard, O.; Casagli, F.; Jeison, D. A Half-Century of Research on Microalgae-Bacteria for Wastewater Treatment. Algal. Res. 2022, 67, 102828. [Google Scholar] [CrossRef]
- Yong, J.J.J.Y.; Chew, K.W.; Khoo, K.S.; Show, P.L.; Chang, J.S. Prospects and Development of Algal-Bacterial Biotechnology in Environmental Management and Protection. Biotechnol. Adv. 2021, 47, 107684. [Google Scholar] [CrossRef]
- Sátiro, J.; Cunha, A.; Gomes, A.P.; Simões, R.; Albuquerque, A. Optimization of Microalgae–Bacteria Consortium in the Treatment of Paper Pulp Wastewater. Appl. Sci. 2022, 12, 5799. [Google Scholar] [CrossRef]
- Zhang, Y.; Zha, M.; Gao, M.; Wang, X. How Weak Static Magnetic Field Contributes to Rapid Granulation and Better Performance of Microalgal-Bacterial Granular Sludge? Chem. Eng. J. 2022, 450, 138162. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Chen, S.; Guo, N.; Xiang, P.; Lin, S.; Bai, Y.; Hu, X.; Zhang, Z. Evaluating the Role of Algae in Algal-Bacterial Granular Sludge: Nutrient Removal, Microbial Community and Granular Characteristics. Bioresour. Technol. 2022, 365, 128165. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xin, Y.; Tong, Z.H.; Zheng, Y.M.; Xie, J.F.; Zhao, Q.B.; Yu, H.Q. Storage Strategy of Aerobic Algae-Bacteria Granular Consortia in Photo-Sequencing Batch Reactor. J. Clean. Prod. 2022, 363, 132410. [Google Scholar] [CrossRef]
- Alegbeleye, O.O. Bioremediation of Wastewaters. In Rhizobiont in Bioremediation of Hazardous Waste; Springer: Singapore, 2021; pp. 483–509. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, H.; Zhang, C.; Xie, Y.; Ho, S.H. Emerging Biological Wastewater Treatment Using Microalgal-Bacterial Granules: A Review. Bioresour. Technol. 2022, 351, 127089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Shen, Q.; Chen, X.; Li, F.; Wang, J.; Zhang, Z.; Lei, Z.; Yuan, T.; Shimizu, K. Energy Saving and Rapid Establishment of Granular Microalgae System from Tiny Microalgae Cells: Effect of Decrease in Upflow Air Velocity under Intermittent Aeration Condition. Bioresour. Technol. 2022, 363, 127860. [Google Scholar] [CrossRef] [PubMed]
- Azarpour, A.; Zendehboudi, S.; Mohammadzadeh, O.; Rajabzadeh, A.R.; Chatzis, I. A Review on Microalgal Biomass and Biodiesel Production through Co-Cultivation Strategy. Energy Convers. Manag. 2022, 267, 115757. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. A Self-Sustaining Synergetic Microalgal-Bacterial Granular Sludge Process towards Energy-Efficient and Environmentally Sustainable Municipal Wastewater Treatment. Water Res. 2020, 179, 115884. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspecies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.S. Microalgae-Based Wastewater Treatment—Microalgae-Bacteria Consortia, Multi-Omics Approaches and Algal Stress Response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef]
- Purswani, J.; Llorente, C.P. Nitrification and Denitrification Processes: Environmental Impacts. In Nitrogen Cycle; CRC Press: Boca Raton, FL, USA, 2021; pp. 60–81. [Google Scholar] [CrossRef]
- Yadav, A.; Rene, E.R.; Sharma, M.; Jatain, I.; Mandal, M.K.; Dubey, K.K. Valorization of Wastewater to Recover Value-Added Products: A Comprehensive Insight and Perspective on Different Technologies. Environ. Res. 2022, 214, 113957. [Google Scholar] [CrossRef]
- Lyu, W.; Zhang, S.; Xie, Y.; Chen, R.; Hu, X.; Wang, B.; Guo, W.; Wang, H.; Xing, J.; Zhou, D. Effects of the Exogenous N-Acylhomoserine Lactones on the Performances of Microalgal-Bacterial Granular Consortia. Environ. Pollut. Bioavailab. 2022, 34, 407–418. [Google Scholar] [CrossRef]
- Abbew, A.W.; Amadu, A.A.; Qiu, S.; Champagne, P.; Adebayo, I.; Anifowose, P.O.; Ge, S. Understanding the Influence of Free Nitrous Acid on Microalgal-Bacterial Consortium in Wastewater Treatment: A Critical Review. Bioresour. Technol. 2022, 363, 127916. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, H.; Li, H.; Lu, Q.; Sun, Y. Does Exogenous Carbon Source Always Promote Algal Biomass and Nutrients Removal in Algal-Bacterial Wastewater Remediation? J. Clean. Prod. 2021, 281, 125371. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal Co-Cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. BioEnergy Res. 2021, 15, 1–26. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biol. 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Devi, T.E.; Parthiban, R. Hydrothermal Liquefaction of Nostoc Ellipsosporum Biomass Grown in Municipal Wastewater under Optimized Conditions for Bio-Oil Production. Bioresour. Technol. 2020, 316, 123943. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Katam, K.; Show, P.L.; Gadhamshetty, V.; Upadhyayula, V.K.K.; Bhattacharyya, D. A Comprehensive Review on the Use of Algal-Bacterial Systems for Wastewater Treatment with Emphasis on Nutrient and Micropollutant Removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Pei, H. Algal–Bacterial Consortia for Bioproduct Generation and Wastewater Treatment. Renew. Sustain. Energy Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.; Tian, C.; Liu, S.; Wang, Q.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.J. Ionic Response of Algal-Bacterial Granular Sludge System during Biological Phosphorus Removal from Wastewater. Chemosphere 2021, 264, 128534. [Google Scholar] [CrossRef]
- Van Nguyen, B.; Yang, X.; Hirayama, S.; Wang, J.; Zhao, Z.; Lei, Z.; Shimizu, K.; Zhang, Z.; Le, S.X. Effect of Salinity on Cr(VI) Bioremediation by Algal-Bacterial Aerobic Granular Sludge Treating Synthetic Wastewater. Processes 2021, 9, 1400. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, Z.; Yang, X.; Lei, Z.; Shimizu, K.; Zhang, Z.; Lee, D.J. Response and Recovery of Mature Algal-Bacterial Aerobic Granular Sludge to Sudden Salinity Disturbance in Influent Wastewater: Granule Characteristics and Nutrients Removal/Accumulation. Bioresour. Technol. 2021, 321, 124492. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, D.; Huang, W.; Cai, W.; Zhang, Z.; Lei, Z. Achieving Partial Nitrification and High Lipid Production in an Algal-Bacterial Granule System When Treating Low COD/NH4–N Wastewater. Chemosphere 2020, 248, 126106. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.A.; Guimarães, L.B.; Magnus, B.S.; Leite, W.R.; Vilar, V.J.P.; da Costa, R.H.R. How Volumetric Exchange Ratio and Carbon Availability Contribute to Enhance Granular Sludge Stability in a Fill/Draw Mode SBR Treating Domestic Wastewater? J. Water Process. Eng. 2021, 40, 101917. [Google Scholar] [CrossRef]
- Shi, Y.J.; Yang, L.; Liao, S.F.; Zhang, L.G.; Liao, Z.C.; Lan, M.Y.; Sun, F.; Ying, G.G. Responses of Aerobic Granular Sludge to Fluoroquinolones: Microbial Community Variations, and Antibiotic Resistance Genes. J. Hazard. Mater. 2021, 414, 125527. [Google Scholar] [CrossRef]
- Xian, C.; Gong, C.; Lu, F.; Wu, H.; Ouyang, Z. The Evaluation of Greenhouse Gas Emissions from Sewage Treatment with Urbanization: Understanding the Opportunities and Challenges for Climate Change Mitigation in China’s Low-Carbon Pilot City, Shenzhen. Sci. Total Environ. 2023, 855, 158629. [Google Scholar] [CrossRef]
- Mittapalli, S.; Sharma, H.B.; Dubey, B.K. Hydrothermal Carbonization of Anaerobic Granular Sludge and Co-Pelletization of Hydrochar with Yard Waste. Bioresour. Technol. Reports 2021, 14, 100691. [Google Scholar] [CrossRef]
- Guven, H.; Ersahin, M.E.; Ozgun, H. Energy Self-Sufficiency in Wastewater Treatment Plants: Perspectives, Challenges, and Opportunities. Clean Energy Resour. Recover. Wastewater Treat. Plants Biorefineries 2022, 2, 105–122. [Google Scholar] [CrossRef]
- Kosar, S.; Isik, O.; Cicekalan, B.; Gulhan, H.; Cingoz, S.; Yoruk, M.; Ozgun, H.; Koyuncu, I.; van Loosdrecht, M.C.M.; Ersahin, M.E. Coupling High-Rate Activated Sludge Process with Aerobic Granular Sludge Process for Sustainable Municipal Wastewater Treatment. J. Environ. Manag. 2023, 325, 116549. [Google Scholar] [CrossRef]
- Markowski, M.; Białobrzewski, I.; Zieliński, M.; Debowski, M.; Krzemieniewski, M. Optimizing Low-Temperature Biogas Production from Biomass by Anaerobic Digestion. Renew. Energy 2014, 69, 219–225. [Google Scholar] [CrossRef]
- Zielinski, M.; Debowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, 14, 590. [Google Scholar] [CrossRef]
- Ali Abd, A.; Roslee Othman, M. Biogas Upgrading to Fuel Grade Methane Using Pressure Swing Adsorption: Parametric Sensitivity Analysis on an Industrial Scale. Fuel 2022, 308, 121986. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Biogas Production and Applications in the Sustainable Energy Transition. J. Energy 2022, 2022, 8750221. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, D.; Liu, Y.; Wang, Q.; Ni, B.J.; Chen, F.; Yang, Q.; Li, X.; Zeng, G.; et al. Free Nitrous Acid Promotes Hydrogen Production from Dark Fermentation of Waste Activated Sludge. Water Res. 2018, 145, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wang, J. Biohydrogen Production by Co-Fermentation of Sewage Sludge and Grass Residue: Effect of Various Substrate Concentrations. Fuel 2019, 237, 1203–1208. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Mechanisms of Enhanced Hydrogen Production from Sewage Sludge by Ferrous Ion: Insights into Functional Genes and Metabolic Pathways. Bioresour. Technol. 2021, 321, 124435. [Google Scholar] [CrossRef]
- Karapinar, I.; Gokfiliz Yildiz, P.; Pamuk, R.T.; Karaosmanoglu Gorgec, F. The Effect of Hydraulic Retention Time on Thermophilic Dark Fermentative Biohydrogen Production in the Continuously Operated Packed Bed Bioreactor. Int. J. Hydrogen Energy 2020, 45, 3524–3531. [Google Scholar] [CrossRef]
- Jahn, L.; Saracevic, E.; Svardal, K.; Krampe, J. Anaerobic Biodegradation and Dewaterability of Aerobic Granular Sludge. J. Chem. Technol. Biotechnol. 2019, 94, 2908–2916. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Yu, Z.; Lu, J.; Li, D.; Wang, G.; Li, Y.; Wu, Y.; Li, S.; Xu, F.; et al. Effect of Inoculum and Substrate/Inoculum Ratio on the Performance and Methanogenic Archaeal Community Structure in Solid State Anaerobic Co-Digestion of Tomato Residues with Dairy Manure and Corn Stover. Waste Manag. 2018, 81, 117–127. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical Conversion of Sewage Sludge for Energy and Resource Recovery: Technical Challenges and Prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the Application of Gasification and Combustion Technology and Waste-to-Energy Technologies in Sewage Sludge Treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Syamsiro, M.; Dwicahyo, M.S.; Sulistiawati, Y.; Ridwan, M.; Citrasari, N. Development of a Rotary Kiln Reactor for Pyrolytic Oil Production from Waste Tire in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 245, 012044. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Effect of Process Parameters on the Production of Pyrolytic Products from Biomass Through Pyrolysis. Liq. Biofuels Fundam. Charact. Appl. 2021, 231–284. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Menezes, L.N.B.; Silveira, E.A.; Mazzoni, J.V.S.; Evaristo, R.B.W.; Rodrigues, J.S.; Lamas, G.C.; Suarez, P.A.Z.; Ghesti, G.F. Alternative Valuation Pathways for Primary, Secondary, and Tertiary Sewage Sludge: Biochar and Bio-Oil Production for Sustainable Energy. Biomass Convers. Biorefinery 2022, 1, 1–14. [Google Scholar] [CrossRef]
- Wang, P.; Pu, G.; Liu, Q.; Xiong, W. Alkali Metal Modified Iron-Nickel Oxygen Carrier to Produce Hydrogen-Rich Synthesis Gas by Chemical Looping Gasification with Pine Sawdust. Int. J. Energy Res. 2021, 45, 5165–5176. [Google Scholar] [CrossRef]
- Cameretti, M.C.; Cappiello, A.; de Robbio, R.; Tuccillo, R. Comparison between Hydrogen and Syngas Fuels in an Integrated Micro Gas Turbine/Solar Field with Storage. Energies 2020, 13, 4764. [Google Scholar] [CrossRef]
- Xu, X.J.; Wang, W.Q.; Chen, C.; Xie, P.; Liu, W.Z.; Zhou, X.; Wang, X.T.; Yuan, Y.; Wang, A.J.; Lee, D.J.; et al. Bioelectrochemical System for the Enhancement of Methane Production by Anaerobic Digestion of Alkaline Pretreated Sludge. Bioresour. Technol. 2020, 304, 123000. [Google Scholar] [CrossRef]
- Ardakani, M.N.; Badalians Gholikandi, G. Microbial Fuel Cells (MFCs) in Integration with Anaerobic Treatment Processes (AnTPs) and Membrane Bioreactors (MBRs) for Simultaneous Efficient Wastewater/Sludge Treatment and Energy Recovery -A State-of-the-Art Review. Biomass Bioenergy 2020, 141, 105726. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Das, I.; Ghangrekar, M.M.; Pant, D. Moving towards Practical Applications of Microbial Fuel Cells for Sanitation and Resource Recovery. J. Water Process. Eng. 2020, 38, 101566. [Google Scholar] [CrossRef]
- Xia, C.; Van Le, Q.; Chinnathambi, A.; Salmen, S.H.; Alharbi, S.A.; Tola, S. Role of ZnO and Fe2O3 Nanoparticle on Synthetic Saline Wastewater on Growth, Nutrient Removal and Lipid Content of Chlorella Vulgaris for Sustainable Production of Biofuel. Fuel 2021, 300, 120924. [Google Scholar] [CrossRef]
- Bernat, K.; Cydzik-Kwiatkowska, A.; Wojnowska-Baryła, I.; Karczewska, M. Physicochemical Properties and Biogas Productivity of Aerobic Granular Sludge and Activated Sludge. Biochem. Eng. J. 2017, 117, 43–51. [Google Scholar] [CrossRef]
- Guo, H.; Felz, S.; Lin, Y.; van Lier, J.B.; de Kreuk, M. Structural Extracellular Polymeric Substances Determine the Difference in Digestibility between Waste Activated Sludge and Aerobic Granules. Water Res. 2020, 181, 115924. [Google Scholar] [CrossRef]
- Huang, F.; Yu, Y.; Huang, H. Temperature Influence and Distribution of Bio-Oil from Pyrolysis of Granular Sewage Sludge. J. Anal. Appl. Pyrolysis 2018, 130, 36–42. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Buisman, C.J.N. Alcohol Production through Volatile Fatty Acids Reduction with Hydrogen as Electron Donor by Mixed Cultures. Water Res. 2008, 42, 4059–4066. [Google Scholar] [CrossRef]
- He, Y.; Cassarini, C.; Marciano, F.; Lens, P.N.L. Homoacetogenesis and Solventogenesis from H2/CO2 by Granular Sludge at 25, 37 and 55 °C. Chemosphere 2021, 265, 128649. [Google Scholar] [CrossRef]
- Cruz-Méndez, A.; Suárez-Vázquez, S.I.; Reyna-Gómez, L.M.; Cruz-López, A. Comparative Study between Compost and Granular Sludge Inoculums as Promising Microbial Consortia Sources for Biohydrogen Production from Food Industry Wastewater. Biofuels 2022, 13, 1173–1182. [Google Scholar] [CrossRef]
- Zhao, N.; Treu, L.; Angelidaki, I.; Zhang, Y. Exoelectrogenic Anaerobic Granular Sludge for Simultaneous Electricity Generation and Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 12130–12140. [Google Scholar] [CrossRef]
- Liu, L.; Hong, Y.; Ye, X.; Wei, L.; Liao, J.; Huang, X.; Liu, C. Biodiesel Production from Microbial Granules in Sequencing Batch Reactor. Bioresour. Technol. 2018, 249, 908–915. [Google Scholar] [CrossRef]
- Cui, B.; Chen, Z.; Guo, D.; Liu, Y. Investigations on the Pyrolysis of Microalgal-Bacterial Granular Sludge: Products, Kinetics, and Potential Mechanisms. Bioresour. Technol. 2022, 349, 126328. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xi, L.; Liu, D.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Effects of Light Intensity on Oxygen Distribution, Lipid Production and Biological Community of Algal-Bacterial Granules in Photo-Sequencing Batch Reactors. Bioresour. Technol. 2019, 272, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huo, H.; Meng, F. Partial Nitrification Algal-Bacterial Granule System Cultivation: Performance, Lipid Production and Biological Community. Water. Air. Soil Pollut. 2020, 231, 236. [Google Scholar] [CrossRef]


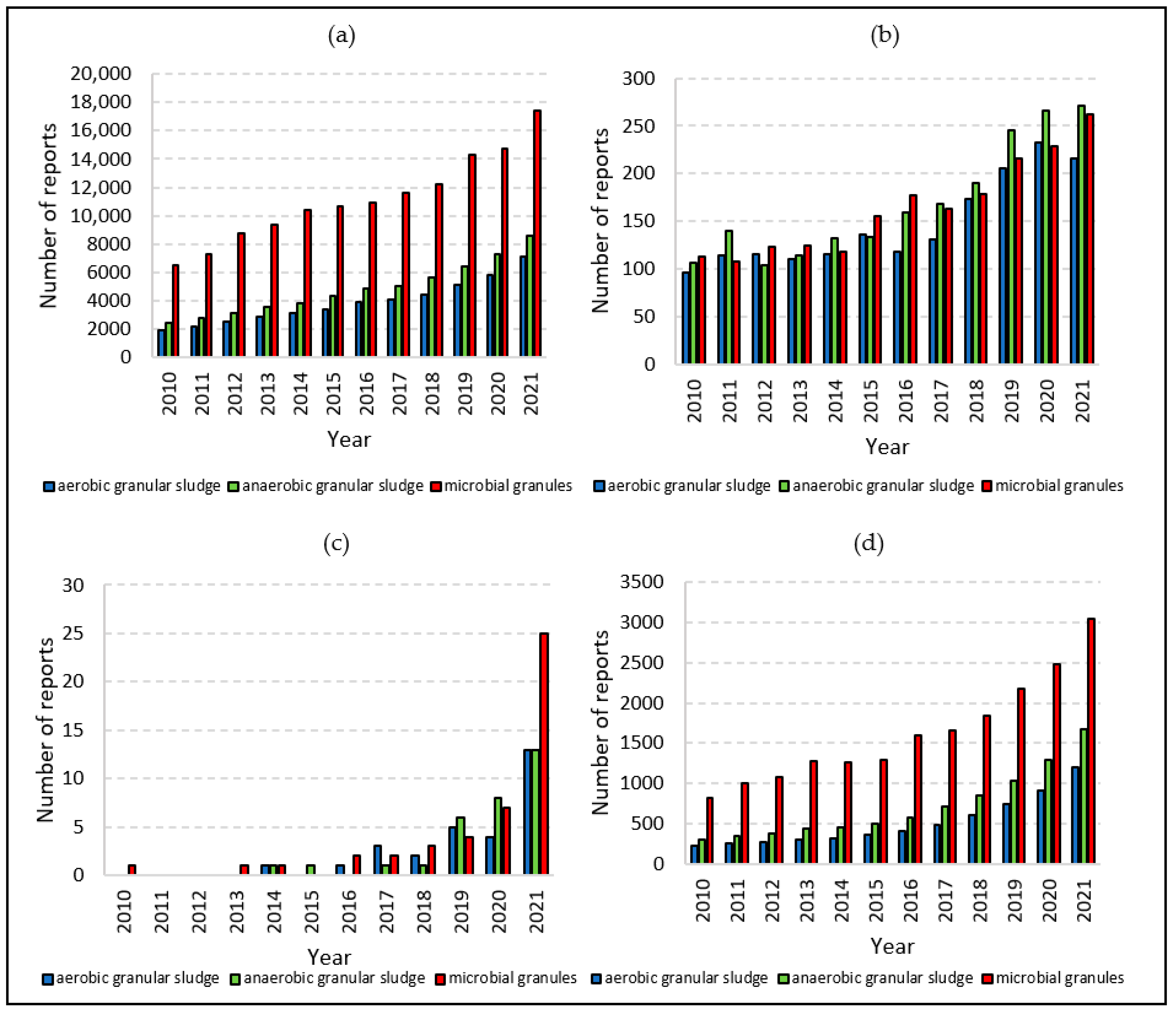
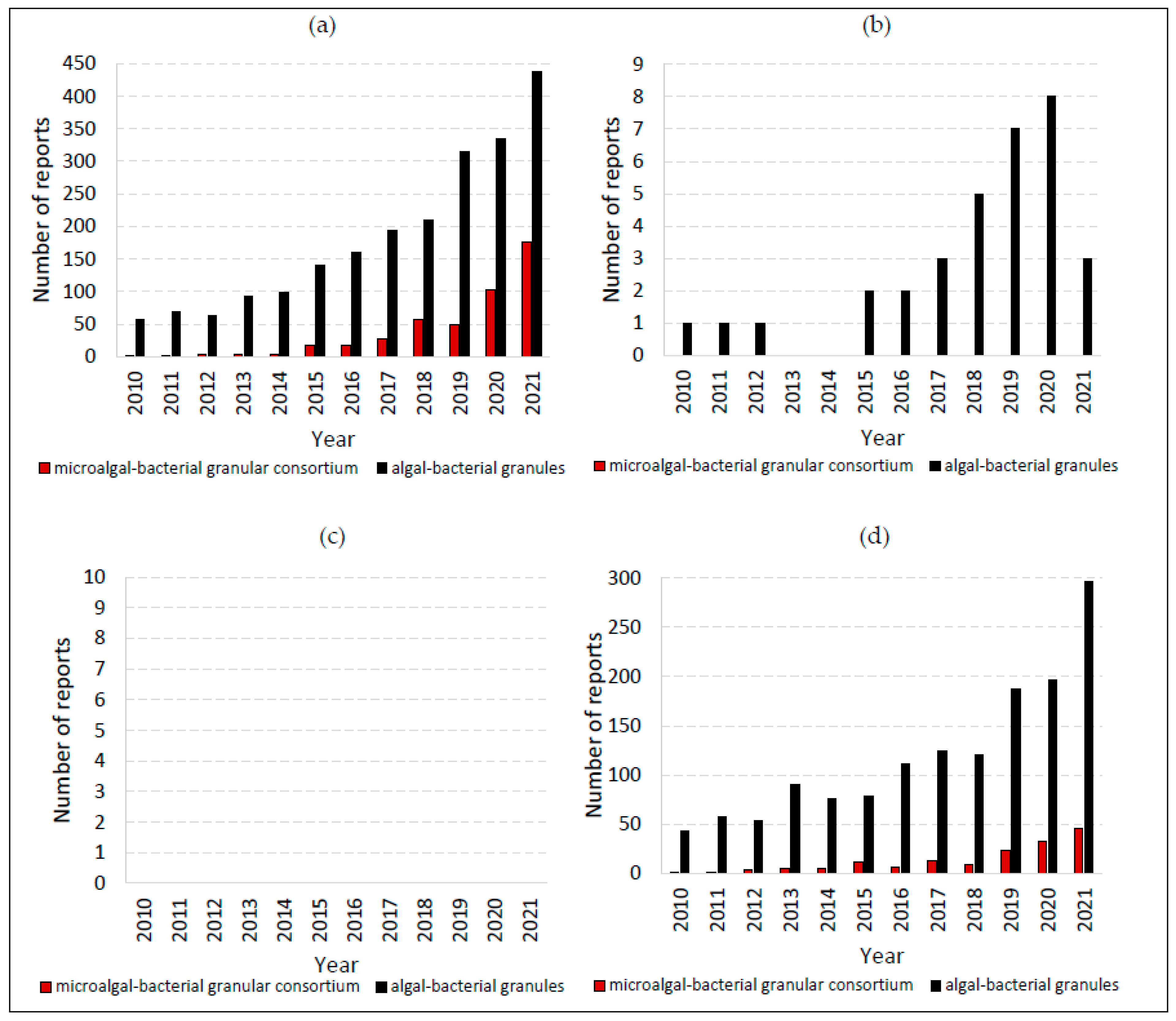
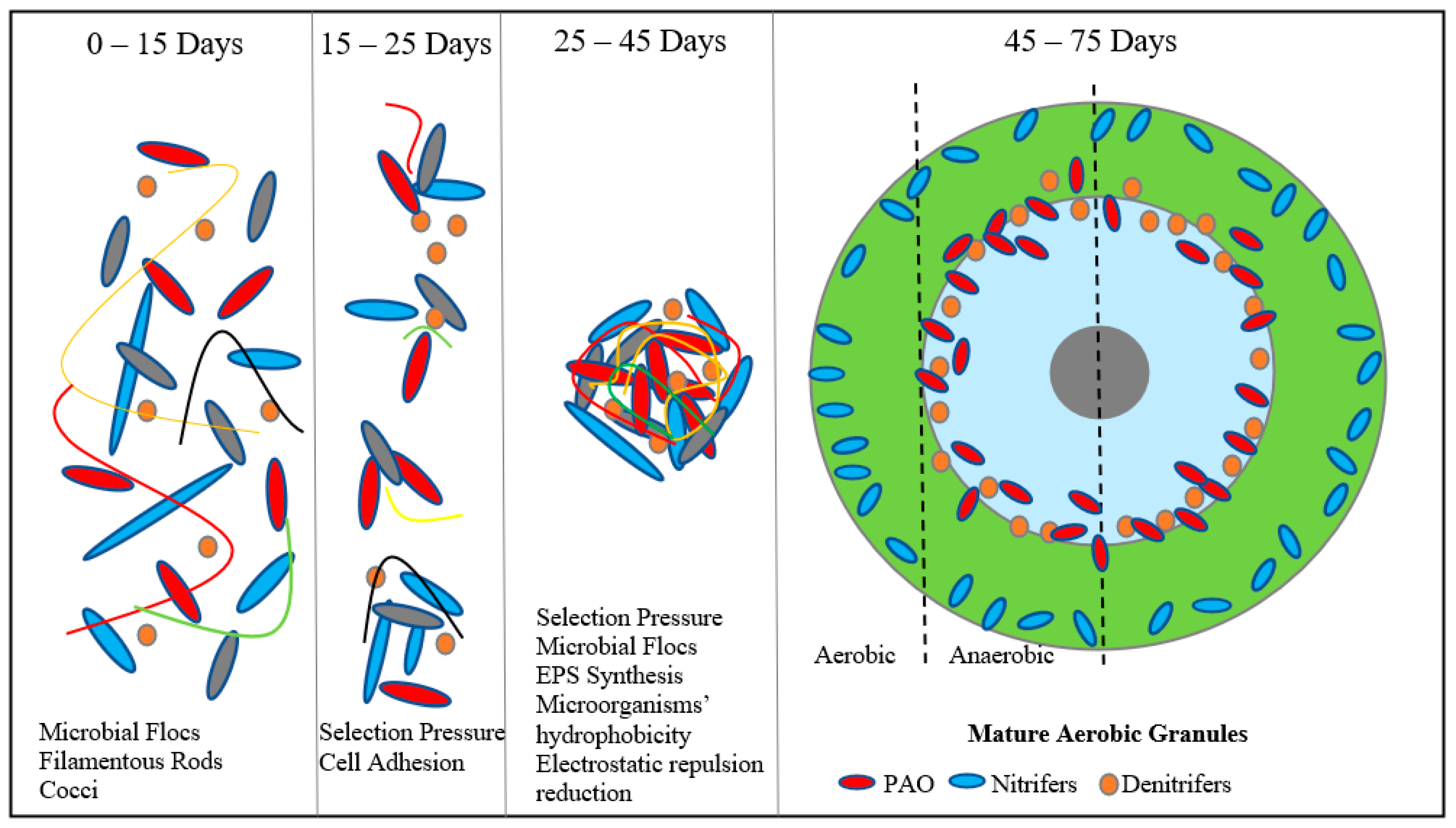


| Parameter | Aim |
|---|---|
| Quality of treated effluent | Compliance with limits for discharged treated effluent |
| Variable loads in the wastewater | Tolerance to variations in hydraulic and organic loads |
| Toxic chemicals and/or metals | Tolerance to toxic pollutants |
| Reliability | Long-term stability and sustainable treatment |
| Operation and maintenance | Flexibility, simplicity, minimal complexity, and low cost |
| Capital costs | Minimum and optimal use |
| Operating costs | Lower energy consumption |
| Space requirements | Minimal space requirements |
| Functional capabilities |
|---|
| High retention of biomass for faster treatment |
| No problems with sludge-bulking |
| Combined COD, N, and P removal from wastewater |
| Simple process flow for N and P removal |
| P removal through increased biological removal of P |
| Removal of pollutants through biological redox reactions |
| Tolerance to shock loads, medium loads, and toxic pollutants |
| Strengths |
| Reduced sludge production and easy sludge dewatering |
| Lower energy cost due to minimal recirculation flows |
| Compact and fast-settling biomass, enabling reduced bioreactor volume |
| No secondary clarifiers |
| Lower space requirements and capital costs |
| Pollutant Removal Steps | Technological Parameters |
|---|---|
| Dephosphatation | Oxygen content: about 0.2 mg O2/dm3 pH: 6.5–8.0 Temp.: optimum 18–20 °C Availability of carbon source (raw sewage) Holding time: about 1 h |
| Nitrification | Dissolved oxygen content > 2 mg O2/dm3 and theoretically 4 mg O2/mg NH4+ pH: 5.5–9 (optimally 7.5) Temp.: 20 °C Microelements: Ca, Fe, Cu, Mg, P Gaseous ammonia with a concentration below 1 mg/dm3 (toxic to nitrifiers) No other toxic compounds (phenols, antibiotics, etc.) Neutralization of the formed nitrous acid, which inhibits both phases of nitrification The presence of carbon dioxide or carbonates as a carbon source for autotrophs |
| Denitrification | Dissolved oxygen content < 0.5 mg O2/dm3 pH: 6.5–7.5 Temp.: 20 °C BZT5: Nog. > 4–6 |
| Aerobic Granular Sludge | |||
|---|---|---|---|
| Type of Wastewater | Operational Conditions | Results | Ref. |
| Piggery wastewater | SBR: Working volume: 30 L Air flow rate: 80–100 L/h Cycle time: 3 h pH: 7.0–7.5 | COD removal: 98% Ammonia removal: 97% TN removal: 92% Antibiotics: 5.2% discharged 62.5% degraded 32.3% adsorbed | [72] |
| Pulp mill wastewater | SBR: Working volume: 4.5 L Upflow air velocity: 2.2 cm/s | COD removal: 73% TN removal: 74% Phosphorus removal: 52% Tannin/lignin removal: 54% Phenols removal: 70% | [73] |
| Synthetic wastewater | SBR: Working volume: 3.5 L Superficial air flow velocity: 1.3 cm/s | EE2: 16.09 µg/g, 77% NP: 20.05 µg/g, 93% CBZ: 10% | [74] |
| Synthetic wastewater | Lab-scale SBR: Working volume: 1.3 L Cycle time: 6 h DO: 6–8 mg/L SRT: 10 days HRT: 12 h | Granular size: Rb 1.18 mm and Rv 0.92 mm SVI5: 32 mL/g(Rb) and 38 mL/g (Rv) Ammonia removal: > 99% Carbon removal: > 95% P removal: 70%–75% in Rb and 44% in Rv | [75] |
| Domestic wastewater | SBR: Cycle time: 3 h Working volume: 3 L 50 days with addition of acetate as carbon source 125 days without additional carbon source | Complete granulation after 51 days Average diameter: 1.5–2.0 mm | [76] |
| Anaerobic granular sludge | |||
| Type of wastewater | Operational conditions | Results | Ref. |
| Olive mill wastewater | Upflow anaerobic sludge blanket (UASB): Working volume: 6 L Total volume: 6.2 L Continuous recirculation for gentle mixing of the bioreactor’s content using an upflow velocity of 1 m/h. The anaerobic granular sludge, consisting of uniformed granules (1–3 mm), was acquired from a full-scale UASB digester treating dairy wastewater. Temperature: 37 ± 1 °C HRT: 9 d OLR: 4.21 g COD/(LR.d) | COD removal: 32 ± 12.7% Phenols removal: 69 ± 14 % | [77] |
| Olive mill wastewater | HUASB (hybrid-UASB): Working volume: 6 L Total volume: 6.2 L The plastic biomass carriers with an active area of 800 m2/m3 (actual size of 2.5 cm diameter and 0.3 cm height) were packed in the upper part of the bioreactor. Continuous recirculation instead of agitation, for gentle mixing of the bioreactor’s content using an upflow velocity of 1 m/h. The anaerobic granular sludge, consisting of uniformed granules (1–3 mm), was acquired from a full-scale UASB digester treating dairy wastewater. Temperature: 37 ± 1 °C HRT: 9 d OLR: 4.21 g COD/(LR.d) | COD removal: 32 ± 6.3% Phenols removal: 46 ± 14% | [77] |
| Vinasse effluent | UASB reactor inoculated with granular sludge Volume: 40.5 L HRT: 2.8 d OLR: 0.2–7.5 g COD/L·d Upflow velocity: 0.019 m/h | The average COD removal: 49–82% | [78] |
| Vinasse effluent | UASB reactor inoculated with granular sludge Volume: 21.5 L HRT: 2.8 d for 219 days and then decreased to 1.8 d OLR: 0.2–11.5 g COD/L·d Upflow velocity: 0.018 m/h | The average COD removal: 49–82% | [78] |
| Synthetic wastewater | A lab-scale plexiglass UASB reactor with height of 71 cm, diameter of 6.8 cm, and total volume of 3.5 L. In stage I (days 1–65), the UASB reactor was started, and the sludge was domesticated with a hydraulic retention time (HRT) of 24 h. In stages II (days 66–91) and III (days 92–112), the HRT was gradually decreased to 6 h for increasing the upflow velocity. In stages IV (days 113–127) and V (days 128–143), 150% and 300% recycling were added to alleviate the antibacterial effect of allicin and increase the upflow velocity. The UASB reactor was fed by a peristaltic pump from the feed tank. The operational temperature was controlled at 30 ± 2 °C. | COD removal: 93.26% EPS enhanced AnGS formation and allicin resistance under allicin stress. The bacterial community contained Acinetobacter and Petrimonas as dominant allicin-resistant genera in AnGS formation process cooperating with the EPS producers Comamonas and Thauera, which improved AnGS tolerance to allicin. | [49] |
| Limitations |
|---|
| Slow granulation or start-up time Poor long-term granule stability AGS systems became less stable when high suspended (floccular) biomass fractions occur The limitation of mass transfer is serious in granules with high density and big size, which affect the specific COD removal rate negatively Various industrial wastewater characteristics negatively impact the sludge granulation process or even lead to de-granulation and loss of biomass Although anaerobic granules were discovered first back in 1976, some definite limitations have been identified such as long start-up period, high operation temperature, and unsuitability for low-strength organic wastewater In a UASB reactor, granule flotation and loss of structure can occur leading to biomass leaching |
| Shortcomings |
| Novel technology requires more understanding and research Technical bottlenecks such as long granulation period and long-term granule instability limit the rapid commercialization of this biotechnology The lack of clear design and performance considerations for implementing AGS-based full-scale reactors which remain the major issues impeding the adoption of AGS in the municipal WWTPs |
| Type of Wastewater | Operational Conditions | Results | Ref. |
|---|---|---|---|
| Synthetic wastewater | Sequencing batch reactor: Working volume of 0.92 L Temp.: 20 °C Illumination of 45 Lx (12 h/12 h) Volume exchange of 50% Operation time: 4 h 2 min feeding + 60 min non-aeration + 172 min aeration + 3 min settling + 2 min decanting + 1 min idle period. | COD removal: 98% TN removal: 78% TP removal: 71% | [117] |
| Synthetic wastewater | Stirring batch reactors: Working volume: 250 mL R1: no salinity R2: 1% salinity Temp.: 25 ± 2 °C Operating cycle: 6 h (7 min feeding + 60 min of non-aeration + 282 min aeration + 2 min settling + 8 min decanting + 1 min of idling) Volume exchange rate (VER): 50% Aeration: 0.81 cm/s Illumination: 6000 lx Retention time: 30–40 days | COD removal: 96.5% TN removal: 78–85% TP removal: 80.8% | [118] |
| Synthetic wastewater | Glass bioreactor: Working volume: 50 mL Temp.: 26 °C Illumination intensity: 210 μ mol/m2s | COD removal: 70.5% TN removal: 80.7% TP removal: 73% | [30] |
| Synthetic wastewater | Sequencing batch reactor: Working volume: 500 mL Temp.: 25 °C 4 h operational time Volume exchange ratio of 50% Aeration of 0.87 cm/s Illumination: 3600 lx | COD removal: NM TN removal: 66% TP removal: 70% | [119] |
| Simulated wastewater | Sequencing batch reactor: Working volume: 2.0 L 4 h cycle (2 min feeding + 232 min aeration + 4 min settling + 2 min decanting) Volume exchange ratio: 50.0% HRT: 8.0 h DO: 7.0–9.0 mg/L Aeration: 1.2 cm/s (2.0 L/min) Temp.: 18.0–23.0 °C Illumination: 1531 mmol/m2s COD/NH4-N: 309.4 /213.6 mg/L | COD removal: NM TN removal: 96.5% TP removal: NM | [120] |
| Simulated wastewater | Glass bottles: Working volume: 50 mL In open environment 12 h/12 h | COD removal: during day 59.9 ± 6.8%, during dark 47.6% N removal: during day NH4+-N: 78%, during dark 56% P removal: during day 61%, during dark 74% | [91] |
| GS | Type of Wastewater | Operational Conditions | Results | Ref. |
|---|---|---|---|---|
| AGS | Municipal wastewater | Glass bottles (OxiTop system): Temp.: 36 ± 1 °C HRT: 21 days OLR: 2, 4, 6 kg VS/m3·d | CH4: 272.5–357 L/kg VS | [149] |
| Municipal wastewater | Flasks with a volume of 2 L Temp.: 35 ± 1 °C Time: 44 days 120 rpm | CH4: 197 ± 11 L/kg VS | [150] | |
| Synthetic wastewater | Flasks with a volume of 525 mL: Temp.: 37 °C OLR: 0.7–0.9 gCOD/ L·d SRT: 15–>40 d | CH4: 245–285 L/kg VS | [135] | |
| Municipal wastewater | Quartz cylindrical reactor time Pyrolysis time: 4 h Temp.: 500–800 °C with a heating rate of 3 °C/min | Maximum yield of bio-oil: 43.6% of weight-lost percent during GSS pyrolysis | [151] | |
| AnGS | Olive mill wastewater | Upflow anaerobic sludge blanket (UASB): Working volume: 6 L Total volume: 6.2 L Continuous recirculation for gentle mixing of the bioreactor’s content using an upflow velocity of 1 m/h. The anaerobic granular sludge, consisting of uniformed granules (1–3 mm), was acquired from a full-scale UASB digester treating dairy wastewater. Temp.: 37 ± 1 °C HRT: 9 OLR: 4.21 g COD/(LR.d) | Biogas Production Rate: 0.91 ± 0.25 LB/(LR.d) CH4 content: 34.07 ± 8.20% Yield: 0.21 ± 0.07 L CH4/g COD converted | [77] |
| Olive mill wastewater | HUASB (hybrid-UASB): Working volume: 6 L Total volume: 6.2 L The plastic biomass carriers with an active area of 800 m2/m3 (actual size of 2.5 cm diameter and 0.3 cm height) were packed in the upper part of the bioreactor. Continuous recirculation instead of agitation for gentle mixing of the bioreactor’s content using an upflow velocity of 1 m/h. The anaerobic granular sludge, consisting of uniformed granules (1–3 mm), was acquired from a full-scale UASB digester treating dairy wastewater. Temperature: 37 ± 1 °C HRT: 9 OLR: 4.21 g COD/(LR.d) | Biogas Production Rate: 1.01 ± 0.23 LB/(LR.d) CH4 content: 35.93 ± 5.53% Yield: 0.27 ± 0.08 L CH4/g COD converted | [77] | |
| Vinasse effluent | UASB reactor inoculated with granular sludge Volume: 40.5 L HRT: 2.8 d OLR: 0.2–7.5 g COD/L·d Upflow velocity: 0.019 m/h | Average conversion efficiencies of the removed COD into methane: 48–58%. The largest methane yield values: 0.181 L CH4/gCODremoved. These values were attained after 140 days of operation with an OLR of 5.0–7.5 g COD/L·d. | [78] | |
| Vinasse effluent | UASB reactor inoculated with granular sludge Volume: 21.5 L HRT: 2.8 d for 219 days and then decreased to 1.8 d OLR: 0.2–11.5 g COD/L·d Upflow velocity: 0.018 m/h | Average conversion efficiencies of the removed COD into methane: 39–65%. The largest methane yield values: 0.185 L CH4/gCODremoved. These values were attained after 140 days of operation with an OLR of 5.0–7.5 g COD/L·d. | [78] | |
| Synthetic wastewater | Bottles Volume: 125 mL Time: 21 d Temp.: 30 °C Gas pressure: 0.5 bar | Ethanol production: 17.1 mM Propanol: 8.08 ± 0.85 mM n-butanol: 3.66 ± 0.05 mM | [152] | |
| Dairy wastewater | Bottles Volume: 125 mL Time: 408 h Temp.: 25 °C Gas pressure: 1.8 bar | Ethanol production 17.1 mM | [153] | |
| Food industry wastewater | Batch bioreactors Temp.: 35 °C pH: 5.5 ± 0.3 | Biohydrogen: 72.9 ± 5.7 mL H2/g CODrem | [154] | |
| Municipal wastewater | AnGS was successfully demonstrated as a novel and efficient biocatalyst in METs such as microbial fuel cells. Three different strategies were explored to shift the microbial composition of AGS from methanogenic to exoelectrogenic microbes, including varying the external resistance and organic loading and manipulating the anode potential | The significantly high current response: 10.32 A/m2 and 100% removal of organic carbon from wastewater. | [155] | |
| M-BGs | Synthetic wastewater | Photo sequencing batch reactors (PSBRs) Illumination incubator (12 h light/12 h dark, 6000 ± 200 lux) Influent feeding 1 min, aeration 356 min, sedimentation 2 min, effluent withdrawal 1 min Aeration intensity: 4 L/min Temp.: 26 ± 1 °C pH: 7.5 ± 0.1 | Biodiesel production: 66.21 ± 1.08 mg/g·SS | [156] |
| Synthetic wastewater | Fixed bed reactor: Temp.: 673–1073 K Time: 1 h | Biooil: 39.5–45.4 wt% Biochar with a nitrogen content of 3.7–7.0 wt% | [157] | |
| Municipal wastewater | Lab-scale identical SBR reactors made of acrylic plastic Working volume: 2.0 L Dark/light cycle: 12 h/12 h Light intensity: 0–225 µmol/ m2·s1 Temp.: 23 ± 2 °C HRT: 8 h | Lipid content: 31.2–59.6 mg/g-SS | [158] | |
| Synthetic wastewater (Ammonium-rich wastewater) | Lab-scale sequencing batch reactors (SBRs) made of transparent acrylic plastic Effective working volume: 2.0 L Average light illuminance: 190 μmol/m2/s with a constant dark/light (12 h/12 h) cycle 3 h cycle: 2 min of feeding, 20 min of non-aeration, 152 min of aeration, 4 min of settling, and 2 min of decanting Temp.: 20–23 °C HRT: 6 h | Lipid content: 57.4 mg/g-SS | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Microbial Granule Technology—Prospects for Wastewater Treatment and Energy Production. Energies 2023, 16, 75. https://doi.org/10.3390/en16010075
Kazimierowicz J, Dębowski M, Zieliński M. Microbial Granule Technology—Prospects for Wastewater Treatment and Energy Production. Energies. 2023; 16(1):75. https://doi.org/10.3390/en16010075
Chicago/Turabian StyleKazimierowicz, Joanna, Marcin Dębowski, and Marcin Zieliński. 2023. "Microbial Granule Technology—Prospects for Wastewater Treatment and Energy Production" Energies 16, no. 1: 75. https://doi.org/10.3390/en16010075
APA StyleKazimierowicz, J., Dębowski, M., & Zieliński, M. (2023). Microbial Granule Technology—Prospects for Wastewater Treatment and Energy Production. Energies, 16(1), 75. https://doi.org/10.3390/en16010075









