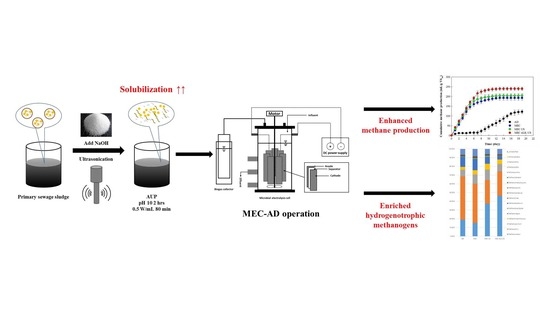
Development of a Primary Sewage Sludge Pretreatment Strategy Using a Combined Alkaline–Ultrasound Pretreatment for Enhancing Microbial Electrolysis Cell Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sludge Preparation and Disintegration Experiment
2.2. Reactor Construction and Operation
2.3. Analytical Methods
2.4. 16S rRNA Gene Sequencing and Taxonomic Assignment
3. Results and Discussion
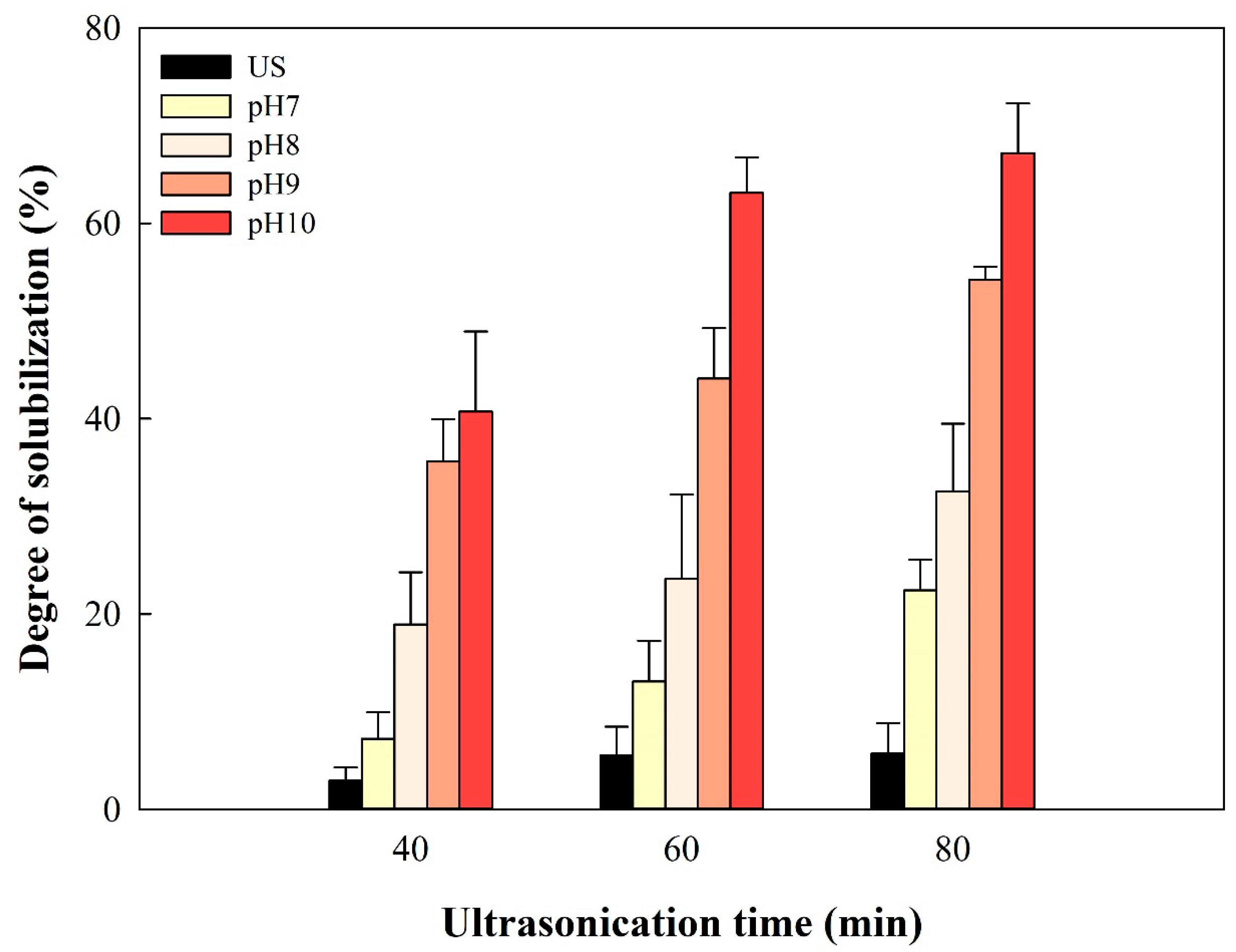
3.1. Sludge Solubilization Experiment
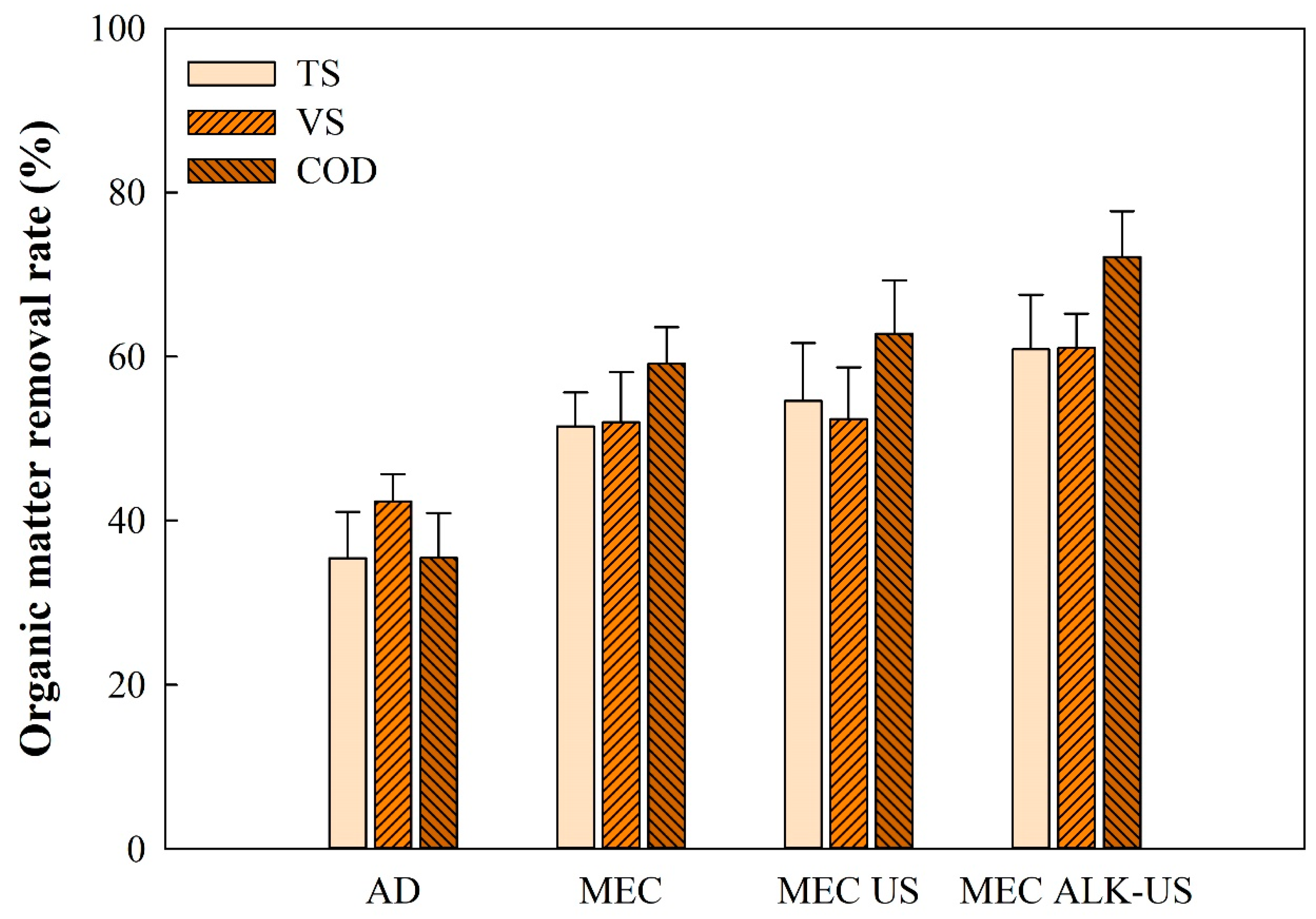
3.2. Organic Matter Removal Rate
3.3. Biogas Production and Modified Gompertz Model
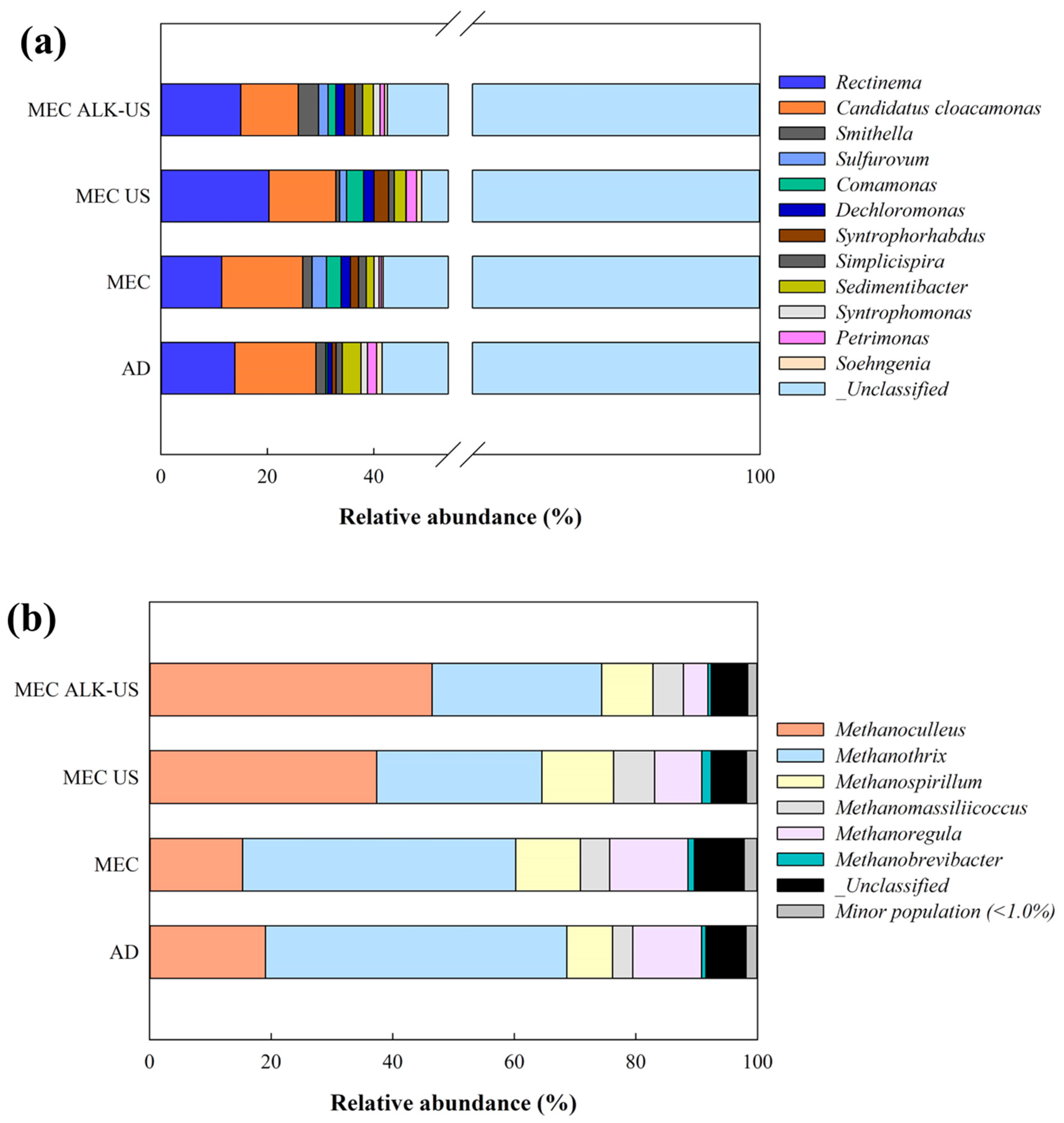
3.4. Microbial Taxonomy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| AUP | Combined alkaline–ultrasound pretreatment |
| DIET | Direct interspecies electron transfer |
| DS | Degree of solubilization, |
| EAB | Electroactive bacteria |
| EMA | Electromethanogenic archaea |
| λ | Lag phase |
| MEC | Microbial electrolysis cell |
| MEC-AD | MEC assisted anaerobic digester |
| MEC US | MEC fed with ultrasound pretreated sludge |
| MEC ALK-US | MEC fed with combined alkaline–ultrasound-pretreated sludge |
| Rm | Maximum methane production rate |
References
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.P.; Havukainen, J. Anaerobic Digestion: Factors Affecting Anaerobic Digestion Process. In Waste Bioremediation; Varjani, S.J., Gnansounou, E., Gurunathan, B., Pant, D., Zakaria, Z.A., Eds.; Springer: Singapore, 2018; pp. 343–359. [Google Scholar]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal Pretreatment of Sewage Sludge to Enhance Anaerobic Digestion: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonication of wastewater sludge—Consequences on biodegradability and flowability. J. Hazard. Mater. 2009, 163, 891–898. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Ji, M. Mechanisms and kinetics models for ultrasonic waste activated sludge disintegration. J. Hazard. Mater. 2005, 123, 145–150. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Liu, W.; Zou, S. Optimized alkaline pretreatment of sludge before anaerobic digestion. Bioresour. Technol. 2012, 123, 189–194. [Google Scholar] [CrossRef]
- Kim, J.; Yu, Y.; Lee, C. Thermo-alkaline pretreatment of waste activated sludge at low-temperatures: Effects on sludge disintegration, methane production, and methanogen community structure. Bioresour. Technol. 2013, 144, 194–201. [Google Scholar] [CrossRef]
- Grübel, K.; Suschka, J. Hybrid alkali-hydrodynamic disintegration of waste-activated sludge before two-stage anaerobic digestion process. Environ. Sci. Pollut. Res. 2015, 22, 7258–7270. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Herrera, R.R.; Pedreguera, A.Z.; Ruiz Espinoza, J.E. Effects of ultrasonic pretreatment on the solubilization and kinetic study of biogas production from anaerobic digestion of waste activated sludge. Int. Biodeterior. Biodegrad. 2017, 123, 1–9. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, E.; Oh, S.-E.; Shin, H.-S. Combined (alkaline + ultrasonic) pretreatment effect on sewage sludge disintegration. Water Res. 2010, 44, 3093–3100. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Yin, C.; Shen, Y.; Yuan, R.; Zhu, N.; Yuan, H.; Lou, Z. Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbial electrolysis cell for methane production. Bioresour. Technol. 2019, 284, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.; Witarsa, F.; Guo, X.; Yong, L.; Lansing, S.; Qiu, L. Next generation digestion: Complementing anaerobic digestion (AD) with a novel microbial electrolysis cell (MEC) design. Int. J. Hydrogen Energy 2017, 42, 28681–28689. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Quan, X.; Chen, S.; Afzal, S. Enhanced anaerobic digestion of organic contaminants containing diverse microbial population by combined microbial electrolysis cell (MEC) and anaerobic reactor under Fe(III) reducing conditions. Bioresour. Technol. 2013, 136, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, W.; Sun, Y.; Ding, W.; Cheng, S. Enhancing hydrogen production with Ni–P coated nickel foam as cathode catalyst in single chamber microbial electrolysis cells. Int. J. Hydrogen Energy 2017, 42, 3641–3646. [Google Scholar] [CrossRef]
- Cho, S.-K.; Lee, M.-E.; Lee, W.; Ahn, Y. Improved hydrogen recovery in microbial electrolysis cells using intermittent energy input. Int. J. Hydrogen Energy 2019, 44, 2253–2257. [Google Scholar] [CrossRef]
- Wang, X.-T.; Zhao, L.; Chen, C.; Chen, K.-Y.; Yang, H.; Xu, X.-J.; Zhou, X.; Liu, W.-Z.; Xing, D.-F.; Ren, N.-Q.; et al. Microbial electrolysis cells (MEC) accelerated methane production from the enhanced hydrolysis and acidogenesis of raw waste activated sludge. Chem. Eng. J. 2021, 413, 127472. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A.; Kakde, A.; Liu, P.; Li, X. A review on the applications of microbial electrolysis cells in anaerobic digestion. Bioresour. Technol. 2018, 255, 340–348. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Dhar, B.R. Progress towards catalyzing electro-methanogenesis in anaerobic digestion process: Fundamentals, process optimization, design and scale-up considerations. Bioresour. Technol. 2019, 289, 121738. [Google Scholar] [CrossRef]
- Cusick, R.; Bryan, B.; Parker, D.; Merrill, M.; Mehanna, M.; Kiely, P.; Liu, G.; Logan, B. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, Z.J.; Huang, C.; Liu, B.; Ren, N.; Xing, D. Multiple syntrophic interactions drive biohythane production from waste sludge in microbial electrolysis cells. Biotechnol. Biofuels 2016, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Chen, W.; Jia, S.-Q.; Wang, W.; Han, F. Enhanced Degradation of Waste Activated Sludge in Microbial Electrolysis Cell by Ultrasonic Treatment. Front. Microbiol. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Joicy, A.; Seo, H.; Lee, M.-E.; Kim, D.-H.; Cho, S.K.; Ahn, Y. Enhanced methane production using pretreated sludge in MEC-AD system: Performance, microbial activity, and implications at different applied voltages. Int. J. Hydrogen Energy 2022, 47, 40731–40741. [Google Scholar] [CrossRef]
- Song, Y.-C.; Feng, Q.; Ahn, Y. Performance of the Bio-electrochemical Anaerobic Digestion of Sewage Sludge at Different Hydraulic Retention Times. Energy Fuels 2016, 30, 352–359. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.-C. Surface Modification of a Graphite Fiber Fabric Anode for Enhanced Bioelectrochemical Methane Production. Energy Fuels 2016, 30, 6467–6474. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H.; Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Gong, L.; Yang, X.; Wang, Z.; Zhou, J.; You, X. Impact of hydrothermal pre-treatment on the anaerobic digestion of different solid–liquid ratio sludges and kinetic analysis. RSC Adv. 2019, 9, 19104–19113. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef]
- Lane, D. 16S/23S rRNA sequencing. In Nucleic acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Rhee, C.; Park, S.-G.; Yu, S.I.; Dalantai, T.; Shin, J.; Chae, K.-J.; Shin, S.G. Mapping microbial dynamics in anaerobic digestion system linked with organic composition of substrates: Protein and lipid. Energy 2023, 275, 127411. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Gonze, E.; Pillot, S.; Valette, E.; Gonthier, Y.; Bernis, A. Ultrasonic treatment of an aerobic activated sludge in a batch reactor. Chem. Eng. Process. Process Intensif. 2003, 42, 965–975. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Bao, H.; Yang, H.; Zhang, H.; Liu, Y.; Su, H.; Shen, M. Improving methane productivity of waste activated sludge by ultrasound and alkali pretreatment in microbial electrolysis cell and anaerobic digestion coupled system. Environ. Res. 2020, 180, 108863. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yi, J.; Luo, K.; Jing, X.; Li, X.; Liu, Y.; Zeng, G. Improving disintegration and acidification of waste activated sludge by combined alkaline and microwave pretreatment. Process Saf. Environ. Prot. 2013, 91, 521–526. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Li, Y.-Y.; Zhao, Y. Combined electrical-alkali pretreatment to increase the anaerobic hydrolysis rate of waste activated sludge during anaerobic digestion. Appl. Energy 2014, 128, 93–102. [Google Scholar] [CrossRef]
- Lu, D.; Xiao, K.; Chen, Y.; Soh, Y.N.A.; Zhou, Y. Transformation of dissolved organic matters produced from alkaline-ultrasonic sludge pretreatment in anaerobic digestion: From macro to micro. Water Res. 2018, 142, 138–146. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Removal of volatile fatty acids and ammonia recovery from unstable anaerobic digesters with a microbial electrolysis cell. Bioresour. Technol. 2016, 219, 348–356. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. Prot. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Beegle, J.R.; Borole, A.P. An integrated microbial electrolysis-anaerobic digestion process combined with pretreatment of wastewater solids to improve hydrogen production. Environ. Sci. Water Res. Technol. 2017, 3, 1073–1085. [Google Scholar] [CrossRef]
- Wang, A.; Liu, W.; Cheng, S.; Xing, D.; Zhou, J.; Logan, B.E. Source of methane and methods to control its formation in single chamber microbial electrolysis cells. Int. J. Hydrogen Energy 2009, 34, 3653–3658. [Google Scholar] [CrossRef]
- Asztalos, J.R.; Kim, Y. Enhanced digestion of waste activated sludge using microbial electrolysis cells at ambient temperature. Water Res. 2015, 87, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Su, M.; Li, D. Removal of Sulfide and Production of Methane from Carbon Dioxide in Microbial Fuel Cells-Microbial Electrolysis Cell (MFCs-MEC) Coupled System. Appl. Biochem. Biotechnol. 2014, 172, 2720–2731. [Google Scholar] [CrossRef] [PubMed]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Basséguy, R.; Délia, M.L.; Erable, B.; Bergel, A. 5—Electroactive biofilms. In Understanding Biocorrosion; Liengen, T., Féron, D., Basséguy, R., Beech, I.B., Eds.; Woodhead Publishing: Oxford, UK, 2014; pp. 107–143. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Wang, W.; Liu, G.; Chen, C. Assessment of pretreatment effects on anaerobic digestion of switchgrass: Economics-energy-environment (3E) analysis. Ind. Crops Prod. 2020, 145, 111957. [Google Scholar] [CrossRef]
- Ma, S.-J.; Ma, H.-J.; Hu, H.-D.; Ren, H.-Q. Effect of mixing intensity on hydrolysis and acidification of sewage sludge in two-stage anaerobic digestion: Characteristics of dissolved organic matter and the key microorganisms. Water Res. 2019, 148, 359–367. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Parameswaran, P.; Kang, D.W.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef]
- Dong, X.; Greening, C.; Brüls, T.; Conrad, R.; Guo, K.; Blaskowski, S.; Kaschani, F.; Kaiser, M.; Laban, N.A.; Meckenstock, R. Fermentative Spirochaetes mediate necromass recycling in anoxic hydrocarbon-contaminated habitats. ISME J. 2018, 12, 2039–2050. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, H.; Chen, Y.; Gou, M.; Xia, Z.; Hu, B.; Nie, Y.; Tang, Y. Identification of Novel Butyrate- and Acetate-Oxidizing Bacteria in Butyrate-Fed Mesophilic Anaerobic Chemostats by DNA-Based Stable Isotope Probing. Microb. Ecol. 2020, 79, 285–298. [Google Scholar] [CrossRef]
- Juste-Poinapen, N.; Turner, M.; Rabaey, K.; Virdis, B.; Batstone, D. Evaluating the potential impact of proton carriers on syntrophic propionate oxidation. Sci. Rep. 2015, 5, 18364. [Google Scholar] [CrossRef]
- Solli, L.; Håvelsrud, O.; Horn, S.; Rike, A. A metagenomic study of the microbial communities in four parallel biogas reactors. Biotechnol. Biofuels 2014, 7, 146. [Google Scholar] [CrossRef]
- Ariesyady, H.D.; Ito, T.; Yoshiguchi, K.; Okabe, S. Phylogenetic and functional diversity of propionate-oxidizing bacteria in an anaerobic digester sludge. Appl. Microbiol. Biotechnol. 2007, 75, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Ariesyady, H.D.; Ito, T.; Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007, 41, 1554–1568. [Google Scholar] [CrossRef]
- Barret, M.; Gagnon, N.; Kalmokoff, M.L.; Topp, E.; Verastegui, Y.; Brooks, S.P.J.; Matias, F.; Neufeld, J.D.; Talbot, G. Identification of Methanoculleus spp. as Active Methanogens during Anoxic Incubations of Swine Manure Storage Tank Samples. Appl. Environ. Microbiol. 2013, 79, 424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, G.; Li, L.; Sun, Y. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manag. 2018, 79, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Xu, C.; Shi, X.; Yang, S.; Li, L.; Peng, X.; Song, L. Acetoclastic methanogenesis pathway stability despite the high microbial taxonomic variability in the transition from acidogenesis to methanogenesis during food waste anaerobic digestion. J. Clean. Prod. 2022, 372, 133758. [Google Scholar] [CrossRef]


| ADseed a | Sludge b | UP c | AUP d | |
|---|---|---|---|---|
| TS (g L−1) | 25.65 | 44.12 ± 3.00 | 46.60 | 48.00 |
| VS (g L−1) | 17.20 | 29.13 ± 0.40 | 29.30 | 29.20 |
| TCOD (g L−1) | 20.74 | 44.88 ± 2.03 | 44.49 | 43.94 |
| SCOD (g L−1) | 1.25 | 1.47 ± 0.43 | 4.31 | 12.93 |
| pH | 8.3 | 5.78 ± 0.26 | 5.83 | 6.97 |
| Alkalinity (mg L as CaCO3−1) | 5945 | 710 ± 119 | 680 | 1610 |
| TVFAs (mg L as HAc−1) | 1030 | 784 ± 32 | 899 | 1328 |
| No. | Types of Sludge | Pretreatment | DS (%) | Reference | |
|---|---|---|---|---|---|
| Methods | Conditions | ||||
| 1 | WAS a | Electrical + alkaline | pH 12.2 + 20 V 40 min | 54.7 | [37] |
| 2 | WAS a | Alkaline + thermal | pH 11 18 h + 90 °C 1.5 h | 53.1 | [24] |
| 3 | WAS a | Alkaline + microwave | pH 11 + 38,400 kJ/kg TS | 65.9 | [36] |
| 4 | WAS a | Alkaline + ultrasound | pH 12 0.5 h + 24 kJ g TS−1 | 36.9 | [38] |
| 5 | WAS a | Ultrasound + alkali | 0.5 kW L−1 10 min + pH 10 0.5 h | 35.0 | [35] |
| 6 | Primary sewage sludge | Alkaline + ultrasound | pH 10 2 h + 0.5 W mL−1 80 min | 67.2 | This study |
| Yield (mL g VSin−1) | Rm e (mL g VSin−1 d−1) | λ f (day) | |
|---|---|---|---|
| AD a | 122 ± 8.4 | 10.2 ± 2.7 | 5.6 ± 0.3 |
| MEC b | 193 ± 11.8 | 34.7 ± 3.0 | 0.4 ± 0.4 |
| MEC US c | 204 ± 9.4 | 38.3 ± 1.4 | 0.4 ± 0.2 |
| MEC ALK-US d | 240 ± 6.4 | 40.0 ± 2.1 | 0.2 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Joicy, A.; Lee, M.E.; Rhee, C.; Shin, S.G.; Cho, S.-K.; Ahn, Y. Development of a Primary Sewage Sludge Pretreatment Strategy Using a Combined Alkaline–Ultrasound Pretreatment for Enhancing Microbial Electrolysis Cell Performance. Energies 2023, 16, 3986. https://doi.org/10.3390/en16103986
Seo H, Joicy A, Lee ME, Rhee C, Shin SG, Cho S-K, Ahn Y. Development of a Primary Sewage Sludge Pretreatment Strategy Using a Combined Alkaline–Ultrasound Pretreatment for Enhancing Microbial Electrolysis Cell Performance. Energies. 2023; 16(10):3986. https://doi.org/10.3390/en16103986
Chicago/Turabian StyleSeo, Hwijin, Anna Joicy, Myoung Eun Lee, Chaeyoung Rhee, Seung Gu Shin, Si-Kyung Cho, and Yongtae Ahn. 2023. "Development of a Primary Sewage Sludge Pretreatment Strategy Using a Combined Alkaline–Ultrasound Pretreatment for Enhancing Microbial Electrolysis Cell Performance" Energies 16, no. 10: 3986. https://doi.org/10.3390/en16103986